Abstract
Objectives: The purpose of this study was to investigate the effects of mechanical fascia manipulation techniques as a noninvasive method to treat cellulite.
Study Design: This study is designed to be a clinical study looking at the effects of a non-invasive treatment for the appearance of cellulite. Methods: Thirty-three adult women in the experimental (FT) group with thigh cellulite were instructed on fascia manipulation techniques (FMT), using the FasciaBlaster® devices (FBD) 5 days a week for 12 weeks. Measurements of subcutaneous adipose thickness (SAT) were measured using ultrasonography, along with measurements of body composition, metabolism, and routine blood chemistries at 4-week intervals.
Results: In the FT group, SAT was lower from baseline at Wk4, Wk8, and Wk12. Resting energy expenditure (REE) was greater at Wk12 compared to baseline. Carboxyterminal Propeptide of Type I Procollagen (PICP) was higher at Wk4 compared to baseline. Also compared to baseline, serum irisin levels were significantly higher at Wk4, Wk8, and Wk12. No significant differences were detected in routine blood chemistries or total body composition during study period in both the FT group and the control group (CON).
Conclusions: The present study provides evidence that fascia manipulation techniques (FMT), through use of the FasciaBlaster® devices (FBD), can decrease subcutaneous adipose tissue (SAT), and the appearance of cellulite in adult women over 12 weeks. Evidence also shows improved collagen remodeling and the overall safety of fascia manipulation and the FasciaBlaster® devices.
PUBLIC INTEREST STATEMENT
Cellulite is a major cosmetic concern, which affects up to 85% of females. There are many different causes and treatments for cellulite but there is little research on how to effectively treat it. This article describes how our body creates cellulite and more importantly, a non-invasive way to address it. Not only did the fascia therapy device improve the appearance of cellulite, it has the potential for a large variety of other health improvements such as inflammation, rehabilitation, recovery, anti-aging, and metabolism.
1. Background
Cellulite is the commonly used term for the dimpled lumpy appearance of the skin, usually in the thigh and buttock region, that affects greater than 85% of post‐pubertal females of all ethnicities (Bayrakci Tunay, Akbayrak, & Bakar et al., Citation2010). For this reason, cellulite is a major cosmetic concern for a large percentage of the female population. This significance of this study is to research a specific non-invasive technique, fascia manipulation specifically with FasciaBlaster® devices, as an effective way to reduce the appearance cellulite. Our hypothesis is that by using the FasciaBlaster® devices to provide a method of fascia manipulation, that the appearance of cellulite can be reduced.
Mechanistically, cellulite is likely caused by excess adipose tissue retention with fibrous septae that sequester fat in discrete packets along with vertically oriented fascial bands that are anchored to the deep fascia, giving the skin its distinctive puckered, irregular, “orange-peel” appearance (Alster & Tanzi, Citation2005; Romero, Caballero, & Herrero et al., Citation2008). There are many factors that may affect the formation and appearance of cellulite, including genetic factors, hormonal disturbances, disturbances in lymph and blood microcirculation, dietary habits, psychological factors, pregnancy, sedentary lifestyle and alcohol consumption (Bayrakci Tunay et al., Citation2010; Lucassen, Sluys, & Herk et al., Citation1997). Cellulite is a clinical condition that is not well understood, despite being such a common problem for women, and there are few effective non-invasive treatments available.
Romero et al. (Romero et al., Citation2008) found that mechanical massage provides a non-invasive method, which has been demonstrated to treat cellulite in a similar manner to procedures used in a clinical plastic surgery setting. Similarly, Bayrakci Tunay et al. (Citation2010) found that both mechanical massage and fascia manipulation resulted in an improvement in thinning of the subcutaneous fat after the treatment and decreased thigh circumference. While the mechanism has yet to be fully elucidated, it is likely that fascia manipulation frees fat cells from fibrous septae, thereby decreasing projections in the dermis (Romero et al., Citation2008). In addition, random histological analyses performed by Romero et al. (Romero et al., Citation2008) found that mechanical massage improved dermal firmness, fiber compaction, and tightening of skin layers, all of which are possible reasons for the effects achieved. This hypothesis has been validated by Lucassen et al. (Lucassen et al., Citation1997) who found that mechanical manipulation of fascia resulted in significant smoothing of the dermis-hypodermis junction, as monitored by ultrasound imaging.
