Abstract
Objective: This study evaluated the effect of food-simulating media associated with brushing and coffee staining on color stability of different composite resins.
Materials and methods: Eighty specimens were prepared for each composite: Grandio SO (Voco), Amaris (Voco), Filtek Z350XT (3M/ESPE), Filtek P90 (3M/ESPE). They were divided into four groups according to food-simulating media for 7 days: artificial saliva (control), heptane, citric acid and ethanol. The composite surface was submitted to 10,950 brushing cycles (200 g load) in an automatic toothbrushing machine. The specimens were darkened with coffee solution at 37 °C for 24 h. After each treatment, color measurements were assessed by spectrophotometry, using CIE L*a*b* system. The overall color change (ΔE) was determined for each specimen at baseline (C1) and after the treatments (food-simulating media immersion/C2, brushing/C3 and dye solution/C4). Data were analyzed by two-way repeated measures ANOVA and Tukey’s tests (p < .05).
Results: The results of RM-ANOVA showed significant differences for composites (p = .001), time (p = .001) and chemical degradation (p = .002). The mean of ΔE for composites were: Z350XT (5.39)a, Amaris (3.89)b, Grandio (3.75)bc, P90 (3.36)c. According to food-simulating media: heptane (4.41)a, citric acid (4.24)a, ethanol (4.02)ab, artificial saliva (3.76)b. For the treatments: dye solution (4.53)a, brushing (4.26)a, after food-simulating media (3.52)b.
Conclusions: The composite resin Filtek Z350XT showed significantly higher staining than all other composite resin tested. The immersion in heptane and citric acid produced the highest color alteration than other food-simulating media. The exposure of samples to brushing protocols and darkening in coffee solution resulted in significant color alteration of the composite resins.
Keywords:
Introduction
Composite resins are the most widely restorative esthetic material used and still represent a challenge in dental practice [Citation1]. The composite resin restorations are intended to simulate the tooth color; however, when they are subjected to prolonged exposure under oral conditions, their original color can change [Citation2]. One of the most important reasons for replacement of composite resin restorations is color alteration [Citation3,Citation4]. In the long-term, color can be altered due to intrinsic discoloration and extrinsic staining factors [Citation4,Citation5].
The extrinsic staining may be a result of the insufficient polymerization, heat, water sorption or adsorption of dye substances from dietary habits and oral hygiene [Citation6,Citation7]. Under oral conditions, composite resins are exposed either intermittently or continuously to chemical agents found in saliva, food and drinks [Citation8]. Previous studies have shown that some dietary foods and beverages that are chemically acid can cause surface degradation of restorative materials [Citation9,Citation10]. Furthermore, although brushing plays an important role in oral hygiene, abrasion constitutes another important issue on dental materials wear processes that can result in loss of contour, alterations in surface roughness, staining and plaque retention [Citation11,Citation12].
On the other hand, the intrinsic discoloration may be related to the type of resin matrix and, the size and distribution of the filler’s particle. Composite resins are generally classified according to the size, content and filler type, such as nanoparticles, nanohybrid, hybrid, microhybrid, microfill and macrofill. Most of them are composed by methacrylate monomers, such as BisGMA, UDMA and TEGDMA (organic phase), inorganic filler particles (dispersed phase), photoinitiator system and other minor additions including stabilizers and coloring pigments [Citation13,Citation14]. Also, as an alternative to the methacrylate-based composite resin, there is a composite resin based on ‘silorane’, obtained from the reaction of oxirane and siloxane molecules [Citation4,Citation15,Citation16], which is characterized by low polymerization shrinkage and hydrophobicity [Citation16,Citation17]. Previous studies have shown a decreased water sorption, solubility and associated diffusion coefficient of silorane composite as compared to conventional composite resins based on methacrylate [Citation4,Citation15,Citation17].
Then, improvements on the mechanical properties of composite resins associated with increased esthetic demands have resulted on the enlarged use of direct resin composite restorations [Citation8,Citation18], providing more strength and durability to the resin composite. Although the degradation of composite resins has been previously reported [Citation19], the combined effect of the food-simulating solvents associated with habits that the patients are daily exposed, as brushing and staining, on color stability in methacrylate-based resin compared to silorane resin is still unknown.
