Abstract
Tropical dry forest is an important ecosystem that offers different good and services to communities. However, despite their importance, these ecosystems are the least studied and have been impacted by human activity in the last decades. Moreover, future projections show an additional impact caused by climate change. For this reason, the understanding of anthropogenic and climate change impacts will help to improve adaptation strategies for a better species conservation. Consequently, we analyzed the impact of climate change and stressors on species distribution from dry forest ecosystem in southern Ecuador. We identified the variables that could help to buffer the potential impacts. Indicator species of climate change impacts were chosen using a species selection index considering an importance index value, density, rarity and altitudinal range. The selected species were Cavanillesia platanifolia, Cordia macrantha (C. macrantha), Erythrina velutina, Handroanthus chrysanthus (H. chrysanthus) and Terminalia valverdeae (T. valverdeae). Species current and future distribution models were generated with information from Herbarium ‘Reinaldo Espinosa’ of the National University of Loja and permanent plots that were established between 2005 and 2009. We used climate data from WorldClim under two climate change scenarios based on two IPCC Representative Concentration Pathways; RCP 2.6 and RCP 8.5. To know if the species distribution will be placed in regions with high sensitivity or adaptive capacity, 13 stressor variables and 3 buffers were selected. The sensitivity and adaptive capacity maps were intersected with species future distribution models under RCP 2.6 and RCP 8.5. Four of five species in the analysis showed a reduction in their future distribution: C. macrantha, T. valverdeae and H. chrysanthus. These were reduced due to increasing niche restriction. Between 18 and 26% of species future distribution area were located in zones with high sensitivity. In addition, among 14% to 46% of species future distribution area could be in sites with high adaptive capacity. In these areas, conservation strategies can serve as buffers against climate change; however, nowadays there is a conservation gap that needs to be addressed through the implementation of different strategies oriented to reinforce the adaptive capacity of these species in dry forest ecosystems. Species distribution models showed no migration to higher altitudes, but we observed a reduction in areas with high probability of presence. Additionally, sites with high likelihood of species presence have been influenced by deforestation, fragmentation, land use and other stressors, that will likely diminish their ability to adapt to climate warming and increased rainfall variability.
Introduction
The climate and its variations have a direct influence on ecosystems and the natural distribution of species [Citation1–5]. Consequently, abrupt changes in the climate system will have serious negative impacts on species structure, composition, diversity and their distribution, resulting in a decrease in their habitat and species migration [Citation3,6–9]. Modeling studies on species behavior in recent years have become useful tools for conservation, natural resources management and risk assessments [Citation10–12]. Research suggest that changes in distribution could be due to a migration of species to higher elevations, to more favorable habitat conditions [Citation13–15]. This is only possible if dispersal mechanisms and biological corridors allow these movements [Citation16]. If temperature continues rising in lowland areas as projected by IPCC (2013), there would likely not be individuals or populations available that can repopulate these areas, causing a biotic attrition of ecosystems and loss of diversity [Citation13].
Dry forest in Ecuador is located on the western flank of the Andes in Esmeraldas, Manabí, Guayas, El Oro and Loja provinces. These ecosystems are primarily deciduous during dry season [Citation17], with little presence of shrubs and herbs [Citation18]. It is estimated that at least 50% of area of dry forest ecosystems have disappeared because of human interventions [Citation18], mainly due to forest products extraction and deforestation [Citation19,20]. In this study, the analysis focused on dryland ecosystems of southern Ecuador (El Oro and Loja provinces) which have a high diversity and an intermediate endemism level [Citation21,22]. Although these ecosystems are the largest and best preserved of the country, they are threatened by human activities [Citation17,20,23]. For this reason, it is necessary to identify, establish and maintain actions for restoration and conservation [Citation21].
The aim of this study was to project changes of dry forest species distribution (Cavanillesia platanifolia (C. platanifolia), Cordia macrantha (C. macrantha), Erythrina velutina (E. velutina), Handroanthus chrysanthus (H. chrysanthus ) and Terminalia valverdeae) in southern Ecuador under climate change scenarios, as well as anthropogenic (deforestation, land use, fragmentation, mining and so on.) and intrinsic (fires probability, water stress and so on.) stressors. We analyzed adaptive capacity of species with regard to these stressors and climate change. This work, in contrast to other studies [Citation13–15,24], not only predicts species future distributions under climate change scenarios, but also analyzes whether the future location of species will be in areas with high levels of sensitivity, adaptive capacity or both. Our results will help to identify priority zones for conservation actions aimed to maintain species niches in coming decades. Thereby, we could secure the coexistence of ecologically important species for dry forest functionality.