While mechanical stimulation of fascia appears to have a positive impact on the appearance of cellulite, there is a need for safe and effective devices that can be used for this treatment. The purpose of this study was to investigate the effects of such fascia manipulation techniques (FMT), using non-invasive FasciaBlaster® devices (FBD), (FasciaBlaster®, Mini2™, and FaceBlaster®, Ashley Diana Black International Holdings, LLC; Houston, TX) to treat cellulite. A secondary purpose was to investigate the impact of FMT with FBD on the metabolism and connective tissue remodeling, and to establish its overall safety as a potential method to reduce the appearance of cellulite.
2. Methods
2.1. Subjects
A total of 43 women volunteered to participate in this controlled study, with 33 women in the experimental group, referred to collectively as (FT) and 10 similar women in the control group, referred to collectively as (CON). Vital statistics (age, height and weight) were gathered from all 43 volunteers. The 33 women of FT group were very similar (age: 40 ± 6 years, height: 165.51 ± 2.66, weight: 76.67 ± 14.79 kg) to the 10 women volunteers in the CON group (age: 43 ± 2 years, height: 165.10 ± 4.02 cm, weight: 73.80 ± 23.45 kg). All test subjects were instructed to not change their diet or exercise routine for the duration of the study (12 weeks). All subjects were screened for the following inclusion criteria: age 25 to 60 years old, a Body Mass Index (BMI) of 20.0 to 30.0 kg/m2, visually apparent cellulite on the thighs, non-habitual caffeine users (defined as less than 2 cups of coffee per day), no use of a thermogenic or weight loss supplement (for at least 4 weeks prior to and through the 12-week duration of the study). All subjects were subsequently screened and were excluded from the study if they: had metabolic or musculoskeletal disorders; had cardiovascular abnormalities; had experienced a musculoskeletal injury within the previous 6 months; had a history of smoking or recreational drug use; excessively consumed alcohol (more than 2 drinks per day); were pregnant or planning to be pregnant; were breastfeeding; had experienced weight loss exceeding 10lbs within the previous 6 months; were subjects in another research study with the last 12 months. Each subject signed an informed consent form that was approved by an Institutional Review Board (IntegReview, LLC; Austin, TX, USA). All subjects underwent all of the following testing procedures at baseline (BL), week 4 (Wk4), week 8 (Wk8), and week 12 (Wk12) of the study: ultrasound imaging, metabolic measurements, body composition analysis and hematologic studies. All FT subjects also underwent visual photography at BL, Wk4, Wk8, and Wk12 of the study.
2.2. FT protocol
All 33 FT subjects were instructed to complete a 90-day home protocol of FMT, which consisted of self-administered brisk scrubbing movements over the skin with pressure into the myofascial tissue ranging from light to moderate using the FBD. Subjects were instructed on FMT by a trained technician immediately following baseline measurements. The subjects started the protocol by sitting in a portable sauna for approximately 20 minutes to warm-up the subcutaneous fascial network. Following the sauna warm-up, subjects generously applied oil (Blaster Oil®, Ashley Diana Black International Holdings, LLC; Houston, TX) to the targeted areas to allow for smooth gliding. Thereafter, subjects performed FMT for 20 minutes on each leg, and 5 minutes on the abdominals for a total of 45 minutes. Subjects isometrically contracted the targeted area after it was manipulated. Subjects performed the protocol 5 days per week, but not more than 2 consecutive days. Instructional videos, personalized coaching, and opportunities to ask questions were available to all subjects for the duration of the study.