Thus, the aim of this study was to evaluate the effects of chemical degradation using food-simulating media associated with brushing and coffee immersion on color stability (ΔE) of different composite resins. It is hypothesized that the immersion in food-simulating media, brushing and dye solutions are able to modify the color stability of the tested materials.
Materials and methods
Specimens preparation
Four direct restorative materials were tested, as described in .
Table 1. Compositions of the composite resin tested.
Eighty cylindrical specimens of each composite resin were fabricated (shade A3) using a metallic matrix (2 mm in height and 3 mm in diameter) and cured on the top surface using LED photocuring unit (Elipar Freelight 2, 3M/ESPE, St. Paul, MN) at 1200 mW/cm2 power density, activated for 40 s. A mylar strip was placed over the resin composite and pressed with a glass plate to provide a flat surface, being removed after curing.
After curing, the specimens were stored in individual containers with deionized water for 24 h. Then, they were polished using a sequence of 1200, 2400 and 4000 grit aluminum oxide abrasive disks (Extec, Enfield, CT) in a polishing device (DP-10, Panambra Industrial e Técnica, São Paulo, Brazil). All the samples were stored in individual containers in deionized water at 37 °C for 24 h.
All groups of composite resins were submitted to the same treatment protocol: (1) immersion in food-simulating liquids, (2) brushing protocol and (3) staining in coffee immersion. After each treatment, the color was evaluated.
Initial color measurement
The color of each specimen was assessed under standardized ambient conditions according to the Commission Internationale de l’Eclariage (CIE) L*a*b* system, using a spectrophotometer (CM2600d, Konica Minolta, Osaka, Japan). The device was adjusted to a small area view (SAV), and the observer angle was set at 2°. The D65 standard light source with the reflectance mode and the 100% UV was included.
The specimens were carefully dried with an absorbent paper (not desiccated) and immediately placed into a custom-made holder with a 3 mm diameter reading window. The color of each specimen was measured three times and averaged. The results of color measurements were quantified in terms of three coordinate values (L*, a*, b*), as established by CIE, which the L* axis represents the degree of lightness and ranges from 0 (black) to 100 (white); the a* plane represents the degree of green/red color, while the b* plane represents the degree of blue/yellow color within the sample [Citation20].
Food-simulating media immersion
According to that recommended by FDA (1976), the present study used four solutions as food-simulating liquids [Citation21]. The specimens of each composite resin were randomly divided according to the food-simulated substances tested (n = 20):
Heptane P.A. (Synth – Labsynth Produtos para Laboratório Ltda, Diadema, São Paulo, Brazil) was used to simulate butter, fat meals and vegetable oils.
Citric acid 0.02M (Synth – Labsynth Produtos para Laboratório Ltda., Diadema, São Paulo, Brazil) simulated acid beverages, vegetables, fruits and candies.
Ethanol 70% (Zulu Hospitalar 70% – Companhia Nacional de Álcool, Piracicaba, São Paulo, Brazil) simulated alcoholic beverages and mouth rinses.
Artificial saliva prepared according to Gohring et al. [Citation22], used as control group.
The specimens were kept in individual vials with 2 mL of each solution during 7 days at 37 °C. After this period, the specimen was rinsed with deionized water and color measurement was performed (C2).
Brushing protocols
After exposure to food-simulating liquids, the same specimens were subjected to brushing abrasion in an automatic toothbrushing machine (ODEME Biotechnology – Joaçaba, Santa Catarina, Brazil), which imparted reciprocating motion to six soft bristle toothbrush heads (Sanifill Ultraprofissional, Hypermarcas – São Paulo, Brazil). This apparatus provides linear brushing movements across the specimens at a speed of 120 cycles per min at 37 °C, with a double pass of the brush head over the surface.
