ABSTRACT
Valid body core temperature measurements are essential for diagnosing and monitoring athletes with exertional heat stroke (EHS). Experts question the validity of body temperature sites that vary by >±0.27°C from the gold standard, rectal temperature (TREC). No research has established the validity of body temperature sites when American football uniforms (PADS) are worn during simulated EHS scenarios. Thirteen men (age, 22 ± 2 y; mass, 77.5 ± 8.8 kg; height, 181.3 ± 5.7 cm) donned PADS and entered an environmental chamber (38.7 ± 0.8°C, 38.9 ± 2.4% relative humidity). We compared TREC to a forehead liquid crystal temperature monitor (TFHD), axillary (TAXL), oral (TORL), and aural temperatures (TEAR) 34 times over four consecutive periods: 10-minutes of rest; exercise until participants TREC was between 39.7°C and 39.8°C; cold-water immersion (CWI, 10.0 ± 0.1°C) until all temperature sites indicated ≤38°C; and a 15-minute post-immersion recovery period. Body temperatures varied between sites during all periods (F36,432 ≥ 2.5, P ≤ 0.001). TAXL and TORL statistically differed from TREC and exceeded the 0.27°C bias threshold at all 34 measurement times (100%). TFHD differed from TREC eight times during rest; eight times during exercise; 0 times during CWI; and twice during recovery (18/34, 53%). TFHD exceeded the bias threshold 28 times (82%). TEAR differed from TREC five times during rest; 0 times during exercise; five times during CWI; and once during recovery (11/34, 32%). TEAR exceeded the 0.27°C bias threshold 15 times during testing (44%). TAXL, TFHD, TEAR, and TORL should not be used to diagnose or monitor American football players with EHS.
Abbreviations ANOVA: analysis of variance; CWI: cold water immersion; EHS: exertional heatstroke; PADS: full American football uniform consisting of a helmet; shoulder, knee, thigh, hip and tailbone pads; a jersey top; undergarments; and half-length pants; TAXL: axillary temperature; TEAR: aural temperature; TFHD: liquid crystal temperature monitor; TORL: oral temperature; TREC: rectal temperature
Introduction
Obtaining an accurate assessment of body core temperature is essential for diagnosing and treating life-threatening exertional heat stroke (EHS). The current standard of care for EHS includes obtaining a valid assessment of body core temperature (e.g., rectal temperature [TREC]); confirmation of central nervous system dysfunction; and aggressive whole body cooling by any means available before transport to advanced medical care [Citation1,Citation2]. Without a valid assessment of body core temperature, clinicians are unable to differentiate EHS from other potentially life-threatening medical conditions (e.g., hyponatremia) or know when to safely discontinue cooling. Mismanagement and/or misdiagnosis can lead to inappropriate medical decisions and disastrous outcomes for patients with EHS [Citation3–5]. In contrast, rapid recognition of EHS followed by cold water immersion (CWI) has resulted in 100% survival rates[Citation6].
TREC is the “gold standard” method for estimating body core temperature when exercise-induced hyperthermia and EHS are suspected [Citation2,Citation7–9]. Unfortunately, administrative barriers, privacy concerns of doing TREC (especially in adolescents or children), proficiency with the technique, and budgetary limitations are often cited as reasons why clinicians cannot or do not utilize TREC as part of their standard of care in an EHS emergency [Citation10–12]. Consequently, some clinicians utilize other body temperature sites like the mouth (TORL), axillae (TAXL), ear (TEAR), or forehead (TFHD) to assess body temperature and believe these sites are valid[Citation13]. However, the indirect physiological responses for heat dissipation (i.e., skin wittedness due to increased skin blood flow, the thermal gradient created between the core and skin) coupled with the external environmental conditions during exercise make the validity of temperature estimates from these sites questionable [Citation7,Citation8,Citation14,Citation15]. In fact, some EHS experts consider a temperature bias (i.e., difference between TREC and the other body sites) greater than ±0.27°C to be an invalid measurement for exercising, hyperthermic individuals [Citation7,Citation16].