Methods
Study area
The study area is located at the southern region of Ecuador, between Loja and El Oro Provinces. This area is considered as one of the biggest continuous dry forest in the Western Andes [Citation22]. The annual precipitation is between 300 and 700 mm; its annual mean temperature ranges from 20 to 26 °C, while the slopes could reach up to 60° [Citation18,25]. Southern Ecuadorian dry forest is a highly diverse ecosystem and has intermediate endemism levels, compared to other areas in South America [Citation21,22,26]. Dry forests from southern region of Ecuador are situated in areas with the presence of human populations, suitable for crops and cattle ranching. These activities have resulted in a high levels of habitat loss and degradation [Citation18,20]. These deciduous ecosystems do not necessarily correspond to old growth forests; instead, they are more commonly composed by second growth forests [Citation22], depending on the human intervention level. They are characterized by deciduous species that in certain times of year lose their foliage [Citation17,18].
Analysis
In order to determine primary impacts of climate change on dry forest, we selected five species that could serve as indicators of these impacts. For species selection, information of permanent plots located in La Ceiba, Laipuna, Algodonal and Las Cochas registered by Herbarium Loja of Universidad Nacional de Loja was used. We selected four plots from a network of 1.0 ha established between 2005 and 2009. The plots were set considering a completely randomized design. We used all individuals with a diameter at breast height ≥5 cm. Species were selected based on parameters such as: (i) Importance Value Index (IVI) [Citation27,28]; (ii) Density (D) [Citation29]; (iii) Rarity (R); and (iv) Altitudinal gradient range (AG) (Table ). In each plot and for each species we calculated the IVI and density; the averaged values of these parameters were used to identify species with high ecological importance values.
Table 1. Species selection index equations.
In addition, we identified species that might be more susceptible to climate change based on its rarity (R), considering rare species as the ones which are present in unique habitats [Citation30]. Rarity of each species was determined through expert elicitation, rating the presence of species in different habitats such as slope, edge, riparian, flat and depression. Species with presence in fewest habitats were considered as the rarest and the most important for our analysis. To determine the altitudinal gradient range (AG) of each species, we used secondary information from [Citation63] in order to know the range of distribution; we considered that those species with a more restricted altitudinal gradient could be more susceptible to climate change.
Each of the parameters (IVI, D, R and AG) was standardized on a scale 0–1. With these parameters we built a Species Selection Index – SSI that was standardized between values 0 (lowest)–4 (high). Based on the SSI, we selected ecologically important species that are more susceptible to climate change.
The final species selection was based on the SSI and expert elicitation (Table ). The five selected species were C. platanifolia, C. macrantha, E. velutina, H. chrysanthus and Terminalia valverdeae (Figure ). We used presence models of Maxent software [Citation31], for both current and future distributions (time horizon 2050).
Table 2. Summary of species selection index (SSI).
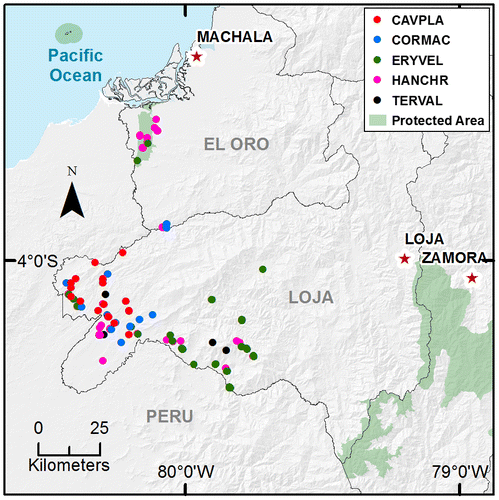
Figure 1. Species presence locations in the Southern Region of Ecuador. Cavanillesia platanifolia (CAVPLA), Cordia macrantha (CORMAC), Erythrina velutina (ERYVEL), Handroanthus chrysanthus (HANCHR), Terminalia valverdeae (TERVAL).
We assumed that one of central impacts of climate change on species is related to changes in their distribution. Thus, we ran a species distribution model (SDM), to determine the optimal areas for the five species in the next years (2050). For current and future distributions, only presence points were used, based on information of databases from Herbarium Loja, permanent plots and studies executed by Universidad Nacional de Loja; and, botanical databases of the Ministry of Environment of Ecuador. Each one of the species collection (voucher) from the databases were collected by random. Additionally, WorldClim database was used for a time horizon to 2050, considering two climate change scenarios: (i) Representative Concentration Pathways 2.6 (RCP 2.6); and (ii) Representative Concentration Pathways 8.5 (RCP 8.5). One scenario represents an optimistic projection (RCP 2.6) characterized by a very low concentration and emissions levels of greenhouse gases, a medium rate of population growth, the radiative forcing peaks at 3 W/m2 before 2100 and then decrease. The second scenario was selected because it represents a pessimistic projection (RCP 8.5) with high levels of concentrations and emissions of greenhouse gases, a high rate of population growth and the radiative forcing reaches 8.5 W/m2 by the end of the century, this scenario does not implement climate change policies [Citation32–34]. In each of these scenarios, we used eight General Circulation Models (GCMs) (BCC-CSM1-1, CCSM4, HadGEM2-AO, HadGEM2-ES, IPSL-CM5A-LR, MIROC5, MRI-CGCM3, NorESM1-M) of the Coupled Model Intercomparison Project Phase 5 (CMIP5) and a spatial resolution of 30 s from WorldClim platform. For the selection of GCMs we considered models with the lowest values of relative error (≤0.2) [Citation35,36]. For climate scenarios (RCP 2.6 and RCP 8.5) and each bioclimatic variable we used an assembly with eight selected climate models in order to reduce uncertainty of these models and have a better representation than with individual [Citation35,37–42].