The surface of each composite resin was submitted to 10,950 brushing stokes under a vertical load of 200 g with an abrasive mixture, simulating one year of clinical situation [Citation23]. The abrasive slurry consisted of fluoridated dentifrice (Colgate Tripla Ação, Colgate-Palmolive Ind. e Com. Ltda., São Paulo, Brazil) and artificial saliva, in a ratio of 1:3, by weight [Citation24]. Toothbrushes were replaced after the completion of each brushing cycle. At the end of the brushing period, the color was measured again (C3).
Dye solution
After brushing protocols, the specimens were sequentially immersed in 2 ml of coffee solution at 37 °C for 24 h, which simulates about 1 month of coffee consumption [Citation25]. The solution was prepared with 10 g of soluble coffee (Nescafé Original, Nestlé, Araras, São Paulo, Brazil) dissolved in 200 ml of boiling water. The obtained solution was left at room temperature for cooling before use.
After this period, the color was measured again (C4).
Final color measurement
The differences in the values of L* (ΔL), a* (Δa), and b*(Δb) were determined for each specimen, comparing the baseline values (C1) with the values obtained after the treatments (food-simulating media immersion/C2, brushing/C3 and dye/C4). The overall change in color (ΔE) was calculated for each period using the following formula: ΔE*ab = [(ΔL*)2 + (Δa*)2 + (Δb*)2]0.5.
Statistical analysis
Data were submitted to statistical analysis using the computer software Statistica for Windows (Statsoft, Tulsa, OK). The descriptive statistics consisted of the calculation of the means and standard deviations. The inferential statistics consisted of two-way repeated measures ANOVA (composite resins; food-simulating media and treatments), in which the variable composite resins were considered as a repeated factor, followed by Tukey’s test. The significance level used was p < .05.
Results
The overall color change (ΔE) mean values of the composite resins for all experimental conditions are shown in . The application of RM-ANOVA showed significant differences for composites (p = .001), time (p = .001) and chemical degradation (p = .002).
Figure 1. Mean values of color alteration (ΔE) of the composite resins for all experimental conditions.
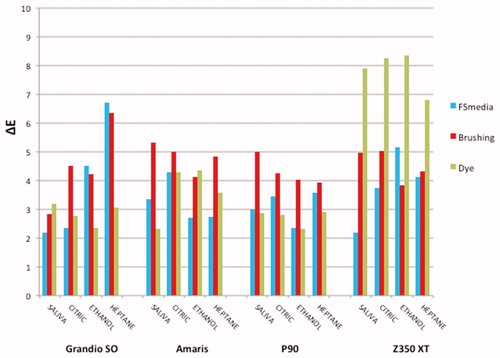
When color stability of composite resins was compared, Filtek P90 and Grandio SO showed the lowest color change (ΔE), followed by Amaris. However, among the composites resins mean values, Filtek Z350 XT exhibited the highest color alteration ().
Table 2. Results of color alteration (ΔE) for the composite resins tested.
Regarding the food-simulating media, as shown in , the immersion in heptane and citric acid resulted in higher color variation compared to artificial saliva. 70% ethanol showed an intermediate behavior.
Table 3. Results of color alteration (ΔE) for food-simulating solutions.
shows the results of the surface treatments as a factor. The color changes after dye solution and brushing protocol were significantly higher than after food-simulating media immersion.
Table 4. Results of color alteration (ΔE), according to the treatments.
Discussion
Under oral conditions, composite resins are exposed to different chemical and physical agents present in saliva, foods, drinks and oral hygiene habits. These materials should be resistant and show minimal changes inside the mouth. However, it can be assumed that interactions among these agents in the oral environment can degrade age composite materials [Citation26].
According to the results of this study, the artificial saliva promoted a slight color alteration (ΔE= 3.76 ± 2.37). Similar results were obtained by Domingos et al. [Citation27] and Omata et al. [Citation28]. Color change should be greater than 3.5 when assessed with the CIE L*a*b* scale in order to be detected clinically by a naked eye [Citation25]. As artificial saliva has no pigments, the small color change might be attributed to some water sorption of the organic matrix [Citation29], and was significantly lower than the other food-simulating media tested.
