Abstract
Objective: To assess the impact on postpartum hemorrhage (PPH) of a smartphone training application called “Safe Delivery”, distributed to midwives. Design: A cluster randomized controlled trial. Setting: Greater Accra Region, Ghana. Population: 146 midwives were randomly allocated to intervention (70 midwives, seven hospitals, 1,665 deliveries) or control (76 midwives, eight hospitals, 1,746 deliveries). Methods: The intervention group received Safe Delivery which is a smartphone training tool in Emergency Obstetric and Neonatal Care. Main Outcome Measures: The primary outcome was PPH. Secondary outcomes were severe PPH and the relative difference in postpartum blood loss. Results: The intervention was associated with an insignificant lower incidence of PPH (odds ratio 0.86 (95% confidence interval (CI) 0.59 to 1.25)) and an insignificant lower incidence of severe PPH: odds ratio .95 (95% CI: .65 to 1.40). The relative difference in blood loss between intervention and control arms was: 5.3% (95% CI: −3.2%, to13.8%). The intra-class correlation for hospitals was .016, and for midwives within hospitals: .026. Conclusions: Safe Delivery was associated with an insignificant lower incidence of PPH. The validity of the blood loss data was a concern since the data collection was not completely uniformly and this imprecision could not be evaluated. The study questions whether Safe Delivery works as a stand-alone tool to improve basic emergency obstetric and neonatal care.
PUBLIC INTEREST STATEMENT
Every year more than 300,000 women die during pregnancy and childbirth. A main cause of maternal death is postpartum hemorrhage. In a cluster randomised trial, where 146 midwives in 15 hospitals were observed while conducting a total of 3,411 deliveries, we tested the Safe Delivery app, which contains instruction films with correct management. About half of the midwives received smartphones with the app.
Safe Delivery was associated with an insignificant lower incidence of PPH of 14% and an insignificant lower incidence of severe PPH of 5%. The quality of care in the Greater Accra Region, Ghana, was high, and it is likely the app would have a greater effect in rural settings and as integrated part of other efforts to improve quality of delivery care.
1. Introduction
Every year, more than 300,000 women worldwide die during pregnancy and childbirth (World Health Organization, Citation2015). In low-income countries postpartum hemorrhage (PPH) is the leading cause of maternal mortality (Sentilhes et al., Citation2016). In Ghana, one in four maternal deaths are due to hemorrhage, including PPH (McCormick, Citation2002). In 2013, the Ghana Health Service published a strategy for the prevention and management of PPH with the goal to reduce the contribution of hemorrhage to maternal mortality within five years (Ministry of Health, Citation2013). The report highlights poor quality of maternal care services as a major contributing factor to PPH. For instance, a survey from 2007 showed that even though 33% of the study hospitals had guidelines that mentioned the use of oxytocin, only three per cent used the drug properly (Ministry of Health, Citation2013).
Improving competency of health care workers such as midwives will improve pregnancy outcomes. One of the approaches to improve competency of health workers is to conduct training courses. However, several logistical and financial barriers can make this approach unattainable (Msemo et al., Citation2013; Sorensen et al., Citation2011). Given the availability of mobile phone, it is suggested that specially developed phone apps that provide essential obstetric training through videos can overcome these barriers and improve pregnancy outcomes (Agarwal & Labrique, Citation2014; Agarwal, Perry, Long, & Labrique, Citation2015; Nilsson, Sørensen, & Sørensen, Citation2014). A number of studies have investigated the effectiveness of mobile health (mHealth) interventions in maternal health initiatives in low-income countries. However, so far, no studies on the contribution of mHealth to improve quality of care during labor have been published (Lee et al., Citation2016). In general, the evidence for effectiveness of mHealth interventions, particularly in low-income countries, is weak. In particular, conclusions need to be drawn about impacts on patient health outcomes. Therefore, there is a need for evidence of mHealth intervention benefits to patient’s outcomes such as PPH.
Recently, a free smartphone application (app), “Safe Delivery”, was developed. The app aims to improve the professional competence of birth attendants in low- and middle-income countries by means of animated instructional videos (Video S1). We investigated whether Ghanaian midwives’ use of Safe Delivery could lower the incidence of PPH.
