Abstract
Abstract: Thirty-three-year-old Caucasian male underwent initiation of levetiracetam following witnessed generalized seizure activity at the same time presenting with a right MCA territory ischemic stroke. He then developed elevated CPK and myalgias are highly suspicious for rhabdomyolysis following levetiracetam. Subsequent improvement noted following complete cessation of medication. At follow-up patient reported complete resolution of hemiparesis and myalgias and no new neurological deficits while tolerating valproic acid. This case exemplifies potential rare adverse effect of levetiracetam. Levetiracetam is an antiepileptic medication effective for both generalized and focal types of epilepsy by affecting a broad spectrum of neurotransmitter release via calcium channels GABA receptors and synaptic vessel protein 2A (SV2A). Typical adverse effects include mild dizziness, headache, nausea, somnolence and sometimes hostility. This case provides further evidence of a rare and potentially life-threatening adverse effect of rhabdomyolysis. Further study is needed to possibly detect the exact mechanism resulting in this rare and dangerous adverse effect.
Keywords:
PUBLIC INTEREST STATEMENT
Levetiracetam is a well-known antiepileptic medication that is well tolerated to be useful as a broad-spectrum treatment for various classifications of epilepsy. This case highlights a potential adverse effect appealing to multiple facets of medical treatment and public knowledge. Given its lengthy history of use for antiepileptic purposes and profile which is mainly well tolerated, the aspects of the following report will appeal to many of those receiving this medication or who know someone receiving this medication. As detailed in the report, this case reveals a potentially significant and severe side effect likely to engage a multidisciplinary team in terms of medical treatment. From a nephrology perspective, it yields concern for rhabdomyolysis. In terms of critical care medicine treatment, patients are at significant risk of suffering severe rhabdomyolysis and subsequent sequelae. This topic also pertains directly to neurological treatment. An epileptologist and/or general neurologist may be on frontline of treating seizure and/or status epilepticus.
1. Introduction
Levetiracetam has been approved for clinical use as an antiepileptic drug (AED) in the United States for more than 20 years. It was considered to be a broad-spectrum AED, believed to control neurotransmitter release via modulating calcium channels, gamma-aminobutyric acid (GABA) receptor and synaptic vesicle protein 2A (SV2A) (Brodie, Citation2010). The adverse effect profile mainly encompasses asthenia, dizziness, headache, nausea, somnolence and hostility (Steinhoff et al., Citation2005). Recently, it has been reported that levetiracetam has resulted in a rare side effect of rhabdomyolysis (Moinuddin, Citation2020). Described is a case of levetiracetam-induced rhabdomyolysis in a 33 year old Caucasian male with no significant past medical history experiencing acute ischemic stroke and generalized seizure, providing further evidence for the significant few identified case reports on this subject ().
Table 1. Summary of levetiracetam-induced rhabdomyolysis case reports
2. Case report
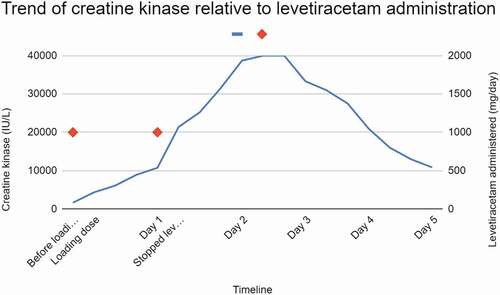
Thirty-three-year-old Caucasian male, without significant past medical history other than self-reported frequent marijuana smoking was admitted after acute onset of right-sided retro-orbital headache occurring prior to a motor vehicle collision in which he was the operator. Upon presentation to the emergency room, he demonstrated clinical generalized tonic-clonic seizure activity and subsequent slurred speech and left hemiparesis. He initially received a fosphenytoin bolus. He was found to have distal right MCA occlusion, but was not a candidate for thrombolytics or thrombectomy. He later underwent formal digital subtraction angiography which revealed spontaneous resolution of the above mentioned distal right MCA occlusion. He was started on levetiracetam. Subsequent diagnostic studies established evidence of PFO and acute right MCA ischemic stroke. Upon admission serum creatine kinase (CK) was 1656 IU/L. After he received an initial 1-g dose of intravenous levetiracetam, approximately 12 hours later, his CK rose to 6111 IU/L. Unfortunately, potentially confounding, he also received haloperidol 5 mg intravenously for agitation prior to sample collection. He received ziprasidone 10 mg via intramuscular injection (IM) several hours later. At approximately 24 hours after the initial dose of levetiracetam, CK rose to 10,761 IU/L, but there was no laboratory or clinical evidence comporting with acute kidney injury (AKI). There was no discoloration of urine. The patient reported subjective myalgia and lumbago. Continuous intravenous fluid (IVF) was introduced in an effort to mitigate the severe rise in CK. All further antipsychotic medications as well as all meds via intramuscular injection (IM) were discontinued. The patient did not receive anymore levetiracetam beyond 28 hours since the initial dose. CK levels continued to rise, beyond the detectable limit of 40,000 IU/L twice at the 60-hour and 64-hour mark since the initial dose. Afterwards, down-trending was noted. On the day of discharge, 5 days after the initial dose of levetiracetam, he still had an elevated CK of 10,852 IU/L, but muscle ache and renal function was unremarkable. His AED regimen was changed to divalproex. At subsequent follow-up, a few weeks later, the patient reported complete resolution of neurological symptoms including left hemiparesis, myalgia and lumbago. The patient also reported no further episodes of seizure-like activity while reporting continued marijuana usage ().