2.3. Ultrasound imaging
Ultrasound imaging has been used by many researchers as a useful way to measure the effectiveness of non-invasive methods to address cellulite. In this study, ultrasound imaging was used to evaluate the subcutaneous fascial architecture and measure the subcutaneous adipose tissue (SAT). During the imaging process, all subjects would lay supine with knees resting comfortably in extension near the natural rest position. An elastic loop band was placed around the feet to permit subjects to relax without hip rotation. All images were taken at 50% of the thigh length from the greater trochanter of the lateral epicondyle of the knee. Each measurement was taken on the right leg and all measurement sites were marked with a permanent marker to ensure that the probe was applied to the proper location. Two-dimensional, B-mode ultrasound (GE LOGIQ P6, General Electric Company, Boston, MA) imaging with a 12 MHz broad-spectrum linear-matrix array transducer (Model ML6-15, General Electric Company, Boston, MA) was used and images of the SAT were captured with the probe oriented in the transverse and longitudinal plane perpendicular to the skin. Water soluble hypoallergenic ultrasonic gel (Aquasonic® CLEAN® Ultrasound Gel, Parker Laboratories, INC., Fairfield, NJ) was applied to the transducer to optimize spatial resolution and then placed at the marked measurement site with minimal force to avoid compression of the SAT layer. Onscreen electronic calipers were used to measure maximal SAT with a single perpendicular line between the muscle-adipose interface to the top of the epidermis at the widest distance. The top of the epidermis is a smooth, flat surface, and was chosen as the apex of the measurement site to avoid possible measurement error from the uneven surface of the dermal-adipose interface. Two images were captured and measured to the nearest 0.01cm. The average of the two images were used for analysis. The coefficient of variation for SAT was determined to be 3.5% prior to the commencement of the study.
2.4. Metabolic measures
All test subjects were instructed to avoid consuming caffeine and stimulants that could alter resting energy expenditure (REE) and respiratory exchange ratio (RER) 12 hours prior to testing. Before testing, subjects were positioned in a chair and instructed to avoid unnecessary movement to achieve a resting state (approximately 2–3 minutes). Metabolic testing was conducted on an indirect calorimeter (CardioCoach; KORR Medical Technologies, Inc., Salt Lake City, Utah) for approximately 15 minutes in a quiet, lit room while subjects breathed normally into a mouthpiece with a nose clip in place. Calibration took place prior to each individual test; this process is automated as the device contains barometric, temperature, and humidity sensors in addition to the oxygen and flowmeter sensors. The breathing hose came from the factory with a bacterial/viral filter inserted between the mouthpiece and gas analyzer for sanitary purposes.
2.5. Body composition
Prior to body composition testing, all subjects were instructed to void or eliminate any flatus in the gastrointestinal tract and urine in the bladder. Body composition was determined by a whole-body scan on a dual-energy x-ray absorptiometry (DXA) device (Horizon DXA System, Hologic Inc., Marlborough, MA). Fat-Free Mass (FFM), Fat Mass (FM), and Body Fat Percentage (BF%) was determined for the total body with the subject lying in a supine position with knees and elbows extended and instructed not to move for the entire duration of the scan (approximately 5 minutes). Results from each scan were uploaded and accessed on computer that was directly linked to the DXA device. Calibration of the DXA device was done against a phantom provided by the manufacturing company prior to testing.
2.6. Hematological measures
The FMT used in this study applied variable amounts of pressure to the skin (the largest organ in the human body), thus compressing the subcutaneous adipose tissue layer, and that pressure is also partially transmitted to the underlying muscle tissue. The subcutaneous adipose layer contains blood vessels, lymphatics, glands, a complex fascial network of proteins and proteoglycans, and adipose cells, which are a warehouse for hormones that can function as an endocrine organ. The fascial network within the subcutaneous layer is composed of the living cells supported by a complex web of proteins, including collagen, and this fascial network also envelops the underlying muscle tissue (Bordoni & Zanier, Citation2014; Schleip, Citation2003). It can therefore be surmised that applying pressure to the subcutaneous adipose layer and underlying muscle may result in changes in blood chemistries, the release of stored hormones, increased protein turnover, and perhaps even inflammation (Schleip, Citation2003). Our study was interested in understanding and quantifying the effects of FMT to all of these tissues and the possible effects on vital organs.