Surface staining of resinous materials is mainly related to the absorption or adsorption of coloring substances, such as those found in the daily diet. Heptane resulted in ΔE values similar to citric acid, and ethanol showed an intermediate behavior, according to . These organic solvents have the potential for polymer damage [Citation8,Citation30]. Previous study [Citation31] reported that the solubility of monomers in organic solvents was higher than that in water, in agreement with the results of this present study. This fact is possibly related to the degradation of the composite surfaces by the solvents. It has been reported that the solvent can penetrate into resin matrix and promote the release of unreacted monomers [Citation31], which facilitates staining by softening the resin matrix [Citation4].
Regarding the treatments, the resin composites behavior was different during the simulation procedures. Dye in coffee solution (ΔE = 4.53 ± 2.25) produced greater color alteration than brushing (ΔE = 4.26 ± 2.69) and immersion in food-simulating media (ΔE = 3.52 ± 1.90). Coffee contains yellow-stain molecule responsible for the staining [Citation6]. Also, the compatibility between this molecule and the resin polymer chain has been suggested and may facilitate the adsorption and penetration of the dye in the composite [Citation32]. Furthermore, the same specimens were initially exposed in food-simulating media and brushing, which could have promoted softening of the resin matrix, increased roughness surface caused by the superficial removal of filler particles from resin matrix due to the abrasion [Citation33], and could have favored the adsorption of the coffee stain molecules on the composite surface [Citation6]. These results are in agreement with the previous studies [Citation25,Citation34,Citation35], wherein coffee solution showed higher ΔE values than the other treatments, presenting the greatest influence on color changes of the resin composites.
In addition to coffee color staining, simulated brushing may influence the composites surface. The simulated brushing in vitro is a parameter used to evaluate the ability of a resinous material to maintain its softness, shine and prevent color alteration [Citation36]. When submitted to brushing protocol, the results of this study showed significant differences in color change. The roughness promoted greater light refraction indexes and lead to changes in color measurements, consequently resulting in darker color of resin composites. However, it was previously demonstrated that the effect of brushing on composites surface differs in relation to the composite composition: particle size, type of resin matrix and the degree of conversion after polymerization [Citation36].
The susceptibility to extrinsic staining of the composite resins after immersion in food-simulating media, brushing, and staining, observed in the present study may be related to the type of resin matrix, due to water sorption and solubility [Citation6]. The phenomena of sorption and solubility produce deleterious effects on the structure and function of a resin matrix, including swelling, plasticization, softening, oxidation and hydrolysis [Citation27,Citation37]. Consequently, it promotes higher susceptibility of staining. The differences in composites composition could be seen in the results of this study.
Filtek P90 showed the highest color stability (ΔE = 3.36 ± 1.71), according to , as described by other studies [Citation4,Citation30]. The silorane molecule formulated by the incorporation of siloxane groups imparted hydrophobicity, thereby reducing water sorption and solubility. Differences in composition and type of particles could be associated to the differences showed in the results among the composites.
Grandio SO composite resin (ΔE = 3.75 ± 2.46) has nanohybrid particles and 89% filler content, which provides rigidity to its structure. Filtek Z350 XT (ΔE = 5.39 ± 2.48) has nanoparticles, approximately 0.6 μm, and 78.5% filler content, which showed the highest levels of color alteration in all experimental conditions. Although both composites were composed by nanotechnology particles, the ΔE values showed statistical differences. On the other hand, Amaris (ΔE = 3.89 ± 2.10) is a microhybrid composite resin with 80% filler content. The differences in ΔE values could be related to the differences in type of particles as well as resin matrix. These three types of composites had the same polymer matrix composition (BisGMA, UDMA and TEGDMA), but the amount and type of filler particles are different. Also, TEGDMA content in resin matrix presents higher hydrophilicity when compared to BisGMA and UDMA. Otherwise, UDMA is more susceptible to dissolution by food-simulating solvents [Citation30].
Then, the composites tested in this study had the same monomer, but they differed in monomer ratios. The TEGDMA present in Filtek Z350 XT may have favored water sorption and increased the solubility of the polymers [Citation33]. Food-simulating substances gets through the polymer matrix, damaging the matrix structure and filler particle interface, resulting in greater color alteration, as observed by Roselino et al. [Citation33] and Yesilyurt et al. [Citation30]. Therefore, the results found in this study correspond with the finding achieved by other studies [Citation38,Citation39], where low concentration of filler content showed higher color change values for methacrylate composites.