Sadly, EHS remains one of the leading causes of death in sport and physical activity, especially amongst American football players [Citation3,Citation5,Citation17]. In fact, American football players have an 11 times higher risk of developing an exertional heat illness than all other sports combined at the secondary school level[Citation18]. The risk profile for EHS in American football is high because of the high-intensity nature of the sport; training beginning during the hottest part of the year in the Northern Hemisphere; and the wearing of heavy, insulating safety equipment usually consisting of a helmet; shoulder, knee, thigh, hip and tailbone pads; a jersey top; undergarments; and half-length pants (PADS). Because PADS cover approximately 75% of the body’s surface area and reduce evaporative capacity[Citation19], thermal strain and body core temperature rises faster when athletes exercise with PADS donned than other protective equipment ensembles (e.g., shorts and t-shirts) [Citation20–22]. Since PADS affect body core and skin temperatures[Citation22], it is possible they also affect the validity of other external body temperature sites.
To date, no research has examined the criterion-validity of common body temperature sites when PADS are worn during exercise-induced hyperthermia development, CWI treatment, and post-immersion recovery scenarios. Based on their popularity and use in clinical settings [Citation13,Citation23], we examined whether TORL, TAXL, TEAR, and TFHD could be used as a surrogate for TREC when men wore PADS during rest, exercise-induced hyperthermia, CWI, and post-immersion recovery. We hypothesized all common body temperature sites would demonstrate unacceptable levels of bias (i.e., bias >±0.27°C) from TREC during all periods of testing.
Materials and methods
Sample size for the experimental design was determined a priori using a statistical software package (G*Power 3.1; Kiel, Germany) with the following parameters: alpha = 0.05, beta = 0.8, effect size = 0.5, and a correlation among repeated measures of 0.8. It was estimated 10 subjects were needed to sufficiently power the study. Individuals were excluded from participating if they self-reported: (1) an injury or illness which impaired their ability to exercise, (2) any neurological, respiratory, gastrointestinal, esophageal, thermoregulatory, or cardiovascular diseases, (3) taking any medications that may have affected fluid balance or temperature regulation, (4) a sedentary lifestyle (defined as exercising <30 minutes, 3 times per week)[Citation24], (5) a history of heat-related illness in the 6 months preceding data collection, or (7) cold allergy. All procedures were approved by Central Michigan University’s institutional review board and participants provided written consent before participation.
Participants reported for one day of testing between 0800 and 1500 hours. Participants were instructed to abstain from exercise, stimulants (e.g., caffeine), and depressants (e.g., alcohol) for at least 24 hours prior to testing. They were also instructed to drink water regularly throughout the day preceding testing to ensure their urine was clear or light yellow, and to fast for 2 hours before the start of testing. Compliance was self-reported prior to testing.
Participants voided their bladders completely and urine specific gravity was measured to assess hydration status (SUR-Ne refractometer, Atago USA Inc., Bellevue, WA). If urine specific gravity was >1.020[Citation25], participants were assumed to be hypohydrated, asked to ingest ~1 L of water, and urine specific gravity was reassessed approximately 1 hour later. If participants were still hypohydrated, they were rescheduled for another testing day at least 24 hours later. If euhydrated, we measured skinfolds at the chest, abdomen, and thigh in triplicate per Pollock, Schmidt, and Jackson [Citation26] (Baseline skinfold caliper #12-1110, Fabricated Enterprises, Inc, White Plains, NY). Skinfolds were averaged at each site and summed to estimate body density [Citation27] and percent body fat[Citation28]. Then, participants were weighed nude to the nearest hundredth of a kilogram (Defender #5000, Ohaus Corp, Parsippany, NJ) and self-inserted a rectal thermistor 15 cm past the anal sphincter (model #401; Advanced Industrial Systems, Prospect, KY)[Citation29]. Body surface area was estimated using Dubois and Dubois’s equation[Citation30]. They then dressed in undergarments, shorts, and socks.
We shaved the left armpit and taped a thermistor (model #409B; Advanced Industrial Systems, Prospect, KY) over the skin in the middle of the axilla. We placed a liquid crystal temperature monitor (Redi-Temp model #5010-H; Trademark Medical LLC, St Louis, MO) in the center of the forehead just above the eyebrows per manufacturer instructions. Then, participants donned a heart rate monitor (model FT1, Polar Electro, Inc, Lake Success, NY) and PADS. For a complete description of PADS, we direct the reader to our prior work [Citation31,Citation32].