In order to avoid an over-parameterization of the niche, principal component analysis was run. We obtained four orthogonal components that explained 90% of the observed variability. Based on these components, bioclimatic variables were selected. We considered variables with the highest contribution and with least or no correlation (Appendix 1) [Citation43]. In cases where pairs of variables had high correlation coefficient, we chose the variable with the greatest biological influence on species [Citation44]. The bioclimatic variables used were the annual mean temperature (BIO1); annual precipitation (BIO12); precipitation of the wettest month (BIO13); precipitation of the coldest quarter (BIO19); precipitation of driest quarter (BIO17); and elevation.
Different parameterizations related to beta values and feature types (auto, linear, quadratic) were applied in the SDM with Maxent. We used 75% of presence data for training and 25% for model validation. In addition, 5,000 interactions and 100 replicates were run for each species model. The current and future distribution used was the average of the 100 generated replicates. The best SDM was selected by AICc criteria (ENMTools software) and values of area under the curve (AUC ≥ 0.84 – Maxent software), with a logistics output format. Higher values indicate better discrimination of suitable areas for species [Citation31].
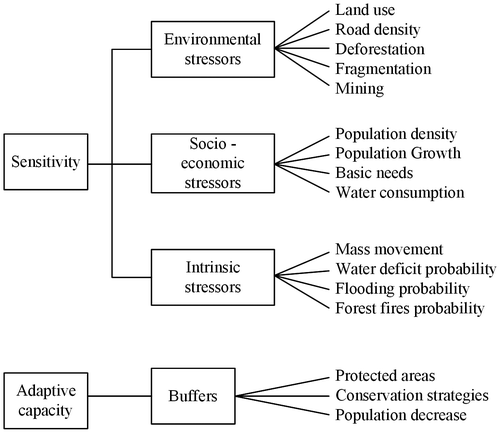
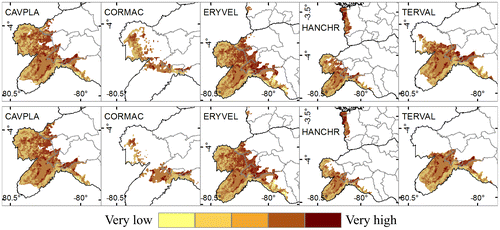
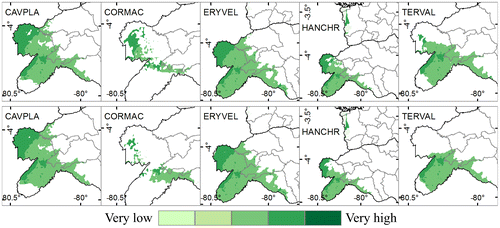
To determine the influence of stressors on species distribution, the results of future distribution under RCP 2.6 and RCP 8.5 were combined with a sensitivityFootnote1 map, taking into account environmental, socioeconomic and intrinsic stressors (Table ; Figure ). Furthermore, future distribution was intersected with adaptive capacity,Footnote2 considering factors that buffer the projected bioclimatic changes [Citation45–48].
Table 3. Sensitivity and adaptive capacity equations.
The stressor and buffer variables use current information and were calculated with the ArcGIS platform; the methodological process for each of them differed depending on its characteristics and the available information. These variables were normalized using map algebra on a 0–100 scale expressed as a percentage; the normalization for categorical variables was based in Equation (3) (Table ), and for continuous variables we used Equation (4). (Table ). We then classified the stressors and buffers into five levels of influence (very low, low, moderate, high and very high) through the method of natural breaks [Citation49]. For categorical variables we assigned weights according to their degree of influence. Analytic hierarchy processes [Citation50,51] through workshops and experts elicitation were conducted for this step. For the total sensitivity (Table ; Equation (1)) we considered the socioeconomic, environmental and intrinsic stressors. The socioeconomic stressors like population density, population growth, basic needs and water consumption, corresponded to conditions of population, cultural, social conflicts and condition of life that influence land use and natural resources. Environmental stressors as land use, road density, deforestation, fragmentation and mining, were related to anthropogenic activities that have impacted in land cover, ecosystems structure and function. Intrinsic stressors as mass movement, water deficit probability, flooding probability and forest fires probability were those that appear naturally and are not influenced by human activity.
Protected areas, conservation tools and population decrease were consider as buffers and determined adaptive capacity (Table ; Equation (2)). Buffers, as categorical variables, were normalized through each internal category. We assigned a weight depending on the degree of contribution to the adaptive capacity of ecosystems. A hierarchical method and expert elicitation processes was used for assigning weights to the categories.