Composite resin includes different types of inorganic fillers. Methacrylate composites containing zinc and barium glass or zirconia/silica glass, which were more susceptible to water sorption [Citation30,Citation40], while silorane composites contained quartz and yttrium fluoride as inorganic fillers. This means that Filtek P90 presents lower unreacted monomer than the methacrylate composites, and consequently, P90 was less affected by immersion in solvents [Citation30]. This fact could also explain the better behavior showed in P90 composite resin than the others composites.
The present study demonstrated that the combined effect of food-simulating solvents, brushing and staining solution promoted differences on color stability according to the type of composite resin used. Then, the hypothesis tested was accepted. The staining protocol in this study was dependent on the type of solvent and the composition of composite resin. So, dietary and hygiene habits of the patients should be considered in studies in order to increase the longevity of esthetic composite resin outcomes.
Conclusions
Within the limitations of this study, it can be concluded that Filtek Z350 XT showed significant higher staining than all other composite resins tested, as well as the immersion in heptane and citric acid. The exposure of composites to brushing protocols and darkening in coffee solution resulted in a significant color alteration of the composite resins.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Sabatini C. Color stability behavior of methacrylate-based resin composites polymerized with light-emitting diodes and quartz-tungsten-halogen. Oper Dent. 2015;40:271–281.
- Ardu S, Braut V, Gutemberg D, et al. A long-term laboratory test on staining susceptibility of esthetic composite resin materials. Quintessence Int. 2010;41:695–702.
- Villalta P, Lu H, Okte Z, et al. Effects of staining and bleaching on color change of dental composite resins. J Prosthet Dent. 2006;95:137–142.
- Arocha MA, Mayoral JR, Lefever D, et al. Color stability of siloranes versus methacrylate-based composites after immersion in staining solutions. Clin Oral Investig. 2013;17:1481–1487.
- Samra AP, Pereira SK, Delgado LC, et al. Color stability evaluation of aesthetic restorative materials. Braz Oral Res. 2008;22:205–210.
- Borges A, Caneppele T, Luz M, et al. Color stability of resin used for caries infiltration after exposure to different staining solutions. Oper Dent. 2014;39:433–440.
- Joiner A. Tooth colour: a review of the literature. J Dent. 2004;32(Suppl 1):3–12.
- Yap AU, Tan SH, Wee SS, et al. Chemical degradation of composite restoratives. J Oral Rehabil. 2001;28:1015–1021.
- Hengtrakool C, Kukiattrakoon B, Kedjarune-Leggat U. Effect of naturally acidic agents on microhardness and surface micromorphology of restorative materials. Eur J Dent. 2011;5:89–100.
- McKenzie MA, Linden RW, Nicholson JW. The physical properties of conventional and resin-modified glass-ionomer dental cements stored in saliva, proprietary acidic beverages, saline and water. Biomaterials. 2003;24:4063–4069.
- Peumans M, Van Meerbeek B, Lambrechts P, et al. The influence of direct composite additions for the correction of tooth form and/or position on periodontal health. A retrospective study. J Periodontol. 1998;69:422–427.
- Yap AU, Wu SS, Chelvan S, et al. Effect of hygiene maintenance procedures on surface roughness of composite restoratives. Oper Dent. 2005;30:99–104.
- Kalachandra S, Taylor DF, Mc Grath JE, et al. Structure- property relationships in dental composites based on polydimethacrylates. Polymer Prepr. 1997;38:94–95.
- Sideridou I, Tserki V, Papanastasiou G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials. 2002;23:1819–1829.
- Ilie N, Hickel R. Macro-, micro- and nano-mechanical investigations on silorane and methacrylate-based composites. Dent Mater. 2009;25:810–819.
- Weinmann W, Thalacker C, Guggenberger R. Siloranes in dental composites. Dent Mater. 2005;21:68–74.