Participants entered an environmental chamber and stood on a treadmill for 10 minutes to adapt to the heat (). During this period, participants had the helmet off so we could record TFHD and TEAR more easily. All body temperatures were recorded every minute during this rest period. Then, participants donned the helmet and completed sequential 5-minute bouts of exercise. Each bout consisted of running for 2 minutes at 90% of their age-predicted maximum heart rate (0% incline) and then walking at 4.8 km·h−1 (3 mph) for 2.25 minutes. After each walk, they removed their helmet and rested for 0.75 minutes. During these short rest intervals, we recorded all body temperatures. Participants were given special instruction to keep their left arm tightly against their side as much as possible before measurements to ensure accurate measurement of TAXL. Similarly, they were to place TORL in the same location under their tongue each time. These 5-minute bouts continued, without interruption, until TREC was between 39.7°C and 39.8°C. All temperatures were recorded every 5 minutes during exercise. TREC was monitored continuously to determine the exact time participants TREC was between 39.7°C and 39.8°C. Due to the large number of repeated measurements and body sites, we recruited several licensed medical providers to aid us with data collection. When possible, the same medical provider collected data from the same body site within and between sessions to reduce investigator measurment variability and improve consistency of measurement technique.
Table 1. Participant demographics and descriptive information
Upon reaching a TREC between 39.7°C and 39.8°C, participants removed their helmet and shoes and had all temperatures recorded. Then, they immersed themselves up to the neck in a 1135.6 L capacity, non-circulating water tub (model #4247, Rubbermaid, Atlanta, GA) with the remaining equipment on since football equipment does not impair CWI cooling rates [Citation31,Citation32]. Participants were instructed to keep their left arm tightly against their side during CWI for TAXL accuracy. A standard stopwatch was started the moment each subject’s foot touched the water and stopped when participants exited the bath. Participants remained immersed until all temperatures read ≤38°C. All body temperatures were recorded every minute during CWI since some devices required long lead times to display a temperature. Participants were asked to self-report if, and when, they noticed any shivering. The water bath was stirred every 2 minutes. A separate model #401 thermistor (Advanced Industrial Systems Inc, Prospect, KY) was secured at 21 cm from the bottom of the water bath. The water bath was kept in the environmental chamber for the duration of testing; water temperature was continuously monitored and maintained at ~10°C by adding ice as necessary. We ensured all ice had melted before participants entered the bath and no ice was added once participants began CWI.
Once all sites displayed a temperature ≤38°C, participants exited the bath, dried their arms, and sat in the heat, with the wet equipment donned, for 15 minutes. All temperatures were recorded every 5 minutes during this recovery period. Following the recovery period, participants exited the environmental chamber, removed the thermistors, towel dried completely, were weighed nude a second time, and excused. No fluids were given to participants once they entered the environmental chamber.
Separate 4600 Precision thermometers (Advanced Industrial Systems Inc, Prospect, KY) were used to measure TREC and TAXL. The 4600 thermometers were certified to be accurate to within ±0.1°C when used between −40°C to 100°C. TFHD was measured with a liquid crystal temperature monitor with a temperature range of 34.4°C to 41.1°C. No manufacturer specifications for accuracy of TFHD could be identified. TORL was measured with a digital thermometer (#15-681-000, Briggs Healthcare, Waukegan, IL) and disposable plastic cover. Manufacturer specifications indicated TORL thermometer accuracy to ±0.4°C. TEAR was measured with a digital ear thermometer and disposable ear cover (#6026, Braun, Kronberg, Germany). Manufacturer specifications indicated TEAR thermometer accuracy to ±0.25°C when body temperature is within 35.5°C to 42°C.
Because total exercise and CWI durations differed between participants, we compared temperatures at 10% increments of participants’ total exercise and CWI times. Cooling rates for each site were calculated, and compared between sites, by taking the difference in body temperatures at the final exercise time from the last body temperature recorded during CWI and dividing it by the amount of time necessary to reduce each temperature site to ≤38°C. Since all sites, except TREC, were not measured continuously, cooling durations were rounded to the nearest minute when the device read ≤38°C. For example, TAXL dropped below 38°C within seconds of CWI for every subject; however, cooling duration was recorded as 1 minute based on this methodological limitation.
All data were reported as means and standard deviations and we checked for normality to ensure normal distribution. A two-way (temperature site x time) repeated measures analyses of variance (ANOVA) was used to determine if differences in raw temperatures existed between body sites for the four testing periods. In total, we statistically compared body temperatures 34 times: 11 times during rest; 10 times during exercise; 10 times during CWI; and 3 times during recovery.
