Abstract
The Cuban gar (Atractosteus tristoechus) is an endemic lepisosteid living in Cuba. Among gars, this species is one of the most threatened and has the smallest natural distribution range. Lepisosteids are air-breathing fishes belonging to the Holostean, a basal non-teleost clade of actinopterygians. Recent studies have indicated that these fishes could be a ‘bridge between tetrapods and teleost biomedical models’. Herein, we sequenced and assembled the first complete mitochondrial genome of A. tristoechus. The total length of the mt genome is 16,290 bp, containing the typical 13 protein-coding genes, two ribosomal RNA (rRNA) genes, 22 transfer RNA (tRNA) genes, and a 537 bp length control region.
The Cuban gar (Atractosteus tristoechus) is an endemic lepisosteid living in marshes and rivers of southwestern Cuba. It has the smallest natural distribution among the members of the Lepisosteidae family. A recent study revealed very low levels of genetic diversity in the species (Ulmo-Díaz et al. Citation2016). Gars have been reported to share convergent genomic characteristics with mammals (Braasch et al. Citation2016; Symonová et al. Citation2016). Herein, we sequenced and assembled the first complete mitogenome of A. tristoechus.
A fin tissue sample from an adult female of A. tristoechus, caught at Zapata Swamp, Cuba and kept in captivity to be used as brood stock at the Center for Native Ichthyofauna Reproduction, was preserved in 96% ethanol and kept at 4 °C. Total DNA was extracted using a salt extraction protocol (Aljanabi and Martinez Citation1997) and stored at Bernatchez Lab. The DNA libraries were constructed with a NEBNext Ultra II DNA library preparation kit (New England Biolabs, Ipswich, MA) and run on Illumina MiSeq (paired-end 300 reads) at IBIS Genomics facility (Université Laval). The de novo assembly was carried out with A5-miseq pipeline (Coil et al. Citation2015). Sequences were prior trimmed for adaptors, minimum length and minimum quality with Trimmomatic v0.36 software (Bolger et al. Citation2014) fixing leading (3), trailing (3), sliding windows (4:15), and minlen (36) parameters. Assembly was further aligned and checked using the complete Atractosteus spatula mitogenome (Genbank accession number: AP004355.1) as reference, using MEGA7 (Kumar et al. Citation2016). Gene annotation was done using MitoAnnotator (Iwasaki et al. Citation2013) and MITOS (Bernt et al. Citation2013) software. Complete mitochondrial genome sequences of other five lepisosteid species were used for phylogenetic analysis. Additionally, sequences of Amia calva and Danio rerio were used as out groups. ClustalW was used for alignments using MEGA7. A maximum-likelihood phylogenetic tree was constructed using the GTR substitution model (Rodríguez et al. 1990) with gamma parameter α = 0.31, in MEGA7.
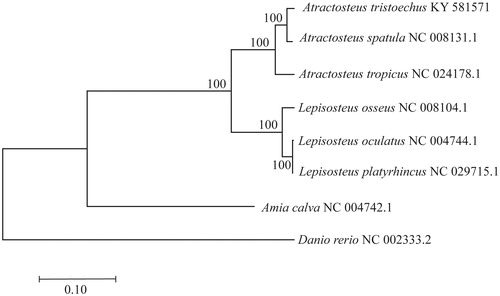
Atractosteus tristoechus complete mitogenome (GenBank accession number: KY581571) has 16,290 pb length and 44% GC content. The mitogenome structure includes 13 protein-coding genes, two rRNA genes (12S and 16S), 22 tRNA genes and a control region (D-loop, located between tRNA-Pro and tRNA-Phe). Main mitogenome features in terms of structure; GC content and gene order were similar to other gar species (Inoue et al. Citation2003; Broughton and Reneau Citation2006; Del Río-Portilla et al. Citation2016; Yu et al. Citation2016). A. tristoechus was phylogenetically close to A. spatula () as shown previously (Wright et al. Citation2012). A slow mitogenome mutation rate is one of the features highlighted in this family (Bernatchez and Wilson Citation1998; Rabosky et al. Citation2013). Notably, Lepisosteus oculatus and Lepisosteus platyrhincus mitogenomes show an extremely low genetic divergence between, with only three variable nucleotides (d = 0.0002 ± 0.00009) over the entire mitogenome. However, genetic distance between these species may vary depending on the geography of the populations sampled. Sipiorski (Citation2011) reported that L. oculatus sampled from Apalachicola River, western Florida, was more closely related to L. platyrhincus than to L. oculatus from other geographically distant populations. This has been explained as result of introgressive hybridization, common in freshwater fish species (Hubbs Citation1955; April et al. Citation2011), including gars species (Herrington et al. Citation2008; Bohn et al. Citation2017).
Figure 1. Full mtDNA maximum likelihood phylogenetic tree of six of the seven extant lepisosteids, obtained using the GTR + G substitution model. Values on nodes are bootstrap values (after 1000 replicates).