3. Discussion
At the time of this submission, a total of 14 published levetiracetam-related rhabdomyolysis reports were available for review (Akiyama et al., Citation2014; Aslan et al., Citation2020; Incecik et al., Citation2016; Isaacson et al., Citation2014; Kubota et al., Citation2017; Di Lorenzo & Li, Citation2017; Moinuddin, Citation2020; Ramon et al., Citation2016; Rastogi et al., Citation2018; Rota et al., Citation2018; Shahbaz et al., Citation2017; Singh et al., Citation2014; Sohn et al., Citation2017; Thomas et al., Citation2019). No confirmatory studies are able to prove definitively the mechanism behind levetiracetam-induced rhabdomyolysis, a study by Chakkalakal et al. suggests the SV2A protein is localized on certain types of muscle fibre (Chakkalakal et al., Citation2010). SV2A targeted by levetiracetam may result in rhabdomyolysis. Upon review of available case studies, there is a correlation between discontinuation of levetiracetam and down-trending creatine kinase (Akiyama et al., Citation2014; Aslan et al., Citation2020; Incecik et al., Citation2016; Isaacson et al., Citation2014; Kubota et al., Citation2017; Di Lorenzo & Li, Citation2017; Moinuddin, Citation2020; Ramon et al., Citation2016; Rastogi et al., Citation2018; Rota et al., Citation2018; Shahbaz et al., Citation2017; Singh et al., Citation2014; Sohn et al., Citation2017; Thomas et al., Citation2019). Reports reveal a down-trending creatine kinase within 24–48 hours of discontinuing levetiracetam similar to clinical evidence reported in this case.
Notably, patients in two of the three pediatric cases reviewed were already being treated with levetiracetam (Aslan et al., Citation2020; Incecik et al., Citation2016). These patients who were already receiving levetiracetam developed rhabdomyolysis after dose escalation of levetiracetam during admission. Case report by Sohn et al. describes a process of re-introduction of levetiracetam where the patient received similar total doses during the first three days (Sohn et al., Citation2017). Collectively, these cases suggested a patient would potentially tolerate levetiracetam if re-introduced at a lower dose, similar to that which is suggested for statin intolerance (Toth et al., Citation2018). Furthermore, it has been reported that levetiracetam does not influence serum concentration or pharmacokinetics of other AEDs (Gidal et al., Citation2005; Otoul et al., Citation2007). Therefore, one may be able to identify it as the precise medication causing the rise in CK in patient on multiple AEDs. Based on existing case reports, it was likely that levetiracetam could cause rhabdomyolysis early in the treatment course. There is no evidence available to suggest marijuana may lead to rhabdomyolysis. While rhabdoyolysis was apparent, there was no evidence of congruent AKI suggesting the phenytoin bolus initially received was less likely the causative agent resulting in rhabdomyolysis (Kim et al., Citation2016). The same circumstances would apply to the contrast material received yielding no evidence of AKI suggesting not the causative agent for vast elevation in CK. Potentially confounding to the accuracy in the rise of CK would be the IM injections received due to the potential side effect of specific antipsychotic medications as well as the muscle injury secondary to IM injection. Noting literature review reveals evidence suggestive of these doses for short duration are less likely to result in significant rhabdomyolysis.