To simplify the statistical analysis of temperature bias, we calculated the average bias for each experimental period and body temperature site. We then used a two-way (temperature site x period) repeated measures ANOVA to examine differences in bias between sites across the four periods. Sphericity was assessed with Mauchly’s test. Geisser-Greenhouse adjustments to P-values and degrees of freedom were made when sphericity was violated. Upon significant interactions or main level effects, Tukey-Kramer post-hoc tests identified differences at each time point.
We also utilized all 34 temperature measurements for each subject to create Bland-Altman plots. These were used to calculate the mean and 95% limits of agreement between body temperature sites and TREC. Significance was accepted when P < 0.05 (Number Cruncher Statistical Software v.2007, Kaysville, UT).
Results
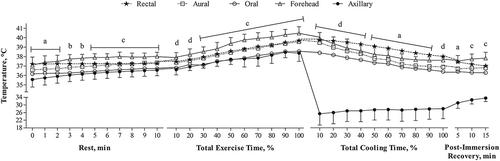
To account for subject attrition and ensure we exceeded a priori power estimates, we recruited a convenience sample of 15 healthy, recreationally active, unacclimated men. Two men discontinued testing during exercise due to the difficulty of the protocol; 13 men completed the study. In addition to demographic information, urine specific gravity, sweat rates, hypohydration levels, shivering onset, and testing conditions are reported for descriptive purposes (). Participants exercised for 42.3 ± 8.9 minutes. The time to reduce body temperature to 38°C (i.e., CWI duration) significantly varied by site (TREC = 7.8 ± 3.6 min; TAXL = 1 ± 0 min; TORL = 3 ± 1 min; TFHD = 3 ± 1 min; TEAR = 4 ± 1; F1,14 = 29.9, P < 0.001) as did cooling rates (TREC = 0.26 ± 0.11°C·min−1; TAXL = 10.4 ± 4.6°C·min−1; TORL = 0.75 ± 0.41°C·min−1; TFHD = 0.89 ± 0.24°C·min−1; TEAR = 0.83 ± 0.34°C·min−1; F1,12 = 59.6, P < 0.001).
We observed significant interactions between body temperatures from the various sites and time in each period (F36,432 ≥ 2.5, P ≤ 0.001, ). TORL and TAXL were statistically different than TREC and above our bias threshold ±0.27°C for all measurement times (34/34, 100%). TFHD was different than TREC eight times at rest, eight times during exercise, 0 times during CWI, and twice during the post-immersion recovery (18/34, 53%). TFHD exceeded our bias threshold 28 times during the study (82%). TEAR was different than TREC at five times during rest, 0 times during exercise, five times during CWI, and once during post-immersion recovery (11/34, 32%). TEAR exceeded our bias threshold 15 times during testing (44%).
Figure 1. Body temperatures measured in the rectum, mouth, axilla, forehead, and ear during rest, exercise, cold-water immersion, and post-immersion recovery in the heat. a = Rectal different than all other measurements except forehead. b = Rectal different than all other measurement sites. c = Rectal different than all other measurements except aural. d = Rectal different than axillary and oral measurements. All data are means ± SD, n = 13. Suprascripts indicate P < 0.05
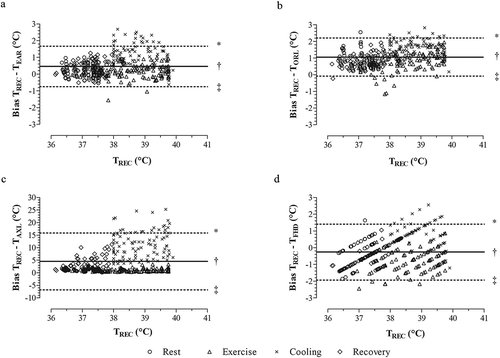
We observed an interaction between temperature site and time for bias (F1,16 = 37.3, P < 0.001, and ). TAXL was different than all other body sites during cooling and post-immersion recovery (P < 0.05). Overall, all the body temperature sites exceeded our bias threshold and ranged from −0.3 ± 0.4°C to 4.7 ± 1.9°C (). Moreover, each temperature site displayed large limits of agreement ranging from −0.3°C to 1.4°C to −6.8°C to 15.9°C ().