To that end, we set out to investigate the effects of FMT on a variety of important hematological markers. A standard comprehensive metabolic panel can give a snapshot of glucose metabolism, kidney and liver function, electrolyte and fluid balance. In addition to dehydroepiandrosterone, which is an important precursor hormone and the most abundant steroid present in the human body, all sex hormones, including estradiol, total testosterone, and progesterone are fat-soluble and are therefore stored in the subcutaneous adipose layer, and can be potentially released in large quantities by FMT. To assess the inflammatory response to FMT, we also measured serum levels of C-reactive protein, which is a substance produced by the liver in response to inflammation and a commonly used marker for inflammation in the human body. Thyroid stimulating hormone is a pituitary hormone that stimulates the thyroid gland to produce T4 and T3, which work together to stimulate the metabolism of almost every tissue in the human body. Irisin is produced in human muscle tissue in response to exercise training and helps convert white fat into brown fat (Timmons, Baar, & Davidsen et al., Citation2012). FMT may increase serum irisin levels. Lipids like triglycerides and free fatty acids may also be released during FMT. Peripheral serotonin (5-HT) in adipose tissue has been shown to affect energy homeostasis by regulating brown and white fatty tissue, and therefore could also be elevated as a result of FMT. Lastly, Type I collagen is the most abundant type of collagen in the human body and Carboxyterminal Propeptide is derived and cleaved from Type I Procollagen during collagen synthesis (Langberg, Skovgaard, & Petersen et al., Citation1999). As such, Carboxyterminal Propeptide of Type I Procollagen (PICP) was measured as the indicator of collagen remodeling of the subcutaneous fascial network as a direct result of FMT (Langberg et al., Citation1999).
Venous blood was extracted by venipuncture of the antecubital vein using a 21-gauge syringe and collected into a 10mL EDTA tube (BD Vacutainer®, Becton, Dickinson and Company, Franklin Lakes, NJ) by a certified phlebotomist. Afterwards, blood samples were centrifuged at 2500 rpm for 10 minutes at 4°C. Resulting serum and plasma samples were then aliquoted and stored at −80°C until analysis. Hematological analysis included the following markers: comprehensive metabolic panel (CMP); C-reactive protein (CRP); thyroid-stimulating hormone (TSH); dehydroepiandrosterone (DHEA); triglycerides; free fatty acids (FFA); irisin; Carboxyterminal Propeptide of Type I Procollagen (PICP); and Serotonin.
2.7. Visual photography
Photographic testing was done at baseline and at week 12, using Kino Flo lighting with daylight bulbs, in all 33 FT subjects only. White paper was used for the background. Kelvin temperature of the lighting was 5500k. The camera used was a Canon 7d with Canon 24-70L shooting 37mm. The camera was placed on a tripod with the lens 29″ off the ground. The distance from camera to the subject was 6′ measured at the sensor. 4 × 4 Kinos to the right and left of cyc paper were used to limit shadowing from the subject. Two separate technicians, one male and one female, both blinded to the source of the photographs, were asked to assess the degree of cellulite in the photos of all test subjects and rate the degree of cellulite on a five-point analog scale, (The Black Cellulite Severity Scale [BCSS]; Table ), to objectively measure changes in cellulite appearance.
Table 1. Ultrasonography, metabolism, & body composition. FT(n = 33), CON(n = 10)
Table 2. Supplemental hematological measures. FT(n = 33), CON(n = 10)
Table 3. Comprehensive metabolic panel. FT(n = 33), CON(n = 10)
Table 4. Objective cellulite appearance (Black Cellulite Severity Scale). n = 33
2.8. Statistical analysis
Prior to performing statistical analysis, data was tested for normality (i.e., Shapiro-Wilk testing). One-way repeated measures analysis of variance (ANOVA) was carried out to test whether there were significant differences among dependent variables over time. In the event that data violated normality testing, a non-parametric Friedman test was used in place of the one-way repeated measures ANOVA. Post-hoc testing was performed with a Bonferroni correction for multiple comparisons. Since two time points (BL and Wk12) assessed the five-point analogue scale of cellulite appearance, paired t-test was used to analyze these data. Significance was set a priori p < 0.05 and data are reported as mean ± standard error unless otherwise stated. The outlier labeling rule was applied to all data sets in accordance to Hoaglin and Iglewicz (Hoaglin and Iglewicz, Citation1987), outliers were excluded from statistical analysis (FT: n = 3; CON: n = 0). Data were analyzed using SPSS software (version 21.0; SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 7 software (GraphPad Software, Inc., La Jolla, CA, USA).
