ABSTRACT
Proline-rich tyrosine kinase 2 (PYK2, also known as Pyk2) and its closely related focal adhesion kinase (FAK) modulate cancer cell invasion by coordinating the balance between focal adhesion-mediated migration and invadopodia-dependent extracellular matrix invasion. Our recent findings present Pyk2 and FAK as novel mediators of breast cancer invasiveness and as potential targets for blocking breast cancer metastasis.
While primary breast tumors can often be removed and are usually responsible for only a small percentage of cancer deaths, complications associated with distant metastasis are the primary cause of mortality from breast cancer. Accordingly, a better understanding of the molecular, cellular, and physiological conditions that help initiate metastasis would be valuable for identifying and improving outcomes of patients with metastatic disease. Metastatic cancer cells invade surrounding tissues and blood vessels by forming F-actin rich protrusions called invadopodia, that localize matrix degrading activity to cell-substrate contact points and in which cell signaling, proteolytic, adhesive, cytoskeletal, and membrane trafficking pathways physically converge to execute cell invasion and consequent metastatic dissemination.Citation1 A major challenge in breast cancer research is to elucidate the mechanisms that trigger invadopodia formation and function.
Proline-rich tyrosine kinase 2 (Pyk2) and its closely related focal adhesion kinase (FAK) define a distinct family of non-receptor tyrosine kinases that exhibit high amino acid identities, common protein domains, and significant structural similarities. Both Pyk2 and FAK are highly expressed in invasive breast cancers, but it was unclear until recently how signaling via these proteins leads to the cytoskeletal rearrangements that are essential for cancer cell invasiveness and consequent metastatic dissemination.
Indeed, previous publications suggested that FAK indirectly regulates invadopodium precursor formation by sequestering Src (SRC, best known as Src) to focal adhesions and consequently controlling the tyrosine phosphorylation balance between invadopodia and focal adhesions in invasive cancer cells.Citation2 Whether and how Pyk2 regulates invadopodium precursor formation and functional maturation in invasive cancer cells was largely unknown until present.
To elucidate the molecular mechanisms by which Pyk2 regulates invadopodia-mediated cellular invasion, we performed high-throughput protein-protein and kinase-substrate microarray screens with purified Pyk2 as a bait. Using this approach, we identified 147 candidates, of which 24 proteins were found as both substrates and interactors of Pyk2. To gain insight into which of the candidates could be a potential invadopodial partner of Pyk2, we performed text mining using the term “invadopodia”. Overlay of the combined text mining proteins with the data obtained in protein microarrays revealed 10 invadopodia-related candidate interactors of Pyk2. Out of these, cortactin (CORTACTIN, also known as cortactin), which is a major component of invadopodia, was found as a novel substrate and interactor of Pyk2. The results of this analysis were verified by in vitro binding assay, in vitro kinase assay, Forster resonance energy transfer (FRET) analysis, and immunofluorescent staining of tyrosine phosphorylated cortactin in invadopodium precursors.Citation3
Src-mediated phosphorylation of the scaffold and Nox organizer protein Tks5 (TKS5, best known as Tks5) leads to reactive oxygen species production, which induces invadopodia formation and activation.Citation4,Citation5 Previous publications suggested that FAK indirectly regulates invadopodium precursor formation by sequestering Src to focal adhesions,Citation2 but the identity of the protein that localizes Src to invadopodium precursors has never been determined. To investigate whether Pyk2 recruits Src to invadopodium precursors, we examined co-localization of fluorescently-labeled Src to cortactin and Tks5-enriched invadopodium precursors. Our findings show, for the first time, that Pyk2 recruits Src kinase to invadopodium precursors. Moreover, we demonstrate that Pyk2 mediates the assembly of these structures by coordinating with FAK on the spatial regulation of Src kinase in breast cancer cells.
We have previously shown that invadopodial Src activates Arg (Abl-related gene ARG, also known as Arg) kinase to induce cortactin phosphorylation in invadopodia, leading to their maturation and activation and consequent actin polymerization, matrix degradation, and tumor cell invasion.Citation6 To determine whether Pyk2 phosphorylates cortactin directly or indirectly via Src recruitment and consequent Arg activation, we expressed either a kinase inactive mutant (Pyk2-KI) or an auto-phosphorylation and Src binding mutant of Pyk2 (Pyk2-Y402F) in Pyk2 knockdown cells. Interestingly, both mutants were unable to rescue cortactin phosphorylation in invadopodia of Pyk2-depleted cells, suggesting that Pyk2-mediated cortactin tyrosine phosphorylation is dependent on both the kinase activity of Pyk2 and recruitment and activation of Src kinase. To confirm the direct and indirect effects of Pyk2 on cortactin phosphorylation, we performed epistasis experiments in which we overexpressed either Pyk2 or Arg and knocked down the other kinase. Knockdown of Pyk2 in cells overexpressing Arg eliminated most of the phosphorylation of cortactin in invadopodia, suggesting that overexpressing of Arg cannot compensate for Pyk2 depletion. Similarly, overexpression of Pyk2 could not rescue cortactin phosphorylation in invadopodia of cells that were knocked down for Arg, confirming that the two kinases work in the same signaling pathway for cortactin phosphorylation. These results suggest that Pyk2 controls cortactin phosphorylation in invadopodia by both direct binding and phosphorylation of cortactin as well as by indirect recruitment and activation of Src and Arg kinases.Citation3 These data indicate that, although Arg and Pyk2 cannot compensate for each other in invadopodium activation, simultaneous activation of both the direct and the Src-Arg mediated pathways is necessary for efficient cortactin phosphorylation at invadopodia.