2. Methods
2.1. Study design and setting
The study took place in 15 hospitals or large polyclinics (hereafter called hospitals) in Greater Accra Region, Ghana, between 14 July and 17 September 2014. Hospitals included had at least 10 midwives employed, a patient flow of 10 deliveries per months and at least 1,200 annual deliveries. The hospitals were randomized to either intervention or control, and the midwives received training accordingly. The study was an un-blinded cluster randomized controlled trial with two parallel arms with an allocation ratio of 1:1. Based on sample size calculations for number of deliveries, we included 140 midwives, and each midwife was expected to conduct 20 deliveries during the study period. Midwives who were fulltime employed on the ward, willing to participate and available for the duration of the study period and on specific dates for meetings were included.
The calculation of statistical power was based on the primary outcome PPH, i.e. a blood loss ≥ 500 ml within the first two hours postpartum. Secondary outcomes were severe PPH (blood loss ≥ 1000 ml) and the relative difference in postpartum blood loss. The null hypothesis was that there was no difference between the intervention and control arm and correlations between births attended to by the same midwife (intra-midwife correlation) and between births attended to by midwives within the same hospital (intra-hospital correlation) were taken into account. Based on previous studies in Sub-Saharan Africa, the risk of PPH in control hospitals was assumed to be 25% (Lars et al., Citation2005; Sorensen et al., Citation2011).
Existing information about correlations from other studies was limited. In a review of five cluster randomized studies in low- and middle-income countries, the intra-community correlations for maternal mortality ranged between 0.00005 and 0.0034 (Sorensen et al., Citation2011). Since maternal mortality is strongly correlated with PPH, we chose to use a value of 0.003 for the intra-hospital correlation. We had no information about the intra-midwife correlation. Therefore, in order to obtain sufficient power, we based our calculations on a relatively high value of 0.1.
Assuming a 40% reduction in the incidence of PPH due to the intervention (from 25% to 15%) (Sorensen et al., Citation2011), equal allocation to intervention and control arms, an intra-hospital correlation of 0.003, an intra-midwife correlation of 0.1, 20 deliveries per midwife and ten midwives per hospital, we estimated that 14 hospitals were needed to achieve a power of 90% at a significance level of five per cent (Teerenstra, Lu, Preisser, van Achterberg, & Borm, Citation2010; World Health Organization, Citation2016). Because some of the included hospitals only had a few midwives available for participation we included a 15th hospital.
2.2. Participants
The hospitals were selected from the hospitals under the Ghana Health Service in Greater Accra with the highest numbers of deliveries in 2013. Nine hospitals provided Basic Emergency Obstetric and Neonatal Care (BEmONC) and six hospitals provided Comprehensive Emergency Obstetric and Neonatal Care (CEmONC). The mobilization of midwives began on 17 June 2014. Because some hospitals enrolled as few as four midwives each midwife should perform 30 deliveries instead of 20. Women who were admitted to the labor ward for vaginal birth were enrolled into the trial after having signed a written informed consent form.
Data collection
Eighty-five research assistants were trained for three days and recorded the socioeconomic and obstetric characteristics of parturient women, delivery outcome, complications and treatment. Fifteen of the research assistants were trained as supervisors, one in each hospital, to assure that the data collection was performed as per protocol.
Immediately after a delivery, and after the gush of amniotic fluid, the assistants placed a blood collection drape under the woman. In case of, for example, suturing, the drape was exchanged for a new drape or pad(s). The drape(s) and pad(s) were collected for two hours and weighed on a scale in grams, and the blood loss calculated by deduction of the blood collection material. Since one ml of blood weighs close to one g, the weight in grams was assumed to be equivalent to the volume in milliliters (Lars et al., Citation2005).
2.3. Intervention
The Safe Delivery app is an Emergency Obstetric and Neonatal Care (EmONC) training tool that provides visual guidance by means of animated videos with clinical instructions (Figure ). It was developed for birth attendants in low- and middle-income countries and aims to improve the quality of maternal and neonatal care. The videos give vivid instructions of, for example, how to administer drugs and perform maneuvers. The version we tested contained videos of: (1) active management of the third stage of labor, (2) manual removal of the placenta, (3) treatment of PPH and (4) neonatal resuscitation.
Figure 1. Users of Safe Delivery can choose to watch full-length videos (5–10 min duration), or a short sequence (1–2 min) from a particular video. The sequence of images to the left is from the postpartum hemorrhage video and illustrates how to perform bimanual compression.