3. Results
3.1. Ultrasound imaging
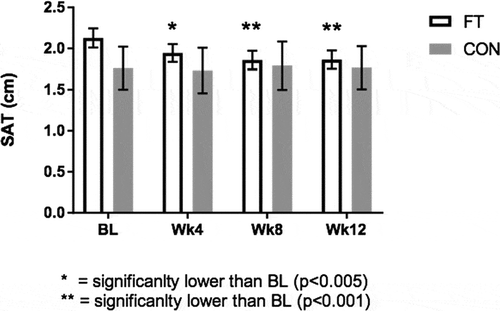
In the FT group, significant differences in SAT of the thigh were observed over time (p < 0.0001, Figure ). Post hoc testing with Bonferroni’s adjustment indicated that SAT thickness was significantly lower from baseline at Wk4 (2.13 ± 0.11 vs 1.95 ± 0.11 cm; 95% CI: −0.32, −0.04; p < 0.005), Wk8 (2.13 ± 0.11 vs 1.86 ± 0.11 cm; 95% CI: −0.40, −0.14; p < 0.001), and Wk12 (2.13 ± 0.11 vs 1.86 ± 0.11 cm, 95% CI: −0.38, −0.15; p < 0.001) in the FT group (Table ). No significant differences in SAT were observed in the CON group.
Figure 1. Ultrasonography results of subcutaneous adipose tissue thickness (cm) at BL, Wk4, Wk8, and Wk12.
Figure Legend. The effects of fascia treatment versus control on subcutaneous adipose tissue (SAT) thickness of the thigh over 12 weeks. The fascia treatment group demonstrated significantly lower SAT thickness at wee 4 (p < 0.005), week 8 (p < 0.001) and week 12 (p < 0.001) relative to baseline whereas no significant differences were detected in control (p > 0.05).
3.2. Metabolic measures
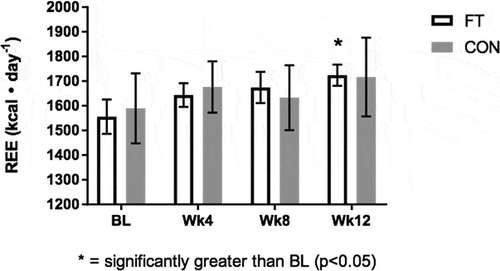
Data for REE violated normality testing (Shapiro Wilk test p < 0.05). Therefore, the non-parametric Friedman test was used in replace of the one-way repeated ANOVA. Friedman testing indicated that REE experienced a significant change over the course of the study (p < 0.005, Figure ). Post hoc analysis with Bonferroni’s adjustment revealed that REE was significantly greater at Wk12 compared to BL for the FT group (1724 ± 43 vs 1556 ± 70 kcal • d−1; 95% CI 10.3, 326.2; p < 0.05). No significant differences were detected in REE for the control group. Neither the FT or CON group demonstrated significant changes in RER (p > 0.05) (Table ).
Figure 2. Resting energy expenditure (kcal • day-1) results at BL, Wk4, Wk8, and Wk12.
Figure Legend. The effects of fascia treatment versus control on resting energy expenditure over 12 weeks. A significant increase in resting energy expenditure was indicated at week 12 (p < 0.05) relative to baseline in the fascia treatment group. No significant differences were detected in control (p > 0.05).
3.3. Body composition analysis
There were no outliers and the data were normally distributed, as assessed by the Shapiro-Wilk test (p > 0.05). No significant differences were detected for any variables of body composition and anthropometry (p > 0.05) (Table ).