Finally, each one of the sensitivity maps and adaptive capacity were crossed with the results of future distribution under RCP 2.6 and RCP 8.5 scenarios. Throughout this process we identified areas where there could be a greater or lesser impact due to climate change and stressors for each species. Moreover, we determined how the buffers could contribute to greater adaptive capacity.
Results
The selected species (Table ) were characterized as typical from dry forest with a high ecological importance. These species had some restrictions like their presence in very specific habitats or a constrained altitudinal gradient [Citation18,52]. These characteristics could cause greater impacts in the future due to climate change. The mean AUC of all species, for both current and future (2050) distribution were between 0.84 and 0.95, indicating that these models had an adequate discrimination for the most suitable presence areas. The species distribution represent areas where there is a high likelihood of presence for the species (≥0.50).
Table 4. Area under ROC curve (AUC) of dry forest species selected.
SDM generally showed a decrease of ecological niches between the current and future distribution. C. platanifolia was the only species that showed an increase in its future distribution (Figures and ) under climate change scenarios RCP 2.6 (2% of area) and RCP 8.5 (3% of area). The other species showed a reduction in the area with a high likelihood (≥0.50) of presence; C. macrantha had the biggest reduction in the area under RCP 2.6 (−69%) and RCP 8.5 (−71%) (Figure ). This reduction may be driven by increase in precipitation and temperature [Citation53] at the study area. For this species and at this zone it will be expected an increment of until 11% on the annual precipitation for an optimistic scenario (RCP 2.6) and 20% considering a pessimistic scenario (RCP 8.5). Temperature also may show an increment of 1.32 °C for an optimistic scenario (RCP 2.) and 2.12 °C for a pessimistic scenario (RCP8.5) [Citation54].
Figure 3. Dry forest species current and future distributions area. Black bars indicate current distribution. Dark gray bars show future distribution with RCP 2.6. Light gray bars illustrate future distribution with RCP 8.5. The time horizon for future distribution was 2050. Cavanillesia platanifolia (CAVPLA), Cordia macrantha (CORMAC), Erythrina velutina (ERYVEL), Handroanthus chrysanthus (HANCHR), Terminalia valverdeae (TERVAL).
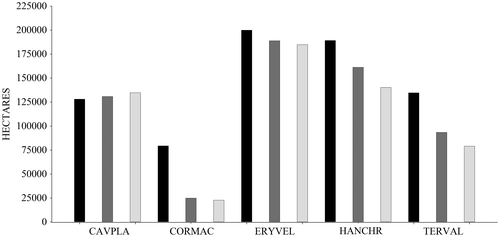
Figure 4. Dry Forest species current and future (time horizon 2050) distribution. Cavanillesia platanifolia (CAVPLA), Cordia macrantha (CORMAC), Erythrina velutina (ERYVEL), Handroanthus chrysanthus (HANCHR), Terminalia valverdeae (TERVAL). Likelihood ≥ 0.50.
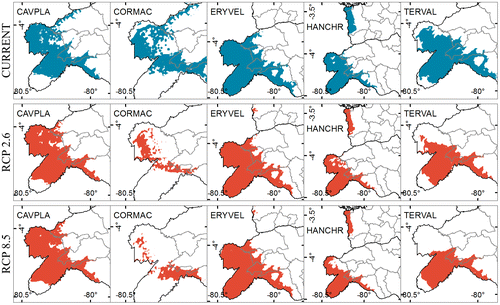
As noted above, climate change will affect the extension of future distribution of analyzed species. Besides to these impacts, the anthropic or intrinsic stressors could act as barriers for species presence. The effect of stressors on species showed moderate sensitivity levels in most future distribution area (39–48%), while areas of potential distribution with high sensitivity levels comprise 18–26% of study site (Figure , Appendix 2).
Figure 5. Species future (time horizon 2050) distribution combined with sensitivity analysis. Above: Future distribution with RCP 2.6. Below: Future distribution with RCP 8.5.Cavanillesia platanifolia (CAVPLA), Cordia macrantha (CORMAC), Erythrina velutina (ERYVEL), Handroanthus chrysanthus (HANCHR), Terminalia valverdeae (TERVAL).
Between 52 and 84% of species future distribution are located in areas with moderate levels of adaptive capacity. Among 14 and 46% of species future distribution could be situated in zones with high adaptive capacity (Figure , Appendix 3). 46% (RCP 2.6) and 23% (RCP 8.5) of C. macrantha future distribution area has a high level of adaptive capacity. This species has the largest area with high adaptive capacity; it is also the one with the greatest predicated reduction in future distribution. Thus, we can assume that high levels of adaptive capacity are not enough to enable a more favorable adaptation to climate change.
Figure 6. Species future (time horizon 2050) distribution combined with adaptive capacity analysis. Above: Future distribution with RCP 2.6. Below: Future distribution with RCP 8.5. Cavanillesia platanifolia (CAVPLA), Cordia macrantha (CORMAC), Erythrina velutina (ERYVEL), Handroanthus chrysanthus (HANCHR), Terminalia valverdeae (TERVAL).