Table 2. Bias from rectal temperature (°C) during four experimental time periods
Figure 2. Bland-Altman plots indicating temperature bias between rectal temperature (TREC) and aural temperature (TEAR, A), oral temperature (TORL, B), axillary temperature (TAXL, C), and forehead temperature (TFHD, D) during rest, exercise, cold-water immersion, and post-immersion recovery in the heat. * = upper 95% limit of agreement; † = mean difference; ‡ = lower 95% limit of agreement
Discussion
EHS is a medical emergency leading to numerous deaths in American football over the last 100 years [Citation3,Citation33]. Thus, we investigated the validity of several commonly used body temperature measurement sites utilized as a surrogate for TREC [Citation13] in men wearing PADS at rest in a hot environment; during exercise in the heat; CWI; and post-immersion recovery. Our data are especially useful since many clinicians do not perform TREC in EHS situations for a variety of reasons (e.g., cost, privacy concerns) [Citation10–12]. Using previously established definitions of temperature bias acceptability (±0.27°C) [Citation7,Citation8,Citation16,Citation34], our findings show TORL, TAXL, TEAR, and TFHD are invalid as a surrogate for TREC during a bout of exercise and cooling while wearing PADS. Thus, we concur with others that these four external body temperature sites should not be used when body temperature estimates are vital to the survivability and prognosis of patients with EHS [Citation1,Citation2,Citation23].
TORL is one of the most commonly utilized body temperature sites due to its ease of access, lack of invasiveness, and tolerance by patients. It is also considered the most valid non-rectal temperature measurement site by some medical professionals[Citation13]. Unfortunately, our data showed TORL consistently exceeded our bias threshold and statistically differed from TREC at all times in the study. Moreover, the mean bias (1.0 ± 0.2°C) and range of bias (0.8°C − 1.6°C) calls into question the validity of TORL as a diagnostic and monitoring tool for athletes undergoing CWI. Ganio et al [Citation8]. and Casa et al [Citation7]. similarly observed TORL underestimated TREC by 1.1°C and 1.7°C in laboratory and field based settings, respectively.
A potential factor that may have influenced the differences found between TORL and TREC was the testing conditions and environment. Similar to the findings of Ganio et al [Citation8]. and Mairiaux et al [Citation35]. we observed mean bias decrease over the duration of the baseline period (baseline time 0 minute = 1.1 ± 0.5°C; baseline minute 10 = 0.5 ± 0.3°C), but increased as exercise continued (10% exercise complete = 0.7 ± 0.4°C; 100% exercise complete = 1.2 ± 0.4°C). Higher ventilation rates during exercise and inhalation of cooler air near the surface of the water likely perpetuated the increase in mean bias between TORL and TREC compared to the other phases of the study.
The validity of TAXL has been previously questioned in clinical studies [Citation36–38] and during exercise in the heat [Citation7,Citation8]. We observed similar findings with TAXL severely underestimating TREC during all phases of the study (ranged from 1°C to 11.8°C). Our mean bias between TAXL and TREC was smaller during baseline and exercise (1.1 ± 0.4°C and 1 ± 0.5°C respectively) than Casa et al [Citation7]. (2.1°C to 2.6°C) but similar to that of Ganio et al [Citation8]. (0.9°C to 1.3°C). We conclude the controlled environment in the climatic chamber reduced the variability of the measures and wearing an American Football uniform provided no further benefit of minimizing the bias between the two measures.
As with TORL, the environment had similar effects on TAXL. For example, during baseline rest, the mean bias declined from 1.7 ± 0.6°C (minute 0) to 0.7 ± 0.3°C (minute 10). This was most likely due to skin temperature increasing as the body was subjected to longer periods of environmental heat stress. Furthermore, during CWI, the mean bias was quite large and more closely resembled the temperature of the water bath rather than the individual’s body core temperature. Consequently, using TAXL to diagnose and monitor EHS patients would likely result in disastrous patient outcomes.