3.4. Hematological measures
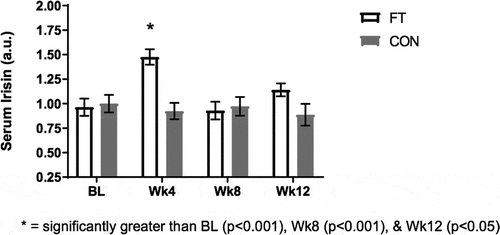
Three outliers were detected in the FT group and removed based on the methods of Hoaglin and Iglewicz (Hoaglin and Iglewicz, Citation1987). Removal of outliers resulted in normally distributed data (Shapiro-Wilk: p > 0.05). Significant changes for PICP and irisin were demonstrated in the FT group but not the CON group (Figure ). Post hoc analysis with Bonferroni’s adjustment indicated that PICP was significantly higher at Wk4 compared to BL (1.20 ± 0.06 vs 0.98 ± 0.04 a.u.; 95% CI: 0.02, 0.41; p < 0.05) In regards to irisin, the FT group’s Wk4 value was significantly higher than BL (1.48 ± 0.08 vs 0.96 ± 0.09 a.u.; 95% CI: 0.19, 0.83; p < 0.001), Wk8 (1.48 ± 0.08 vs 0.93 ± 0.09 a.u.; 95% CI: 0.24, 0.85; p < 0.001) and Wk12 (1.48 ± 0.08 vs 1.14 ± 0.07 a.u.; 95% CI: 0.05, 0.62; p < 0.05, Figure ) (Table ). No other significant differences were detected in hematological metrics (p > 0.05) (Table ).
Figure 3. Serum carboxyterminal propeptide of Type I Procollagen (a.u.) results at BL, Wk4, Wk8, and Wk12.
Figure Legend. Carboxyterminal Propeptide of Type I Procollagen (PICP) as a potential indicator of collagen remodeling of the subcutaneous fascial network. PICP in the serum was significantly higher at week 4 compared to baseline (p < 0.001) in the fascia treatment group whereas no significant changes were detected in control (p > 0.05).
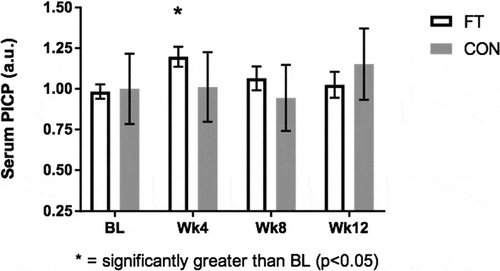
Figure 4. Serum Irisin (a.u.) Results at BL, Wk4, Wk8, and Wk12.
Figure Legend. Irisin is a hormone released from skeletal muscle tissue that aids in conversion of white adipose tissue to brown adipose tissue. Irisin in the serum was significantly higher at week 4 compared to baseline (p < 0.001), week 8 (p < 0.001), and week 12 (p < 0.05) in the fascia treatment group whereas no significant changes were detected in control (p > 0.05).
3.5. Visual cellulite appearance
From BL to Wk12, randomized objective assessments of visual photography from both blinded testers indicated significant improvement in visual reduction of cellulite in the FT group (p < 0.0001, Table ).
4. Discussion
The primary findings of this study are that FMT with the FBD reduced the SAT of the manipulated thigh regions and improve indices of cellulite appearance. In addition, the results showed an increase metabolism and indicators of connective tissue remodeling. Finally both the FMT and the FBD appeared to be safe.
While mechanical stimulation of fascia appears to have a positive impact on cellulite, there is a need for easily-available non-invasive devices that can be used as a self-administered treatment. The purpose of this study was to investigate the effectiveness of FMT using the FBD. A secondary purpose was to investigate the metabolic, connective tissue remodeling, and safety impacts of mechanically manipulating fascia.