Discussion
The generation of reference scenarios will help to improve species adaptation in southern Ecuadorian dry forests. The SDM serves as a guide to prioritize conservation areas, to assess threats and to design new protected areas [Citation12]; for this purpose, we can use information on species distribution and adaptive capacity. Areas with high likelihood of presence and with less degraded ecosystems can be considered a baseline for adaptation measures. These zones could serve as starting points to implement forest landscape restoration to reduce habitat fragmentation and allow species migration. We should emphasize when working with SDMs researchers should be cautious when registering presence or absence points in unsuitable territories, because this information could determine how well models and species distribution can be represented under a territorial climate perspective [Citation31,55]. An important limitation in this study is that we used only presence data because no information has been collected in more areas of our analysis area; also in some cases sample sizes were small and compound the inherent uncertainties in future climates. Uncertainties remain large in such analyses. Monitoring and analysis are necessary for a better understanding of these ecosystems in order to improve planning, design and implementation of strategies focused on increasing their resilience and conservation.
For our study, we selected species with high importance value index and high density, so they are considered as ecologically and structurally significant [Citation27]. On the other hand, these species are characterized as rare because they are located in reduced number of habitats and have a restricted altitudinal gradient. They are both important and likely to face the greatest impacts of climate change due to their narrow distribution.
Some studies suggest a migration of species to higher elevations [Citation13–15,24,56]; notwithstanding, our projections show a reduction in the area with high likelihood of species presence (≥0.50). Perhaps these species are close to the thermal optimum for growth within their niches. Changes in climatic conditions are most often negative for species [Citation2], in many cases showing substantial restriction of distributions. Areas with a high likelihood of future species presence are occupied by other land uses related to anthropic activities, diminishing species ability to adapt to climatic variations. Also unsuitable land uses act as barriers that constrain the possibility of species migration into appropriate habitats.
According to future distribution models, species will be partially located in protected areas like La Ceiba Natural Reserve, Petrified Forest of Puyango, dry forest biosphere reserve and the dry forest closed area; these areas can serve as buffers against climate change; however, more similar strategies should be implemented in order to reduce conservation gaps in dry ecosystems. Additionally, we should evaluate whether these areas in the future will meet requirements for protection and, we should promote the design of dynamic protection networks, to allow species adaptation in time and space [Citation57–59].
As was observed in four of five species analyzed, there is a reduction in the likelihood of presence (p: ≥0.50). Species such as C. macrantha, characterized by a low regeneration rate [Citation52], could face a great niche reduction due to trampling, competition and climatic conditions [Citation52].
We can conclude that C. macrantha, Terminalia valverdeae, E. velutina and H. chrysanthus, show a reduction of their fundamental niche, even under a low-emissions scenario (RCP 2.6). This means that even under optimistic climate change scenarios, species will suffer a residual impact that will be reflected in their distribution and ability to migrate to more suitable habitats. Stressors can decrease the likelihood of species to occupy suitable habitat in distribution models. Other factors that could decrease the distribution probability of species could be: phenology, disease, predation by animals and changes in species composition at an ecosystem level [Citation16,60,61]. We will likely face these impacts, even if we continue implementing conservation strategies (for example, dry forest biosphere reserve).
Dry forests have a great diversity of vascular plants despite the fact the there are few intact areas where these habitats can expand [Citation22]. They face conservation gaps mainly because of low extent of protected areas [Citation22,62]. Therefore, it is a high priority to identify, preserve and, if possible, recover this vegetal formation [Citation21]. Probably the new declaration of dry forest as a biosphere reserve could provide a good opportunity for its conservation, gaining a major interest from governmental and non-governmental organizations that can contribute protection. Also, in recent years civil society started to pay more attention to dry forest due to the incorporation of its benefits into local livelihoods, one example is the guayacane’s flourishing (H. chrysanthus) at the beginning of each year, which promises revitalization for local economies and increases the awareness of protection of these ecosystems.
Associate Editor: Veerle Vanacker
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
We thank to Universidad Nacional de Loja and United States Forest Service for scientific support; to the United States Agency for International Development for the financial support to carry out this research.