- Eick JD, Smith RE, Pinzino CS, et al. Stability of silorane dental monomers in aqueous systems. J Dent. 2006;34:405–410.
- Yanikoglu N, Duymus ZY, Yilmaz B. Effects of different solutions on the surface hardness of composite resin materials. Dent Mater J. 2009;28:344–351.
- Torres CRG, Da Silva TM, Sales ALLS, et al. Influence of chemical degradation and toothbrushing on surface of composites. WJOUD. 2015;6:65–70.
- Ruyter IE, Oysaed H. Conversion in different depths of ultraviolet and visible light activated composite materials. Acta Odontol Scand. 1982;40:179–192.
- Food and Drug Administration. FDA guidelines for chemistry and technology requirements of indirect additive petitions. Washington, 1976.
- Gohring TN, Zehnder M, Sener B, et al. In vitro microleakage of adhesive-sealed dentin with lactic acid and saliva exposure: a radio-isotope analysis. J Dent. 2004;32:235–240.
- Goldstein GR, Lerner T. The effect of toothbrushing on a hybrid composite resin. J Prosthet Dent. 1991;66:498–500.
- Turssi CP, Hara AT, de Magalhaes CS, et al. Influence of storage regime prior to abrasion on surface topography of restorative materials. J Biomed Mater Res Part B Appl Biomater. 2003;65:227–232.
- Ertas E, Güler AU, Yücel AC, et al. Color stability of resin composites after immersion in different drinks. Dent Mater J. 2006;25:371–376.
- Akova T, Ozkomur A, Uysal H. Effect of food-simulating liquids on the mechanical properties of provisional restorative materials. Dent Mater. 2006;22:1130–1134.
- Domingos PA, Garcia PP, Oliveira AL, et al. Composite resin color stability: influence of light sources and immersion media. J Appl Oral Sci. 2011;19:204–211.
- Omata Y, Uno S, Nakaoki Y, et al. Staining of hybrid composites with coffee, oolong tea, or red wine. Dent Mater J. 2006;25:125–131.
- Soares-Geraldo D, Scaramucci T, Steagall-Jr W, et al. Interaction between staining and degradation of a composite resin in contact with colored foods. Braz Oral Res. 2011;25:369–375.
- Yesilyurt C, Yoldas O, Altintas SH, et al. Effects of food-simulating liquids on the mechanical properties of a silorane-based dental composite. Dent Mater J. 2009;28:362–367.
- Zhang Y, Xu J. Effect of immersion in various media on the sorption, solubility, elution of unreacted monomers, and flexural properties of two model dental composite compositions. J Mater Sci: Mater Med. 2008;19:2477–2483.
- Fujita M, Kawakami S, Noda M, et al. Color change of newly developed esthetic restorative material immersed in food-simulating solutions. Dent Mater J. 2006;25:352–359.
- Roselino LMR, Cruvinel DR, Chinelatti MA, et al. Effect of brushing and accelerated ageing on color stability and surface roughness of composites. J Dent. 2013;41S:e54–e61.
- Patel SB, Gordan VV, Barrett AA, et al. The effect of surface finishing and storage solutions on the color stability of resin-based composites. J Am Dent Assoc. 2004;135:587–594.
- Tan BL, Yap AU, Ma HN, et al. Effect of beverages on color and translucency of new tooth-colored restoratives. Oper Dent. 2015;40:E56–E65.
- Heintze SD, Forjanic M, Ohmiti K, et al. Surface deterioration of dental materials after simulated toothbrushing in relation to brushing time and load. Dent Mater. 2010;26:306–319.
- Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater. 2006;22:211–222.
- Lee YK, Powers JM. Color changes of resin composites in the reflectance and transmittance modes. Dent Mater. 2007;23:259–264.
- Schulze KA, Marshall SJ, Gansky SA, et al. Color stability and hardness in dental composites after accelerated aging. Dent Mater. 2003;19:612–619.
- Yap AU, Low JS, Ong LF. Effect of food-simulating liquids on surface characteristics of composite and polyacid-modified composite restoratives. Oper Dent. 2000;25:170–176.