The information regarding the A. tristoechus mitogenome will contribute to the effort for the conservation of this endangered species.
Acknowledgements
The authors thank Alysse Perreault, Guillaume Côté, Cecilia Hernandez and Jerome St-Cyr for lab assistance. GUD stage in Louis Bernatchez laboratory was supported by a grant from Emerging Leaders of America Program (ELAP) of Canada.
Disclosure statement
The authors report no conflicts of interest.
References
- Aljanabi SM, Martinez I. 1997. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 25:4692–4693.
- April J, Mayden RL, Hanner R, Bernatchez L. 2011. Genetic calibration of species diversity among North America's Freshwater Fishes. Proc Natl Acad Sci USA. 108:10603–10607.
- Bernatchez L, Wilson CC. 1998. Comparative phylogeography of nearctic and palearctic freshwater fishes. Mol Ecol. 7:431–452.
- Bernt M, Donath A, Juhling F, Externbrink F, Florentz C, Fritzsch G, Putz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69:313–319.
- Bohn S, Kreiser BR, Daugherty DJ, Bodine KA. 2017. Natural hybridization of lepisosteids: implications for managing the Alligator Gar. N Am J Fish Manage. 37:405–413.
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30:2114–2120.
- Braasch I, Gehrke AR, Smith JJ, Kawasaki K, Manousaki T, Pasquier J, Amores A, Desvignes T, Batzel P, Catchen J, et al. 2016. The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat Genet. 48:427–437.
- Broughton RE, Reneau PC. 2006. Spatial covariation of mutation and nonsynonymous substitution rates in vertebrate mitochondrial genomes. Mol Biol Evol. 23:1516–1524.
- Coil D, Jospin G, Darling AE. 2015. A5-miseq: an updated pipeline to assemble microbial genomes from Illumina MiSeq data. Bioinformatics. 31:587–589.
- Del Río-Portilla MA, Vargas-Peralta CE, Lafarga-De La Cruz F, Arias-Rodriguez L, Delgado-Vega R, Galván-Tirado C, García-de-León FJ. 2016. The complete mitochondrial DNA of the tropical gar (Atractosteus tropicus). Mitochondrial DNA A DNA Mapp Seq Anal. 27:557–558.
- Herrington SJ, Hettiger KN, Heist EJ, Keeney DB. 2008. Hybridization between Longnose and Alligator Gars in captivity, with comments on possible gar hybridization in nature. Trans Am Fish Soc. 137:158–164.
- Hubbs CL. 1955. Hybridization between fish species in nature. Syst Zool. 4:1–20.
- Inoue JG, Miya M, Tsukamoto K, Nishida M. 2003. Basal actinopterygian relationships: a mitogenomic perspective on the phylogeny of the ‘ancient fish’. Mol Phylogenet Evol. 26:110–120.
- Iwasaki W, Fukunaga T, Isagozawa R, Yamada K, Maeda Y, Satoh TP, Sado T, Mabuchi K, Takeshima H, Miya M, et al. 2013. MitoFish and MitoAnnotator: a mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Mol Biol Evol. 30:2531–2540.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33:1870–1874.
- Rabosky DL, Santini F, Eastman J, Smith SA, Sidlauskas B, Chang J, Alfaro ME. 2013. Rates of speciation and morphological evolution are correlated across the largest vertebrate radiation. Nat Commun. 4:1958.
- Rodríguez F, Oliver JL, Marín A, Medina JR. 1990. The general stochastic model of nucleotide substitution. J Theor Biol. 142:485–501.
- Sipiorski JT. 2011. The world according to gars: the molecular systematics and comparative phylogeography of living gars (Actinopterygii: Lepisosteidae) [Doctoral dissertation]. Carbondale (IL): Southern Illinois University Carbondale.
- Symonová R, MajtáNová Z, Arias-Rodriguez L, Mořkovský L, Kořínková T, Cavin L, Pokorná MJ, Doležálková M, Flajšhans M, Normandeau E, et al. 2016. Genome compositional organization in gars shows more similarities to mammals than to other ray-finned fish. J Exp Zool B Mol Dev Evol. [accessed 2017 Mar 3];13. http://onlinelibrary.wiley.com/doi/10.1002/jez.b.22719/pdf.
- Ulmo-Díaz G, Castellanos-Gell J, Ponce de León JL, Casane D, Hurtado A, García-Machado E. 2016. Evidence of very low genetic diversity of Cuban gar (Atractosteus tristoechus). Rev Invest. 36:16–23.
- Wright JJ, David SR, Near TJ. 2012. Gene trees, species trees, and morphology converge on a similar phylogeny of living gars (Actinopterygii: Holostei: Lepisosteidae), an ancient clade of ray-finned fishes. Mol Phylogenet Evol. 63:848–856.
- Yu H, Li J, Ruan Z, Ma X, Zhang J, Wang M, Shi Q, You X. 2016. The complete mitochondrial genome of Florida gar (Lepisosteus platyrhincus). Mitochondrial DNA B Res. 1:128–129.