Notes
1. Sensitivity: degree to which a species is affected by anthropogenic and intrinsic disturbances.
2. Adaptive capacity: ability of a species to resist, recover or adjust to extreme events.
References
- Pearson R, Dawson T. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Glob Ecol Biogeogr. 2003;12:361–371.10.1046/j.1466-822X.2003.00042.x
- McCarty J. Ecological consequences of recent climate change. Conserv Biol. 2001;15:320–331.10.1046/j.1523-1739.2001.015002320.x
- Walther G-R, Post E, Convey P, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395.10.1038/416389a
- Van-der-Putten W, Macel M, Visser M. Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Philos Trans R Soc Lond B Biol Sci. 2010;365:2025–2034.10.1098/rstb.2010.0037
- Hannah L, Betts A, Shugart H. Modeling future effects of climated change on tropical forest. In: Bush M, Flenley J, Gosling W, editors. Tropical rainforest responses to climatic change. 2nd ed. Berlin (Al); 2011. p. 411–429.10.1007/978-3-642-05383-2
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42.10.1038/nature01286
- Ramirez-Villegas J, Cuesta F, Devenish C, et al. Using species distributions models for designing conservation strategies of Tropical Andean biodiversity under climate change. J Nat Conserv. 2014;22:391–404.10.1016/j.jnc.2014.03.007
- Rodrigues P, Silva J, Eisenlohr P, et al. Climate change effects on the geographic distribution of specialist tree species of the Brazilian tropical dry forests. Braz J Biol. 2015;75:679–684.10.1590/1519-6984.20913
- Enquist C. Predicted regional impacts of climate change on the geographical distribution and diversity of tropical forests in Costa Rica. J Biogeogr. 2002;29:519–534.10.1046/j.1365-2699.2002.00695.x
- Guisan A, Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecol Lett. 2005;8:993–1009.10.1111/ele.2005.8.issue-9
- Austin M. Species distribution models and ecological theory: a critical assessment and some possible new approaches. Ecol Modell. 2007;200:1–19.10.1016/j.ecolmodel.2006.07.005
- Franklin J. Mapping species distributions: Spacial inference and prediction; Cambridge University Press; 2013. 320 p.
- Colwell R, Brehm G, Cardelús C, et al. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science. 2009;322:258–261.
- Bush M, Hooghiemstra H. Tropical biotic responses to climate change. In: Lovejoy T, Lee H, editors. Climate change and biodiversity. Michigan (MI): Yale University; 2005. p. 125–141.
- Pearson R. Climate change and the migration capacity of species. Trends Ecol Evol. 2006;21:111–113.10.1016/j.tree.2005.11.022
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2006;37:637–669.
- Pennington R, Prado D, Pendry C. Neotropical seasonally dry forests and Quaternary vegetation changes. J Biogeogr. 2000;27:261–273.10.1046/j.1365-2699.2000.00397.x
- Aguirre Z, Kvist L. Floristic composition and conservation status of the dry forests of south-western Ecuador. Lyonia. 2005;8:41–67.
- Albuja L. Biodiversity of the inter-Andean dry valleys of the Ecuador. In: Albuja L, editor. Escuela Politecnica Nacional. Quito; 2011. 56 p.
- Aguirre Z, Kvist L. Composición florística y estructura de bosques estacionalmente secos en el sur-occidental de Ecuador, provincia de Loja, municipios de Macara y Zapotillo. Arnaldoa. 2009;16:87–99.
- Aguirre Z, Kvist L, Sanchez O. Bosques Secos del Ecuador y su diversidad. Bot Econ los Andes Cent. 2006;162–87.
- Linares-Palomino R, Kvist L, Aguirre-Mendoza Z, et al. Diversity and endemism of woody plant species in the Equatorial Pacific seasonally dry forests. Biodivers Conserv. 2010;19:169–185.10.1007/s10531-009-9713-4
- Jara-Guerrero A, De la Cruz M, Méndez M. Seed dispersal spectrum of woody species in south Ecuadorian dry forests: environmental correlates and the effect of considering species abundance. Biotropica. 2011;43:722–730.10.1111/btp.2011.43.issue-6
- Thuiller W, Albert C, Araújo M, et al. Predicting global change impacts on plant species’ distributions: future challenges. Perspect Plant Ecol Evol Syst. 2008;9:137–152.10.1016/j.ppees.2007.09.004
- Herbario-Loja. Zoning and determination of the types of dry forest in the southwest of the province of Loja. Universidad Nacional de Loja. 2001:144 p.
- Lozano P. Types of forest in the south of Ecuador. In: Aguirre Z, Madsen M, Cotton E, Balslev H, editors. Austroecuatoriana Botany: Studies on plant resources in the provinces of El Oro, Loja and Zamora- Chinchipe. Universidad Nacional de Loja. Loja; 2002. p. 29–49.
- Curtis J, McIntosh R. The interrelations of certain analytic and synthetic phytosociological characters. Ecol Soc Am. 1950;31:434–455.
- Mostacedo B, Fredericksen T. Manual de métodos básicos de muestreo y análisis en ecología vegetal. Proyecto de Manejo Forestal Sostenible (BOLFOR). Santa Cruz; 1999.
- Magurran AE, McGill BJ. Biological diversity: frontiers in measurement and assessment [Internet]. OUP Oxford; 2011. Available from: http://books.google.co.cr/books?id=Q5oURmsTv4wC
- IPCC. Climate Change and Biodiversity. Gineva, Switzerland; 2002. 89 p.