4. Conclusion
In conclusion, it was reported in pediatric literature that classic triad of myalgia, weakness and urine discoloration were only seen in less than 10% of the patients (Elsayed & Reilly, Citation2010). In the case described above, there was no evidence of urine discoloration; no appreciable changes in motor strength on physical exam and no changes in patients overall subjective report of motor strength. There was overt evidence of the elevation in serum CK positively correlated to the notable subjective report of painful myalgia. It is recommended that if suspicion is high in a patient recently initiated on levetiracetam, it will be beneficial to trend creatine kinase and reduce or discontinue the medication. Recognizing that levetiracetam permits high utility by effectively treating multiple types of epileptic seizure in adults and children without serum monitoring, and with a relatively mild adverse effect profile, further studies are suggested to assess whether levetiracetam could potentially be safely and effectively re-introduced for some patients who may not have initially tolerated the medication. Further study may also ultimately yield improved understanding of the mechanism behind this potentially significant adverse effect.
Additional information
Funding
Notes on contributors
Anthony C. Torres
Anthony C. Torres, DO, has studied Osteopathic Medicine at the renowned Philadelphia College of Osteopathic Medicine. Following undergraduate studies in Psychology and Biology at the York College of Pennsylvania, he was surrounded by influence from The Center for Chronic Diseases of Aging while studying at The Philadelphia College of Osteopathic Medicine, where he developed interest in the field of Neurology. Research interests include aspects of stroke and seizure as well as infections associated with brain disease and behavior correlated with cognition and components of language. This case report highlights a case of suspected adverse effect of a well know and typically well-tolerated antiepileptic medication.
References
- Akiyama, H., Haga, Y., Sasaki, N., Yanagisawa, T., & Hasegawa, Y. (2014). A case of rhabdomyolysis in which levetiracetam was suspected as the cause. Epilepsy & Behavior Case Reports, 2, 152–6. https://doi.org/https://doi.org/10.1016/j.ebcr.2014.08.001
- Aslan, N., Yildizdas, D., Huseyınlı, B., Horoz, O. O., Mert, G. G., Ekinci, F., & Ozcanyuz, D. (2020). Levetiracetam treatment-associated acute rhabdomyolysis in an adolescent. Journal of Pediatric Intensive Care, 09(2), 139–140. https://doi.org/https://doi.org/10.1055/s-0039-1700951
- Brodie, M. J. (2010). Antiepileptic drug therapy the story so far. Seizure, 19(10), 650–655. https://doi.org/https://doi.org/10.1016/j.seizure.2010.10.027
- Chakkalakal, J. V., Nishimune, H., Ruas, J. L., Spiegelman, B. M., & Sanes, J. R. (2010). Retrograde influence of muscle fibers on their innervation revealed by a novel marker for slow motoneurons. Development, 137(20), 3489–3499. https://doi.org/https://doi.org/10.1242/dev.053348
- Di Lorenzo, R., & Li, Y. (2017). Rhabdomyolysis associated with levetiracetam administration: Levetiracetam and rhabdomyolysis. Muscle & Nerve, 56(1), E1–E2. https://doi.org/https://doi.org/10.1002/mus.25548
- Elsayed, E. F., & Reilly, R. F. (2010). Rhabdomyolysis: A review, with emphasis on the pediatric population. Pediatric Nephrology, 25(1), 7–18. https://doi.org/https://doi.org/10.1007/s00467-009-1223-9
- Gidal, B. E., Baltès, E., Otoul, C., & Perucca, E. (2005). Effect of levetiracetam on the pharmacokinetics of adjunctive antiepileptic drugs: A pooled analysis of data from randomized clinical trials. Epilepsy Research, 64(1–2), 1–11. https://doi.org/https://doi.org/10.1016/j.eplepsyres.2005.01.005
- Incecik, F., Herguner, O. M., Besen, S., & Altunbasak, S. (2016). Acute rhabdomyolysis associated with levetiracetam therapy in a child. Acta Neurologica Belgica, 116(3), 369–370. https://doi.org/https://doi.org/10.1007/s13760-015-0542-9
- Isaacson, J. E., Choe, D. J., & Doherty, M. J. (2014). Creatine phosphokinase elevation exacerbated by levetiracetam therapy. Epilepsy & Behavior Case Reports, 2, 189–191. https://doi.org/https://doi.org/10.1016/j.ebcr.2014.09.008
- Kim, H., Jo, S., Park, K. W., Han, S. H., & Lee, S. A. (2016, June 30). A case of phenytoin-induced rhabdomyolysis in status epilepticus. Journal of Epilepsy Research, 6(1), 36–38. https://doi.org/https://doi.org/10.14581/jer.16007
- Kubota, K., Yamamoto, T., Kawamoto, M., & Fukao, T. (2017). Levetiracetam-induced rhabdomyolysis: A case report and literature review. Neurol Asia, 22(3), 275-278. http://neurology-asia.org/articles/neuroasia-2017-22(3)-275.pdf
- Moinuddin, I. A. (2020, October 28). Suspected levetiracetam-induced rhabdomyolysis: A case report and literature review. American Journal of Case Reports, 21, e926064. https://doi.org/https://doi.org/10.12659/AJCR.926064. PMID: 33112844; PMCID: PMC7603803.