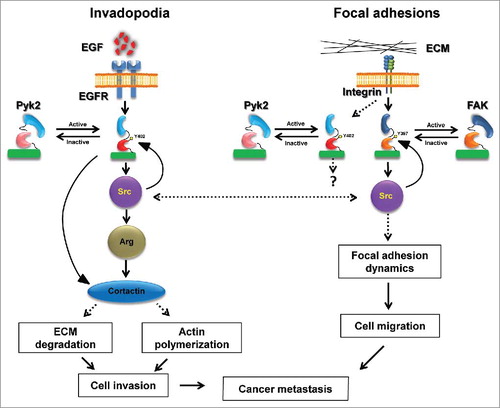
A comparison of the ability of Pyk2 and FAK-depleted cells to migrate on two-dimensional substrates and to invade through Matrigel-coated membranes indicated that both kinases play a role in either type of movement. Based on these data, we suggested a model by which both Pyk2 and FAK regulate tumor cell invasiveness, albeit via different mechanisms (). While Pyk2 regulates invasion mostly by controlling cortactin phosphorylation-dependent maturation of invadopodia and consequent activation of actin polymerization and matrix metalloproteinase (MMP)-dependent matrix degradation, FAK controls extracellular matrix invasion by regulating focal adhesion-dependent motility and functions. Whether Pyk2 and FAK use these distinct mechanisms for regulating in vivo invasiveness and metastatic dissemination, and why does Pyk2 localize to focal adhesions in addition to its localization to invadopodia, is a subject for future investigation.
Figure 1. Pyk2 and FAK coordinate breast cancer cell invasiveness via distinct mechanisms. Following stimulation of epidermal growth factor receptor (EGFR), Pyk2 is recruited to the receptor and activated by auto-phosphorylation on tyrosine 402 (Y402). Activated Pyk2 recruits Src kinase, which leads to complete activation of Pyk2 as well as recruitment and activation of Arg. Both Arg and Pyk2 phosphorylate invadopodial cortactin, leading to MMP-dependent matrix degradation and actin polymerization in invadopodia, and consequent breast cancer cell invasion. At the same time, integrin activation in focal adhesions leads to recruitment and activation of FAK auto-phosphorylation on tyrosine 397 (Y397) and Src, which regulate focal adhesion dynamics by binding and phosphorylation of focal adhesion proteins. This signaling via FAK and Src leads to cancer cell motility-dependent invasiveness. A coordination of both Pyk2-mediated invasion and FAK-mediated migration is necessary for breast cancer cell invasiveness and consequent metastatic dissemination.
Abbreviations
| ARG | = | Abl-related gene |
| EGFR | = | epidermal growth factor receptor. |
| FAK | = | focal adhesion kinase |
| FRET | = | Forster resonance energy transfer |
| MMP | = | matrix metalloproteinase |
| PYK2 | = | Proline-rich tyrosine kinase 2 |
Disclosure of potential conflicts of interest
No potential conflicts of interest are disclosed.
Additional information
Funding
References
- Weaver AM. Invadopodia. Curr Biol. 2008;18:R362–364. doi:10.1016/j.cub.2008.02.028. PMID:18460310
- Chan KT, Cortesio CL, Huttenlocher A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J Cell Biol. 2009;185:357–70. doi:10.1083/jcb.200809110. PMID:19364917
- Genna A, et al. Pyk2 and FAK differentially regulate invadopodia formation and function in breast cancer cells. J Cell Biol. 2018;217:375–95. doi:10.1083/jcb.201702184. PMID:29133485
- Diaz B, Shani G, Pass I, Anderson D, Quintavalle M, Courtneidge SA. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal. 2009;2:ra53. doi:10.1126/scisignal.2000368. PMID:19755709
- Gianni D, Diaz B, Taulet N, Fowler B, Courtneidge SA, Bokoch GM. Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci Signal. 2009;2:ra54. doi:10.1126/scisignal.2000370. PMID:19755710
- Mader CC, Oser M, Magalhaes MA, Bravo-Cordero JJ, Condeelis J, Koleske AJ, Gil-Henn H. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res. 2011;71:1730–41. doi:10.1158/0008-5472.CAN-10-1432. PMID:21257711