After randomization, the midwives in the intervention arm were given a smartphone with the app and they watched the videos once while the principal investigator was present. Thus, all midwives watched the app at least once. In an End of Study Questionnaire filled by the midwives at the trial end, 83% of midwives in the intervention group responded they had watched the videos more than five times. Midwives in the control hospitals provided standard care and received a smartphone at the trial end. Safe Delivery was made inaccessible for download from Ghana before and during the trial.
2.4. Randomization
Prior to the randomization, each hospital was classified into a high or low Key Feature Questionnaire (KFQ) score group, based on whether the majority of the midwives in that hospital were above or below the overall median KFQ score (participating midwives filled in a questionnaire that tested their knowledge of neonatal resuscitation and PPH, treatment of PPH and manual removal of the placenta.). The randomization took place on 14 July 2014 and was stratified by KFQ score (High Score Group or Low Score Group) and hospital type (BEmONC or CEmONC), yielding a total of four strata. On the day of randomization, only 116 midwives had been recruited, therefore, midwives were enrolled until we had reached our target sample size of 140 participants.
2.5. Data analysis
All outcomes were analyzed as intention-to-treat. For the primary outcome, PPH, a mixed-effects logistic regression model was used. The fixed effects were treatment (intervention/control group), type of hospital (BEmONC or CEmONC) and KFQ score group. Clustering was taken into account by considering the hospitals and midwives as nested random effects. Intra-class correlations (ICC) were estimated for hospitals and midwives. Blood loss in milliliters was logarithmically transformed and analyzed in a mixed-effects model with same fixed effects as above and hospitals and midwives as nested random effects. Results were presented with 95% confidence intervals (CI). The model was checked through visual inspection of residual plots and best linear unbiased predictors for the two random effects groups. Statistical significance was defined as P < 0.05. The analyses were performed in Stata version 13.1 (Stata Corp, College Station, TX).
The study received ethical approval from the Ghana Health Service Ethical Review Committee and is registered with ClinicalTrials.gov, protocol record AU201407.
4. Results
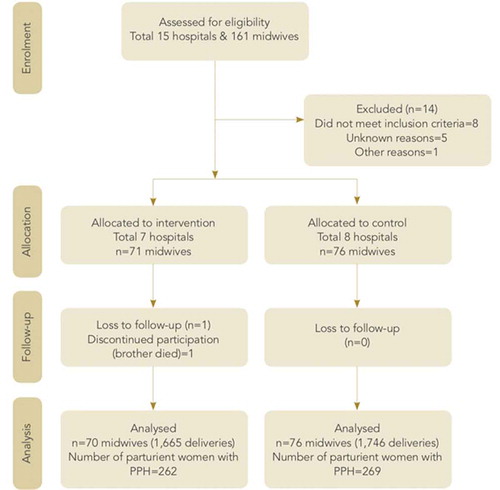
In total, 161 midwives were enrolled, and 3,566 deliveries were recorded (Figure ). Subsequently, eight midwives were excluded from the control group because they did not fulfill the inclusion criteria, four midwives because they never conducted any deliveries, and 1 midwife for other reasons. In the intervention group, 1 midwife was excluded because she did not conduct deliveries. After allocation, 1 midwife opted out of the study because her brother died. One delivery was registered with this midwife’s ID number but it was not certain the midwife had actually performed the delivery. As a consequence, we did not include this midwife in the intention-to-treat analysis and in the results. This left 146 midwives and 3,411 deliveries for inclusion in the analysis.
The characteristics of the parturient women and the midwives were almost similar in the intervention and control arm (Table ). The majority of the parturient women were self-employed women under 30 who had completed primary school. Most midwives were young, had graduated within the last ten years, and had less than ten years of work experience.
Table 1. Baseline characteristics of the study population (n = 146 midwives)
Except for a few variables, the risk factors for PPH were almost similar in the intervention and control group (Table ). Approximately 15% of the women in labor had PPH, five per cent had severe PPH, and about five per cent had experienced PPH before. Three women died after birth in each of the intervention and control arms.
Table 2. Characteristics of the primary and secondary outcome and risk factors for postpartum haemorrhage (PPH)
The variables that varied between the groups were episiotomy (performed in one vs. ten per cent of the deliveries in the intervention and control arm, respectively), suturing of trauma to the birth canal (performed in 23 vs. 31% of the deliveries) and use of misoprostol as part of the AMTSL (performed in 31 vs. 26% of the deliveries).