- Phillips SJ, Robert A, Robert S. Maximum entropy modeling of species geographic distributions. Ecol Modell. 2006;190:231–259.10.1016/j.ecolmodel.2005.03.026
- Riahi K, Rao S, Krey V, et al. RCP 8.5 – a scenario of comparatively high greenhouse gas emissions. Clim Change. 2011;109:33–57.10.1007/s10584-011-0149-y
- van Vuuren D, Edmonds J, Kainuma M, et al. The representative concentration pathways: an overview. Clim Change [Internet]; 2011 Aug 5 [cited 2014 Jul 10];109:5–31. Available from: http://link.springer.com/10.1007/s10584-011-0148-z
- Wayne GP. The beginners’s guide to representative concentration pathways. Skeptical Science; 2013.
- IPCC. Climate change 2013. The physical science basis. Working group I contribution to the fifth assessment report of the Intergovernmental Panel on Climate Change; 2013. 1535 p.
- Sillmann J, Kharin K, Zwiers F, et al. Climate extremes indices in the CMIP5 multimodel ensemble: Part 2. Future climate projections. J Geophys Res Atmos. 2013;118:2473–2493.10.1002/jgrd.50188
- Armenta G, Dorado J, Rodriguez A, et al. Escenarios de Cambio Climático para Precipitación y Temperaturas en Colombia. Instituto de Hidrología, Meteorología y Estudios Ambientales de Colombia Bogota, Colombia. IDEAM:274; 2014.
- Kharin V, Zwiers F. Climate predictions with multimodel ensembles. J Clim. 2002;15:793–799.10.1175/1520-0442(2002)015<0793:CPWME>2.0.CO;2
- Knutti R, Furrer R, Tebaldi C, et al. Challenges in combining projections from multiple climate models. J Clim. 2010;23:2739–2758.10.1175/2009JCLI3361.1
- Knutti R, Sedláček J. Robustness and uncertainties in the new CMIP5 climate model projections. Nat Clim Change. 2012;3:1–5.
- Eguiguren P, Maita J, Aguirre N, et al. Tropical ecosystems vulnerability to climate change in southern Ecuador. Tripical Conserv Sci. 2016:1–17.
- Lambert S, Boer G. CMIP1 evaluation and intercomparison of coupled climate models. Clim Dyn. 2001;17:83–106.10.1007/PL00013736
- Graham C, Loiselle B, Velásquez-Tibatá J, et al. Models of Species Distribution and the Challenge of Forecasting Future Distributions. In: Herzog S, Martinez R, Jorgensen P, Tiessen H, editors. Climate Change and Biodiversity in the Tropical Andes, Instituto Interamericano para la investigación del Cambio global (IAI), São José dos Campos, y Comité Científico sobre Problemas del Medio Ambiente (SCOPE). Paris; 2009. p. 349–368.
- Rissler L, Apodaca J. Adding more ecology into species delimitation: ecological niche models and phylogeography help define cryptic species in the black salamander (Aneides flavipunctatus). Syst Biol. 2007;56:924–942.10.1080/10635150701703063
- IPCC. IPCC 5th assessment report “climate change 2013: the physical science basis.”. Stockholm; 2013.
- IPCC. Climate change 2001. Impacts, adaptation and vulnerability. Parte de la contribución del Grupo de trabajo II al Tercer Informe de Evaluación Grupo Intergubernamental de Expertos sobre el Cambio Climático. Ginebra: Grupo Intergubernamental de Expertos sobre el Cambio Climático; 2001. 93 p.
- Adger N, Arnella N, Tompkins E. Successful adaptation to climate change across scales. Glob Environ Change. 2005;15:77–86.10.1016/j.gloenvcha.2004.12.005
- Liu X, Wang Y, Peng J, et al. Assessing vulnerability to drought based on exposure, sensitivity and adaptive capacity: a case study in middle Inner Mongolia of China. Chin Geogr Sci. 2013;23:13–25.10.1007/s11769-012-0583-4
- Brewer C, Pickle L. Evaluation of methods for classifying epidemiological data on choropleth maps in series. Ann Assoc Am Geogr. 2002;92:662–681.10.1111/1467-8306.00310
- Saaty T. Decision making with the analytic hierarchy process Thomas L. Saaty. Int J Serv Sci. 2008;1:83–98.
- Saaty T. How to make a decision: the analytic hierarchy process. Eur J Oper Res. 1990;48:9–26.10.1016/0377-2217(90)90057-I
- Aguirre Z, Betancourt-Figueras Y, Greada L. Natural regeneration in the dry forests of the province of Loja and useful for local management. Rev CEDAMAZ. 2013;3:54–65.
- Jackson ST, Betancourt JL, Booth RK, et al. Ecology and the ratchet of events: climate variability, niche dimensions, and species distributions. Proc Nat Acad Sci. 2009;106:19685–19692.10.1073/pnas.0901644106
- Aguirre N, Eguiguren P, Maita J, et al. Vulnerability to climate change in the Southern Region of Ecuador: Potential impacts on ecosystems, biomass production and water production. Universidad Nacional de Loja. Loja; 2015. 184 p.