- Otoul, C., De Smedt, H., & Stockis, A. (2007). Lack of pharmacokinetic interaction of levetiracetam on carbamazepine, valproic acid, topiramate, and lamotrigine in children with epilepsy. Epilepsia, 48(11), 2111–2115. https://doi.org/https://doi.org/10.1111/j.1528-1167.2007.01201.x
- Ramon, M., Tourteau, E., Lemaire, N., Gautier, S., & Béné, J. (2016). HyperCKemia induced by levetiracetam. La Presse Médicale, 45(10), 943–944. https://doi.org/https://doi.org/10.1016/j.lpm.2016.05.014
- Rastogi, V., Singh, D., Kaur, B., Arora, P., & Gadikota, J. P. (2018). Rhabdomyolysis: A rare adverse effect of levetiracetam. Cureus, 10(5), e2705. Published online May 29. https://doi.org/https://doi.org/10.7759/cureus.2705.
- Rota, E., Arena, L., Celli, L., Testa, L., & Morelli, N. (2018). Levetiracetam-induced rhabdomyolysis: The first Italian case. Neurological Sciences, 39(9), 1629–1630. https://doi.org/https://doi.org/10.1007/s10072-018-3421-3
- Shahbaz, N., Khan, S. A., Younus, S. M., Khan, M. A., & Memon, M. H. (2017). Levetiracetam induced increase in creatine Phosphokinase levels. Journal of the College of Physicians and Surgeons--pakistan, 27(3), S63-S64. https://pubmed.ncbi.nlm.nih.gov/28302251/
- Singh, R., Patel, D. R., & Pejka, S. (2014). Rhabdomyolysis in a hospitalized 16-year-old boy: A rarely reported underlying cause. Case Reports in Pediatrics, 2, 1–2. https://doi.org/https://doi.org/10.1016/j.ebcr.2014.09.008
- Sohn, S.-Y., Kim, J. G., Kim, D.-H., Jang, S.-H., Yoon, S. J., & Lee, S. J. (2017). Repeated occurrence of HyperCKemia after levetiracetam administration. Published online. 5, 150-154. https://www.ecronicon.com/ecne/pdf/ECNE-08-00250.pdf
- Steinhoff, B. J., Trinka, E., & Wieser, H.-G. (2005). Levetiracetam in patients with refractory epilepsy: Results of the SKATE trial in Austria, Germany and Switzerland. Seizure, 14(7), 490–496. https://doi.org/https://doi.org/10.1016/j.seizure.2005.08.005
- Thomas, L., Mirza, M. M. F., Shaikh, N. A., & Ahmed, N. (2019). Rhabdomyolysis: A rare adverse effect of levetiracetam. BMJ Case Reports, 12(8), e230851. https://doi.org/https://doi.org/10.1136/bcr-2019-230851
- Toth, P. P., Patti, A. M., Giglio, R. V., Nikolic, D., Castellino, G., Rizzo, M., & Banach, M. (2018). Management of statin intolerance in 2018: Still more questions than answers. American Journal of Cardiovascular Drugs, 18(3), 157–173. https://doi.org/https://doi.org/10.1007/s40256-017-0259-7