The median amount of blood loss that occurred among parturient women in the intervention arm was 257ml; interquartile range (IQR) = 222ml. In the control arm the median was 241ml; IQR = 257ml. The intervention was associated with a non-significant lower incidence of PPH of 14% (odds ratio 0.86, 95% CI: 0.59, 1.25) and a non-significant lower incidence of severe PPH of five per cent (odds ratio 0.95, 95% CI: 0.65, 1.40).
We performed likelihood ratio test without treatment in the model and found that there was no significant effect of treatment for both PPH (p = 0.15) and severe PPH (p = 0.16). We therefore kept treatment in the model. The interaction terms such as facility type X KFQ level or treatment X facility type did not show any statistically significant effects when added to the regression models of PPH and severe PPH.
The mean relative difference in blood loss between intervention and control arms was: 5.3% (95% CI: −3.2%, 13.8%). The ICC for hospitals was 0.016. For births attended to by the same midwife the ICC was .026.
5. Discussion
5.1. Main findings
Our study found an insignificant lower incidence of PPH of 14%, an insignificant lower incidence of severe PPH of five per cent and provided valuable information about correlations for calculations of statistical power in future studies. After having carried out the trial, we realized that the prerequisites for our power calculations had been too optimistic and the study turned out to be underpowered (it would have detected a mean relative difference in blood loss of more than 17%).
5.2. Strengths and limitations
One of the limitations of our study was imprecision of the blood collection. Other studies with blood collection have been successful (Lars et al., Citation2005). We discovered, however, that despite training of research assistants, the blood collection was not completely uniform. Blood loss collection was not always initiated immediately after birth and continued for two hours. This lack of uniformity meant that to some extent we did not have the same standard of comparison between the 15 hospitals. However, there is no reason to believe that any differences in blood collection practice are systematically related to the intervention or control group. Another limitation was the lack of blinding after randomization. However, even though the research assistants were not blind to the intervention status, they used the same methods in both arms, and it seems unlikely that bias has ensued as a result of this un-blinding. Since the recruitment of midwives continued after the allocation, a risk of post-randomization recruitment bias existed. The recruitment rate was higher in the intervention arm (n = 22) compared to the control arm (n = 8), however, this imbalance in numbers does not necessarily imply imbalance in characteristics (Eldridge & Kerry, Citation2011). Most likely, the distribution of smartphones to the control group at the trial end helped prevent post-randomization recruitment bias. Also, there is no reason to believe that blood loss should be associated with any desire to be in one group or the other.
Some of the strengths of the study were that the randomization and follow-up was successful, exclusion criteria were kept to a minimum, and the characteristics of the midwives, parturient women, and the risk factors for PPH were almost similar in the intervention and control arm.
5.3. Interpretation
Based on our study results, we cannot conclude that Safe Delivery was a successful intervention. A recent review of the effectiveness of mHealth interventions targeting health care workers to improve pregnancy outcomes could not convincingly demonstrate an effect either (Amoakoh-Coleman et al., Citation2016). It is likely that the app needs a more effective implementation. Instead of being a stand-alone tool to improve BEmONC, the app might work better as an integrated part of other efforts to improve the quality of delivery care. A study from Ethiopia demonstrated a positive impact of an mHealth intervention on delivery care in health facilities possibly through positively influencing the behavior of health workers (Shiferaw et al., Citation2016). The study concluded that future studies need to explore the possible mechanisms of mobile health solutions and evaluate their effectiveness in other health programs. It is likely that Safe Delivery would have a higher effect in a rural setting and/or with a different target group. We chose an urban setting because of time and resource constraints, and because we in view of the general reports on quality problems, expected an effect even in the urban setting (Gohou et al., Citation2004; Kruk et al., Citation2016; Mbaruku, Citation2009; Miller, Citation2003). Several studies have found that primary care facilities, most often located in rural settings, have deficiencies in infrastructure, referral systems, and routine and emergency care practices (Kruk et al., Citation2016). Moreover, understaffing and a low birth volume is a challenge because the quality of care increases with higher delivery volumes (Kruk et al., Citation2016). Our study setting and study population seemed to be in contrast to the descriptions in the literature from Ghana since: (1) staffing seemed sufficient, (2) the quality of care seemed high, since, for instance, almost all women were given prophylactic oxytocin, (3) more than 70% of the midwives had attended training courses, and (4) the patient flow was high. Thus, the quality of care in rural settings in Ghana is probably in great contrast to that of urban settings and that it would have been more relevant to study Safe Delivery in a rural setting. Our study is still important, though, because it addresses the need found by systematic reviews for trials on mobile phone interventions in low- and middle-income countries that have a health outcome as the primary outcome (Free et al., Citation2012; Labrique, Vasudevan, Chang, & Mehl, Citation2013; Tomlinson, Rotheram-Borus, Swartz, & Tsai, Citation2013). There are few studies of mHealth interventions in the area of EmONC to compare our study with. However, the effect of Safe Delivery was recently studied in rural parts of Ethiopia (Lund et al., Citation2016). Safe Delivery was associated with a nonsignificant 24% reduction in perinatal mortality. The study also found a significant increase in health care workers’ knowledge and skills in neonatal resuscitation at six and 12 months after baseline (secondary outcomes). Similar to our study, the study had challenges finding evidence for the effect of the primary outcome. If our study had not been underpowered and we had still found a 14% lower incidence of PPH, the question is if this is a small or a large effect compared to what is achievable in other ways. A study from Tanzania evaluated the impact of a two-day Advanced Life Support in Obstetrics (ALSO) provider course and found a significant reduction of PPH from 32.9 to 18.2%, and of severe PPH from 9.2 to 4.3% (Sorensen et al., Citation2011). In comparison, our study only demonstrated an insignificant lower incidence of PPH of 14% and of severe PPH of five per cent, either because the study had too little power to demonstrate an effect of this size or because there was no effect. However, the starting point in our study was an incidence of PPH of about 15% which was considerably lower than the incidence of PPH of 33% in a rural setting in Tanzania. With a lower starting point it may be more difficult to obtain an effect.
Even though conventional training courses of skilled birth attendants in BEmONC have proved effective to improve health care outcomes (Msemo et al., Citation2013; Sorensen et al., Citation2011), many health care workers face a challenge of inadequate access to reference materials to handle situations that are beyond their skills (Agarwal & Labrique, Citation2014; Agarwal et al., Citation2015). Safe Delivery could possibly overcome this challenge since it is self-explanatory, free of charge, and, once downloaded on a mobile device, does not require network coverage. An increasing number of low-income countries have a framework in place with national mHealth policies, but few mHealth tools are being scaled up and integrated into existing systems (Tomlinson et al., Citation2013). Safe Delivery might be an mHealth tool that could be integrated. The question remains how it would fit in the best. However, even as a stand alone tool, the app training of midwives resulted in a tendency towards a reduction of PPH. Therefore, we recommend midwives to download and use the app. Furthermore, since the trial, the app has been expanded and now includes extensive learning material and several more videos.
6. Conclusion
In conclusion, in this trial, the Safe Delivery app was associated with an insignificant lower incidence of PPH in an urban hospital setting. The trial set-up was ambitious since we collected data from 3,411 deliveries in nine weeks. The validity of the blood loss data was a concern since the data collection was not performed completely uniformly and we could not evaluate the extent of this imprecision. The quality of care in the Greater Accra Region seemed high and it is likely that the Safe Delivery app would have a greater effect in rural settings of low-income countries.
DISCLOSURE OF INTERESTS
All authors declare to have received: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
CONTRIBUTION TO AUTHORSHIP
All authors took part in designing the work and planning of the study. JA and CK performed the sample size calculations. JA registered the study with ClinicalTrials.gov. CK led the recruitment of participants, the randomization and the data collection. JA, RA, UE, SL and BS assisted in this process. JA and CK conducted the statistical analysis. CK drafted the manuscript. JA, RA, UE, SL and BS revised it critically for important intellectual content and approved the final version. All authors had full access to all the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
DETAILS OF ETHICS APPROVAL
The trial received ethical approval from the Ghana Health Service Ethical Review Committee on 10 June 2014, reference number GHS-ERC: 3, and a consultative approval was granted by the Developing-Country Committee set up by the National Committee on Health Research Ethics, Denmark.
Trial registration
ClinicalTrials.gov, AU201407, https://clinicaltrials.gov/ct2/show/NCT02185625?term=AU201407&rank=1
Supplemental Material
Download Zip (23.8 MB)Acknowledgements
The hospitals, midwives and parturient women are kindly thanked for their participation. Bernard Bortei, University of Ghana, is acknowledged for performing the randomization procedure and data management. Ethel M. B. Alexander Mohan, Aarhus University, is acknowledged for her work with the analyses and for critically revising the manuscript for important intellectual content.