4.1. Changes in fat thickness, cellulite appearance, and total fat mass
Cellulite affects the majority of adult females (Alster & Tanzi, Citation2005; Bayrakci Tunay et al., Citation2010; Lucassen et al., Citation1997; Romero et al., Citation2008). Several non-invasive and minimally-invasive surgical treatments exist for cellulite (Bayrakci Tunay et al., Citation2010). One such non-invasive method is mechanical manipulation of the fascia within the area of cellulite. The results of this study indicated that FMT of the lower body with the FBD decreased SAT at the manipulated sites and reduced the visual appearance of cellulite (Figure ). Total fat mass, however, stayed the same (Table ). These results agreed with past studies (Bayrakci Tunay et al., Citation2010; Lucassen et al., Citation1997; Romero et al., Citation2008). For example, Bayrakci Tunay et al. (Bayrakci Tunay et al., Citation2010) used a mechanical fascia technique, which applied suction (negative pressures) and powered rollers (positive pressure) to common cellulite regions. These researchers found decreases in subcutaneous thickness and thigh circumference after just 5 weeks. As with our study there were no changes in total fat mass. Similarly, Lucassen et al. (Lucassen et al., Citation1997) found on average a 56% smoothening of the dermis-hypodermis surface after 3 months of manual massage treatment of the upper thigh. These results indicate that fascia treatment is a body-contouring procedure, which smooths subcutaneous tissue and reduces the appearance of cellulite. Similar to liposuction, the contouring technique appears to be local without decreasing total body fat percentage (Bayrakci Tunay et al., Citation2010).
Figure 5. Visual photographic and ultrasonography images of subject A.
Figure Legend. Images from visual photography and ultrasonography from baseline and week 12 of Subject A (38-year-old female). Visual photography shows smoothening of skin texture and reduction of cellulite dimpling. Ultrasonography image shows reduction in subcutaneous fat tissue thickness of the thigh from baseline (1.54cm) to week 12 (1.13cm).
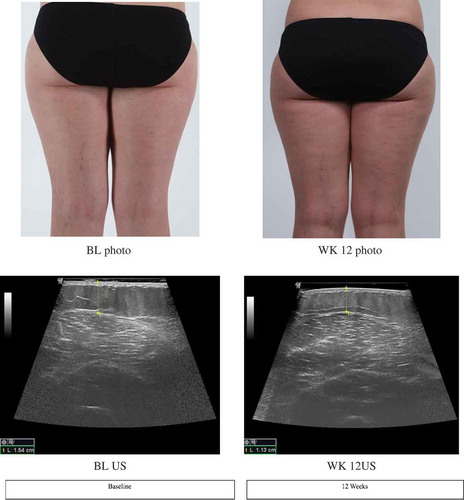
One potential explanation for the results found in this study, along with results from previous studies, is that mechanical massage releases fat cells from fibrous septae, thereby decreasing upward projections of fat cells into the lower dermis, causing a smoother dermal-hypodermal junction, and reducing the outer appearance of cellulite (Romero et al., Citation2008). Our study found that the thickness of the subcutaneous adipose layer is quantifiably and visibly reduced.
4.2. Changes in metabolism and connective tissue remodeling
A very intriguing finding of this study was that the metabolism of the FT group increased by approximately 170 calories per day following the 12 weeks. While the underlying mechanism for these results is uncertain, it may be tied to a change in connective tissue remodeling and/or an increase in circulating irisin. Specifically, FT subjects demonstrated increases in PICP at Wk4 of the research period. PICP is a protein expressed in connective tissue at its peak remodeling stages (Timmons et al., Citation2012). It is plausible that the energy demands of remodeling connective tissue drove the metabolic response experienced by the subjects. In addition, it was also found that the hormone irisin was increased. Irisin is often called the “exercise hormone” (Timmons et al., Citation2012). Irisin is released following intense training bouts and orchestrates an array of physiological processes, which effectively elevate metabolism (Hoaglin and Iglewicz, Citation1987; Schleip, Citation2003). Specifically, powerful evidence indicates that irisin can convert white fat into brown fat, enhancing metabolic uncoupling and, thus, caloric expenditure (Norheim, Langleite, & Hjorth et al., Citation2014). Our results indicate that stimulation of fascia itself may be enough to trigger this response.
4.3. Safety
The most common surgical procedure to treat cellulite is liposuction (Alster & Tanzi, Citation2005). This method effectively removes fat cells. In the process, it results in bleeding, inflammation, and edema, which can take months to recover from (Alster & Tanzi, Citation2005). For these reasons, an alternative non- invasive technique that is safe is highly sought after. This study examined the safety of FMT with FBD via an extensive array of metabolic markers of kidney, liver, and endocrine function (Table ). It was found that none of these markers demonstrated any significant change, indicating that the protocol used was safe. Some FT subjects reported mild symptoms of irritability, nausea, headaches, and bruising. These were found to be minor and all subjects did not report any long-lasting injury or illness to the research team.