Regarding TEAR, we observed it closely approximated TREC during exercise (0.1 ± 0.3°C) and fell below our criterion threshold for validity. While some may interpret this as validation for TEAR in exercising individuals wearing PADS, a few factors must be considered before coming to this conclusion. First, when considering the entirety of the experiment, the overall mean bias between TEAR and TREC (0.5 ± 0.2°C) exceeded our bias threshold. Similarly, Morrissey et al [Citation16]. reported higher amounts of bias with TEAR in 26 runners with EHS. In their field study, TEAR was 2.4 ± 1.0°C lower than TREC. Had TEAR been the only temperature monitored, it is likely several of these athletes would have been misdiagnosed and/or treated incorrectly since their TEAR (38.8 ± 1.1°C) were consistent with the less dangerous condition, exertional heat exhaustion. Second, the device used to assess TEAR had an operating error threshold of ±0.25°C in individuals whose body temperature ranges from 35–42°C. This is a concern as the variability of the temperature device ranges 0.5°C, which is nearly identical to the criterion threshold established for this study. Third, Hansen et al [Citation39]. found that when airflow to the face was minimized, tympanic temperature underestimated TREC by ~0.2°C compared to when airflow was increased (mean bias, 0.7–1.1°C). Since our participants were wearing an American Football helmet for most of the trial, airflow was restricted. In the event of a suspected heat illness, clinicians would remove the helmet to rule out other potentially life threatening conditions before deciding to assess body temperature. Overall, it is clear that the inconsistent nature of the mean bias across all 4 periods between TEAR and TREC, particularly the large bias exhibited during CWI, invalidate this location for exercising, hyperthermic individuals.
Aside from cooling, TFHD overestimated TREC above our criterion threshold for validity. We propose two explanations. First, a microclimate may have been created between the forehead and the internal pad of the helmet thereby increasing the humidity and temperature in this particular space [Citation19] resulting in decreased heat dissipation and a higher than normal temperature that more closely approximated TREC. Supporting this notion is the fact that once CWI began, TFHD underestimated TREC by 0.6 ± 0.5°C which was likely due to the sensor being cooled by the air nearest the water. Prior authors found similar results with the liquid forehead strip either underestimating [Citation7,Citation40,Citation41] or overestimated [Citation8,Citation42] TREC. Second, the sensor relied on color changes for accuracy and only allowed us to measure TFHD in 2°F (1.1°C) increments. Aside from the difficulty determining color in some instances, our team likely interpreted the temperature as the higher of the two temperatures leading to less bias between devices. Regardless, the criterion bias threshold was exceeded in every period and TFHD should not be utilized for patient care due to its insensitivity and high degree of subjectivity.
We acknowledge our study’s limitations. First, to make data collection more manageable, we taped a skin thermistor to the axilla to record TAXL temperature. This likely created a micro-climate in the axilla and may have made TAXL warmer than they actually were. In contrast to our methodology, clinicians working in the field would likely use a small digital thermometer to record TAXL. We believe this practice would result in higher bias since many digital thermometers cannot be safely submerged in water and PADS often interferes with the placement of these thermometers in the axillae. While differences in TAXL applications may exist between laboratory and field settings, the bias of TAXL still prohibits it from meriting any consideration for the assessment of hyperthermia or exertional heat illness. Second, because many of the thermometers required longer lead time to measure (e.g., TORL, TEAR) than others, we could not record these temperatures continuously. This particularly affected CWI durations and, hence, cooling rate estimates. However, we do not believe this affects our interpretation that these common temperature sites should not be used for monitoring EHS patients since numerous, sequential measurements were taken at each site and all data were normalized for between subject comparisons. Third, we tested only one type of American football uniform in a group of healthy men rather than all the possible uniform configurations (e.g., “shells”) utilized by American football athletes. This is important to consider, and worthy of future investigation, as various football ensembles affect thermal insulation and evaporative cooling[Citation19].
In summary, all common body temperature sites measured in this study demonstrated unacceptable levels of bias in hyperthermic individuals wearing PADS and should not be used as surrogates for the gold standard body temperature, TREC. Most concerning was several sites (i.e., TORL, TEAR, TAXL) significantly underestimated TREC. Clinically, this could translate to disastrous outcomes: misdiagnosis of EHS for a condition consistent with illnesses displaying lower body temperatures (e.g., heat exhaustion); initiating incorrect interventions (e.g., fanning, loosening restrictive clothing); or removing an athlete from life-saving CWI too soon (e.g., Gavin Class)[Citation43]. Therefore, our data support the continued, and preferential, use of TREC in assessing body core temperature during exercise in the heat and CWI. Sports medicine professionals must continue to adhere to current best practices in the development and implementation of policies and procedures related to the management of heat-related illness[Citation2]. Without question, TREC is the only valid method of body core temperature assessment in exercising individuals that is also clinically-available in emergency situations. When implemented as part of the emergency action plan for EHS victims, TREC helps saves lives[Citation6].