The following Danish interns are acknowledged for volunteering their time to help with the data collection: Anne Cathrine Jørgensen, Laura Christensen, Louise Hansen, Eva Bisgaard, Frederikke Bilsteen, Josephine Bilsteen, Nathalie Bille, Miriam Ayinza Assago, Michelle Mistry, Golda Fania, Hamida Massaquoi. The following individuals are acknowledged for data entry: Salamatu Issah, Gabriel Boateng, Prince Ennim, Dun-Dery Elvis Junior, Mark Tawiah, Rueben Abladey, William Amoah, Able Narh, Portia Asiedu, Justice Tetteh.
Maternity Foundation is acknowledged for creating the content of the Safe Delivery app in collaboration with the University of Copenhagen and the University of Southern Denmark. Visikon is acknowledged for developing the app.
Supplementary material
Supplementary material for this article can be accessed here.
Additional information
Funding
Notes on contributors

Christina Marie Braüner Klokkenga
Christina Marie Braüner Klokkenga Midwife BSc., MPH, PhD, conducted this trial as her PhD project. She was a PhD scholar at Department of Public Health, Aarhus University, Denmark, and employed as a midwife at Department of Gynecology and Obstetrics, North Denmark Regional Hospital, Hjørring, Denmark. Her supervisors, Ulrika Enemark, associate professor, and Jørn Attermann, associate professor, are also affiliated with the Department of Public Health, Aarhus University. Richard Adanu is a professor of women’s reproductive health, School of Public Health, University of Ghana, Accra, Ghana. Dr. Stine Lund is affiliated with Department of Public Health, University of Copenhagen, Denmark and Department of Pediatrics, Rigshospitalet, Denmark. Dr. Bjarke Lund Sørensen is a chief consultant in obstetrics at Slagelse Hospital, Denmark, and associate professor at Department of Clinical Medicine, University of Copenhagen. Stine Lund and Bjarke Sørensen have also studied the effect of the Safe Delivery app in rural parts of Ethiopia.
References
- Agarwal, S., & Labrique, A. (2014). Newborn health on the line: The potential mhealth applications. Jama, 312(3), 229–13. doi:10.1001/jama.2014.6371
- Agarwal, S., Perry, H. B., Long, L., & Labrique, A. B. (2015). Evidence on feasibility and effective use of mHealth strategies by frontline health workers in developing countries: Systematic review. Tropical Medicine & International Health, 20(8), 1003–1014. doi:10.1111/tmi.12525
- Amoakoh-Coleman, M., Borgstein, A. B., Sondaal, S. F., Grobbee, D. E., Miltenburg, A. S., Verwijs, M., … Klipstein-Grobusch, K. (2016). Effectiveness of mHealth interventions targeting health care workers to improve pregnancy outcomes in low- and middle-income countries: A systematic review. Journal of Medical Internet Research, 18(8), e226. doi:10.2196/jmir.5533
- Eldridge, S., & Kerry, S. M. (2011). A practical guide to cluster randomised trials in health services research. Chichester. West Sussex: John Wiley & Sons. Retrieved from http://ez.statsbiblioteket.dk:2048/login?url=http://site.ebrary.com/lib/stats/Top?id=10524069
- Free, C., Phillips, G., Galli, L., Watson, L., Felix, L., Edwards, P., … Cornford, T. (2012). The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: A systematic review. PLoS Medicine, 10(1), e1001362. doi:10.1371/journal.pmed.1001362
- Gohou, V., Ronsmans, C., Kacou, L., Yao, K., Bohoussou, K. M., Houphouet, B., et al. (2004). Responsiveness to life-threatening obstetric emergencies in two hospitals in abidjan, cote d’ivoire. Tropical Medicine & International Health: TM & IH, 9(3), 406–415. doi:10.1111/j.1365-3156.2004.01204.x
- Kruk, M. E., Leslie, H. H., Verguet, S., Mbaruku, G. M., Adanu, R. M., & Langer, A. (2016). Quality of basic maternal care functions in health facilities of five african countries: An analysis of national health system surveys. The Lancet.Global Health, 4(11), e845–e855. doi:10.1016/S2214-109X(16)30180-2
- Labrique, A., Vasudevan, L., Chang, L. W., & Mehl, G. (2013). H_pe for mHealth: More “y” or “o” on the horizon? International Journal of Medical Informatics, 82(5), 467–469. doi:10.1016/j.ijmedinf.2012.11.016
- Lars, H. J., Cardoso, P., Nielsen, B. B., Hvidman, L., Nielsen, J., & Aaby, P. (2005). Effect of sublingual misoprostol on severe postpartum haemorrhage in a primary health centre in guinea-bissau: Randomised double blind clinical trial. Bmj, 331(7519), 723. doi:10.1136/bmj.331.7519.723
- Lee, S., Nurmatov, U., Nwaru, B., Mukherjee, M., Grant, L., & Cagliari, C. (2016). Effectiveness of mHealth interventions for maternal, newborn and child health in low- and middle-income countries: Systematic review and meta-analysis. Journal of Global Health, 6(1), 010401. doi:10.7189/jogh.06.010401.