- Araújo MB, Guilhaumon F, Neto DR, et al. Impacts, vulnerability and adaptation to climate change of Spanish biodiversity 2. vertebrate fauna; Madrid, Spain; 2011. p. 640.
- Pimm S, Jenkins C, Abell R, et al. The biodiversity of species and their rates of extinction, distribution, and protection. Science. 2014;344:987–999.
- Pliscoff P, Fuentes-Castillo T. Modelación de la distribución de especies y ecosistemas en el tiempo y en el espacio: una revisión de las nuevas herramientas y enfoques disponibles. Rev Geogr Norte Gd. 2011;79:61–79.10.4067/S0718-34022011000100005
- Vos CC, Berry P, Opdam P, et al. Adapting landscapes to climate change: examples of climate-proof ecosystem networks and priority adaptation zones. J Appl Ecol. 2008;45:1722–1731.
- Coetzee BWT, Robertson MP, Erasmus BFN, et al. Ensemble models predict Important Bird Areas in southern Africa will become less effective for conserving endemic birds under climate change. Glob Ecol Biogeogr. 2009;18:701–710.
- Walther G-R. Plants in a warmer world. Perspect Plant Ecol Evol Syst Urban Fischer Verlag. 2003;6:169–185.10.1078/1433-8319-00076
- Alberto F, Aitken S, Alía R, et al. Potential for evolutionary responses to climate change – evidence from tree populations. Glob Chang Biol. 2013;19:1645–1661.10.1111/gcb.12181
- Cuesta-Camacho F, Peralvo M, Ganzenmuller A, et al. Identification of gaps and conservation priorities for terrestrial biodiversity in the Continental Ecuador. EcoCiencia, The Nature Conservancy, Conservación Internacional, Ministerio del Ambiente del Ecuador; 2006. Quito, Ecuador. p. 58.
- Jorgensen P, León Yánez S. Catalogue of the vascular plants of Ecuador. Monographs in systematic botany from the Missouri botanical garden; 1999. p. 1181.
Appendix
Appendix 1. Pearson correlation matrix for 19 bioclimatic variables.
BIO1 = Annual Mean Temperature; BIO2 = Mean Diurnal Range (Mean of monthly (max temp – min temp)); BIO3 = Isothermality (BIO2/BIO7) (*100); BIO4 = Temperature Seasonality (standard deviation *100); BIO5 = Max Temperature of Warmest Month; BIO6 = Min Temperature of Coldest Month; BIO7 = Temperature Annual Range (BIO5-BIO6); BIO8 = Mean Temperature of Wettest Quarter; BIO9 = Mean Temperature of Driest Quarter; BIO10 = Mean Temperature of Warmest Quarter; BIO11 = Mean Temperature of Coldest Quarter; BIO12 = Annual Precipitation; BIO13 = Precipitation of Wettest Month; BIO14 = Precipitation of Driest Month; BIO15 = Precipitation Seasonality (Coefficient of Variation); BIO16 = Precipitation of Wettest Quarter; BIO17 = Precipitation of Driest Quarter; BIO18 = Precipitation of Warmest Quarter; BIO19 = Precipitation of Coldest Quarter.
Appendix 2. Dry Forest species sensitivity to anthropogenic and intrinsic stressors.
Figure 7. Dry Forest species sensitivity to anthropogenic and intrinsic stressors. (a) Sensitivity for species future distribution (time horizon 2050) with RCP 2.6 scenario. (b) Sensitivity for species future distribution (time horizon 2050) with RCP 8.5 scenario.
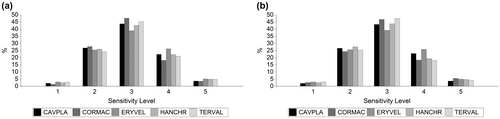
*Sensitivity Level: 1 (Very low), 2 (low), 3 (moderate), 4 (high), 5 (Very High). Cavanillesia platanifolia (CAVPLA), Cordia macrantha (CORMAC), Erythrina velutina (ERYVEL), Handroanthus chrysanthus (HANCHR), Terminalia valverdeae (TERVAL).
Appendix 3. Dry Forest species adaptive capacity.
Figure 8. Dry Forest species adaptive capacity. (a) Adaptive capacity for species future distribution (time horizon 2050) with RCP 2.6 scenario. (b) Adaptive capacity for species future distribution (time horizon 2050) with RCP 8.5 scenario.
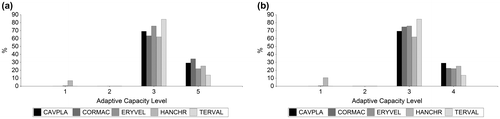
*Adaptive Capacity Level: 1 (Very low), 2 (low), 3 (moderate), 4 (high), 5 (Very High). Cavanillesia platanifolia (CAVPLA), Cordia macrantha (CORMAC), Erythrina velutina (ERYVEL), Handroanthus chrysanthus (HANCHR), Terminalia valverdeae (TERVAL).