Consent to participate
All participant signed an informed consent approved by the Institutional Review Board of Central Michigan University prior to testing.
Consent for publication
We consent to the publishing of this manuscript in Temperature.
Ethics approval
Central Michigan University Institutional Review Board approved this study.
Acknowledgments
We thank Mr. Tyler Truxton, MA, ATC; Mr. Michael Szymanski, MS, ATC; Mr. Jake Taylor, MS, ATC; Mr. Charles Ross, Ms. Alison Fiorini, ATC and Mr. Ethan Launstein, ATC for their help with data collection and Mr. Michael McPike, MS from Central Michigan University’s Athletics Department for donating the football equipment for this study.
Availability of data and material
This data has not been published elsewhere nor is any data set available publicly at this time.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Belval LN, Casa DJ, Adams WM, et al. Consensus statement- prehospital care of exertional heat stroke. Prehospital Emergency Care. 2018;22(3):392–397.
- Casa DJ, DeMartini JK, Bergeron MF, et al. National athletic trainers‘ association position statement: exertional heat illnesses. J Athl Train. 2015;50(9):986–1000.
- Grundstein A, Knox JA, Vanos J, et al. American football and fatal exertional heat stroke: A case study of Korey Stringer. Int J Biometeorol. 2017;61(8):1471–1480.
- Epstein Y, Yanovich R. Heatstroke. N Engl J Med. 2019;380(25):2449–2459.
- Adams WM, Belval LN, Berg AP, et al. Exertional heat stroke of Max Gilpin: A preventable death. Quest. 2020;71(1):102–115.
- DeMartini JK, Casa DJ, Stearns RL, et al. Effectiveness of CWI in the treatment of EHS at the falmouth road race. Med Sci Sports Exerc. 2015;47(2):240–245.
- Casa DJ, Becker SM, Ganio MS, et al. Validity of devices that assess body temperature during outdoor exercise in the heat. J Athl Train. 2007;42:333–342.
- Ganio MS, Brown CM, Casa DJ, et al. Validity and reliability of devices that assess body temperature during indoor exercise in the heat. J Athl Train. 2009;44(2):124–135.
- Armstrong LE, Casa DJ, Millard-Stafford ML, et al. American college of sports medicine position stand: exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556–572.
- Cleary MA, Nottingham S, Kasamatsu T, et al. Using a continuing education workshop to facilitate implementation of evidence-based practices for recognition and treatment of exertional heat stroke in secondary school Athletic Trainers. Athl Train Sports Health Care. 2016;8(3):100–111.
- Miller KC, Casa DJ, Adams W, et al. Round table on preseasonheat safety in secondary school athletics: prehospital care of exertional heatstroke patients. J Athl Train. In press;2020.
- Mazerolle SM, Ruiz RC, Casa DJ, et al. Evidence-based medicine and the recognition and treatment of exertional heat stroke, part I: A perspective from the athletic training educator. J Athl Train. 2011;46(5):523–532.
- Mazerolle SM, Scruggs IC, Casa DJ, et al. Current knowledge, attitudes, and practices of certified Athletic Trainers regarding recognition and treatment of exertional heat stroke. J Athl Train. 2010;2(2):170–180.
- Ronneberg K, Roberts WO, McBean AD, et al. Temporal artery temperature measurements do not detect hyperthermic marathon runners. Med Sci Sports Exerc. 2008;40(8):1373–1375.
- Zehner WJ, Terndrup TE. The impact of moderate ambient temperature variance on the relationship between oral, rectal, and tympanic membrane temperatures. Clin Pediatr. 1991;30(4_suppl):61–72.
- Morrissey MC, Scarneo-Miller SE, Giersch GEW, et al. Aural thermometry does not accurately measure internal temperature in exertional heat stroke patients. J Athl Train. 2020.
- Grundstein AJ, Ramseyer C, Zhao F, et al. A retrospective analysis of American football hyperthermia deaths in the United States. Int J Biometeorol. 2012;56(1):11–20.
- Kerr Z, Casa D, Marshall S, et al. Epidemiology of exertional heat illness among U.S. high school athletes. Am J Prev Med. 2013;44(1):8–14.
- McCullough E, Kenney W. Thermal insulation and evaporative resistance of football uniforms. Med Sci Sports Exerc. 2003;35(5):832–837.