- Lund, S., Boas, I., Bedesa, T., Fekede, W., Nielsen, H., & Sørensen, B. (2016, June). Association between the safe delivery app and quality of care and perinatal survival in ethiopia: A randomized clinical trial. JAMA Pediatrics, 2016, 20.
- Mbaruku, G. G. (2009). Perinatal audit using the 3-delays model in western tanzania. International Journal of Gynecology and Obstetrics, 106(1), 85–88. doi:10.1016/j.ijgo.2009.04.008
- McCormick, M. L. (2002). Preventing postpartum hemorrhage in low-resource settings. International Journal of Gynecology and Obstetrics, 77(3), 267–275.
- Miller, S. S. (2003). Quality of care in institutionalized deliveries: The paradox of the dominican republic. International Journal of Gynecology and Obstetrics, 82(1), 89–103.
- Ministry of Health. (2013). Postpartum hemorrhage prevention and management strategy for ghana. Accra: MoH.
- Msemo, G., Massawe, A., Mmbando, D., Rusibamayila, N., Manji, K., Kidanto, H. L., … Perlman, J. (2013). Newborn mortality and fresh stillbirth rates in tanzania after helping babies breathe training. Pediatrics, 131(2), e353–60. doi:10.1542/peds.2012-1795
- Nilsson, C., Sørensen, B. L., & Sørensen, J. L. (2014). Comparing hands-on and video training for postpartum hemorrhage management. Acta Obstetricia Et Gynecologica Scandinavica, 93(5), 517–520. doi:10.1111/aogs.12372
- Sentilhes, L., Merlot, B., Madar, H., Sztark, F., Brun, S., & Deneux-Tharaux, C. (2016). Postpartum haemorrhage: Prevention and treatment. Expert Review of Hematology, 9(11), 1043–1061. doi:10.1080/17474086.2016.1245135
- Shiferaw, S., Spigt, M., Tekie, M., Abdullah, M., Fantahun, M., & Dinant, G. J. (2016). The effects of a locally developed mHealth intervention on delivery and postnatal care utilization; A prospective controlled evaluation among health centres in ethiopia. PloS One, 11(7), e0158600. doi:10.1371/journal.pone.0158600
- Sorensen, B. L., Rasch, V., Massawe, S., Nyakina, J., Elsass, P., & Nielsen, B. B. (2011). Advanced life support in obstetrics (ALSO) and post-partum hemorrhage: A prospective intervention study in tanzania. Acta Obstetricia Et Gynecologica Scandinavica, 90(6), 609–614. doi:10.1111/j.1600-0412.2011.01115.x
- Teerenstra, S., Lu, B., Preisser, J. S., van Achterberg, T., & Borm, G. F. (2010). Sample size considerations for GEE analyses of three-level cluster randomized trials. Biometrics, 66(4), 1230–1237. doi:10.1111/j.1541-0420.2009.01374.x
- Tomlinson, M., Rotheram-Borus, M. J., Swartz, L., & Tsai, A. C. (2013). Scaling up mHealth: Where is the evidence? PLoS Medicine, 10(2), e1001382. doi:10.1371/journal.pmed.1001382
- World Health Organization. (2015). Trends in maternal mortality: 1990 to 2015: Estimates by WHO, UNICEF, UNFPA, world bank group and the united nations population division. Geneva: WHO Document Production Services.
- World Health Organization. (2016). Ghana: Country profiles. Retrieved from http://www.who.int/gho/countries/gha/country_profiles/en/