5. Conclusions
The present study provides evidence that a non-invasive protocol of mechanically manipulating fascia can decrease subcutaneous thickness of adipose and lower the appearance of cellulite in adult women when applied 5 days a week over 12 weeks. These results indicate that FMT with FBD is generally safe and may be a viable method in treating cellulite. Future research should delve into optimizing the effects produced by FMT with FBD by further studying frequency, intensity, duration, and speed of application.
Acknowledgements
The research in this project can be used to address the issue of cellulite and provide a safe, non-invasive treatment for cellulite. The results also show the improved tissue remodeling capability of the FasciaBlaster® devices, which can be applied to physical rehabilitation and medicine.
Additional information
Funding
Notes on contributors

T Bart Jameson
T Bart Jameson has completed his Bachelors of Science in Exercise and Sports Sciences at the age of 22 years from Texas Tech University and postgraduate studies from Texas Tech University Health Sciences Center where he completed his Masters in Athletic Training. He is a Certified Athletic Trainer (ATC) and a Strength and Conditioning Specialist (CSCS) with experience in NCAA, MLB, and the NFL. The research from this paper not only address the cosmetic condition of cellulite, but can be applied to improvement in rehabilitation and recovery techniques. Email: [email protected]
References
- Alster, T. S., & Tanzi, E. L. (2005). Cellulite treatment using a novel combination radiofrequency, infrared light, and mechanical tissue manipulation device. Journal of Cosmetic and Laser Therapy : Official Publication of the European Society for Laser Dermatology, 7(2), 81–13. doi:10.1080/14764170500190242
- Bayrakci Tunay, V., Akbayrak, T., Bakar, Y. (2010). Effects of mechanical massage, manual lymphatic drainage and connective tissue manipulation techniques on fat mass in women with cellulite. Journal of the European Academy of Dermatology and Venereology : JEADV, 24(2), 138–142. doi:10.1111/j.1468-3083.2009.03355.x
- Bordoni, B., & Zanier, E. (2014). Clinical and symptomatological reflections: The fascial system. Journal of Multidisciplinary Healthcare, 7, 401–411. doi:10.2147/JMDH.S68308
- Hoaglin, D. C., & Iglewicz, B. (1987). Fine-tuning some resistant rules for outlier labeling. Journal of the American Statistical Association, 82(400), 1147–1149. doi:10.1080/01621459.1987.10478551
- Langberg, H., Skovgaard, D., Petersen, L. J. (1999). Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. The Journal of Phsiology, 521(1), 299–306.
- Lucassen, G. W., Sluys, W. V. D., Herk, J. (1997). The effectiveness of massage treatment on cellulite as monitored by ultrasound imaging. Skin Research and Technology : Official Journal of International Society for Bioengineering and the Skin (ISBS) [And] International Society for Digital Imaging of Skin (ISDIS) [And] International Society for Skin Imaging (ISSI), 3(3), 154–160. doi:10.1111/j.1600-0846.1997.tb00180.x
- Norheim, F., Langleite, T. M., Hjorth, M. (2014). The effects of acute and chronic exercise on PGC‐1α, irisin and browning of subcutaneous adipose tissue in humans. The FEBS Journal, 281(3), 739–749. doi:10.1111/febs.12619
- Romero, C., Caballero, N., Herrero, M. (2008). Effects of cellulite treatment with RF, IR light, mechanical massage and suction treating one buttock with the contralateral as a control. Journal of Cosmetic and Laser Therapy : Official Publication of the European Society for Laser Dermatology, 10(4), 193–201. doi:10.1080/14764170802524403
- Schleip, R. (2003). Fascial plasticity- A new neurobiological explanation: Part 1. Journal of Bodywork and Movement Therapies, 7(1), 11–19. doi:10.1016/S1360-8592(02)00067-0
- Timmons, J. A., Baar, K., Davidsen, P. K., et al. (2012). Is irisin a human exercise gene? Nature, 488(7413), E9. doi:10.1038/nature11364