- Armstrong LE, Johnson EC, Casa DJ, et al. The American football uniform: uncompensable heat stress and hyperthermic exhaustion. J Athl Train. 2010;45(2):117–127.
- Kulka T, Kenney W. Heat balance limits in football uniforms: how different uniform ensembles alter the equation. Phys Sportsmed. 2002;30(7):29–39.
- McMurray RG, Smith BW, Ross JL. Physiologic responses during exercise in athletes wearing an American football uniform. Biol Sport. 2002;19:109–119.
- Niven DJ, Gaudet JE, Laupland KB, et al. Accuracy of peripheral thermometers for estimating temperature: A systematic review and meta-analysis. Ann Intern Med. 2015;163(10):768–777.
- Thompson WR, Gordon NF, Pescatello LS. Preparticipation health screening and risk stratification. ACSM’s guidelines for exercise testing and prescription. 8 ed. Baltimore: Lippincott Williams and Wilkins; 2009. p. 18–39.
- Sawka MN, Burke LM, Eichner ER, et al. ACSM position stand: exercise and fluid replacement. Med Sci Sports Exerc. 2007;39(2):377–390.
- Pollock ML, Schmidt DH, Jackson AS. Measurement of cardio-respiratory fitness and body composition in the clinical setting. Compr Ther. 1980;6:12–27.
- Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40(3):497–504.
- Siri WE. Body composition from fluid spaces and density: analysis of methods. In: Brozek J, Henschel A, editors. Techniques for measuring body composition. Washington, DC: National Academies Press; 1961. p. 223–244.
- Miller KC, Hughes LE, Long BC, et al. Validity of core temperature measurements at three rectal depths during rest, exercise, cold-water immersion, and recovery. J Athl Train. 2017;52:332–338.
- DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17(6_2):863–871.
- Miller KC, Long BC, Edwards JE. Necessity of removing American football uniforms from hyperthermic humans before cold-water immersion. J Athl Train. 2015;50(12):1240–1246.
- Miller KC, Swartz EE, Long BC. Cold-water immersion for hyperthermic humans wearing football uniforms. J Athl Train. 2015;50(8):792–799.
- Kucera KL, Klossner D, Colgate B, et al. Annual survey of football injury research: 1931-2018. National Center for Catastrophic Injury Research; 2019. p. 1–36.
- Gant N, Atkinson G, Williams C. The validity and reliability of intestinal temperature during intermittent running. Med Sci Sports Exerc. 2006;38(11):1926–1931.
- Mairiaux P, Sagot JC, Candas V. Oral temperature as an index of core temperature during heat transients. Eur J Appl Physiol. 1983;50(3):331–341.
- Lee SM, Williams WJ, Fortney SM. Core temperature measurement during supine exercise: esophageal, rectal, and intestinal temperatures. Aviat Space Environ Med. 2000;71:939–945.
- Chaturvedi D, Vilhekar KY, Chaturvedi P, et al. Comparison of axillary temperature with rectal or oral temperature and determination of optimum placement time in children. Indian Pediatr. 2004;41:600–603.
- Jensen BN, Jensen FS, Madsen SN, et al. Accuracy of digital tympanic, oral, axillary, and rectal thermometers compared with standard rectal mercury thermometers. Eur J Surg. 2000;2000(11):848–851.
- Hansen RD, Daley WH, Leelarthaepin B. The effect of facial airflow on the estimation of exercise core temperature by infrared tympanic thermometry. Aust J Sci Med Sport. 1993;25:S26–S31.
- Kongpanichkul A, Bunjongpak S. A comparative study on accuracy of liquid crystal forehead, digital electronic axillary, infrared tympanic with glass-mercury rectal thermometer in infants and young children. J Med Assoc Thail Chotmaihet Thangphaet. 2000;83:1068–1076.
- Allen GC, Horrow JC, Rosenberg H. Does forehead liquid crystal temperature accurately reflect “core” temperature? Can J Anaesth. 1990;37(6):659–662.
- Patel N, Smith CE, Pinchak AC, et al. Comparison of esophageal, tympanic, and forehead skin temperatures in adult patients. J Clin Anesth. 1996;8(6):462–468.
- Eichner ER. Fatal exertional heat stroke in football: the coaches are the culprits. Curr Sports Med Rep. 2019;18(7):251–252.
