Abstract
We sequenced the chloroplast genome of Poterioochromonas malhamensis (Pringsheim) R.A.Andersen strain SZCZR2049, which originates from Van Lake in Turkey. This genome is 133,923 bp long, and like those currently available for six phototrophic chrysophytes, it displays a long, gene-rich inverted repeat and a very short single-copy region. Compared to its chrysophyte counterparts, the P. malhamensis inverted repeat differs noticeably in gene content and the whole genome is missing 11 protein-coding genes. The maximum likelihood phylogeny inferred from concatenated protein-coding genes positioned P. malhamensis among the chrysophytes sensu lato as sister to the clade containing the Synurales (Synurophyceae) and Chromulinales (Chrysophyceae).
Poterioochromonas malhamensis (Pringsheim) R.A.Andersen 2017, formerly described as Ochromonas malhamensis (Pringsheim Citation1952), is a mixotrophic flagellated chrysophyte that belongs to the Ochromonadales (Synurophyceae). Photosynthesis was lost multiple times in chrysophytes, prompting studies of the molecular events underlying the transition from phototrophy to heterotrophy (Graupner et al. Citation2018; Dorrell et al. Citation2019). Known for grazing on bacteria (Holen Citation1999), P. malhamensis is also grazing on other microalgae (Zhang et al. Citation1996; Zhang and Watanabe Citation2001), an attribute representing a major threat to massive outdoor production of commercially important microalgae (Ma et al. Citation2017, Citation2018). P. malhamensis is a freshwater species but it can adapt to different salinities (Kauss Citation1974). We isolated this alga from a benthic sample of Van Lake, a saline lake from Eastern Anatolia. Here, we describe the chloroplast genome of this isolate and compare it to those previously reported for six phototrophic chrysophytes: five members of the Synurales (Synurophyceae, GenBank accessions MH795128-MH795132) (Kim et al. Citation2019) and Ochromonas sp. CCMP1393 (Chromulinales, Chrysophyceae, GenBank accession KJ877675) (Ševčíková et al. Citation2015).
A benthic sample was collected at a latitude of 39° 56′ 7.992″ N; 42° 16′ 52.993″ E during the month of February 2020 and was used as inoculum to initiate growth of P. malhamensis under illumination in F/2 medium based on natural freshwater (20 ‰ salinity). A clonal culture of this alga was established and is currently maintained in the Szczecin Culture Collection (http://geocentrum.usz.edu.pl/en/szczecin-diatom-culture-collection-szcz/, contact: Dr Przemysław Dąbek, [email protected]) under the accession number SZCZR2049. DNA was extracted following Doyle and Doyle (Citation1990) and sequenced on the DNBSEQ platform by the Beijing Genomics Institute in Shenzhen. A sample of the DNA preparation is kept at −20 °C at the University of Szczecin. A total of 40-million paired-end reads were assembled using SPAdes 3.14.0 (Bankevich et al. Citation2012). Following identification of chloroplast contigs, the complete chloroplast genome sequence was assembled using Consed (Gordon and Green Citation2013). Genes were identified as previously described (Turmel et al. Citation2017).
The P. malhamensis chloroplast genome (GenBank accession MW175522) is 133,923 bp long and as previously documented for other photosynthetic chrysophytes (Ševčíková et al. Citation2015; Kim et al. Citation2019), it displays two identical copies of long, gene-rich inverted repeat (IR) sequences that are separated from one another by single-copy regions of vastly unequal lengths. At 1,619 bp, the small single copy region (SSC) contains only 5 conserved genes (psaD, rpl21, rpl27, trnM, trnS) in addition to orf133 (divergent ycf54 sequence). The long single-copy region (LSC) is 69,530 bp long and codes for 87 conserved proteins, 3 hypothetical proteins, and 15 tRNAs. The IR is 31,387 bp long and contains 15 conserved protein-coding genes, 5 ORFs, 3 rRNA genes and 7 tRNA genes. Although they were found in conserved gene contexts in the IRs of all previously examined autotrophic chrysophytes, the psaM, petJ, and rpl34 genes appear to be entirely missing from the P. malhamensis genome. On the other hand, the P. malhamensis IR exhibits dnaK and a block of 4 contiguous genes (ilvB, rbcS, rbcL and rps4) that are located in the LSC in the 6 compared chrysophytes. Note here that blastn analysis of P. malhamensis SZCZR2049 rbcL revealed 100% identity with those previously reported for other strains of P. malhamensis (GenBank: EF165169, MH643685 to MH643690, MH643692). With respect to gene content, P. malhamensis also differs from phototrophic chrysophytes by the absence of a number of additional genes (acpP, atpE, ftrB, psbW, rpl29, tsf, ycf12 and ycf36) and by the occurrence of rpoC2 gene as two separate ORFs (the portion corresponding to the 3′ coding region is annotated as orf648). Breakup of the chloroplast rpoC2 into two ORFs has also been reported in green algae belonging to the core Trebouxiophyceae and Chlorophyceae (Turmel and Lemieux Citation2018). The dnaB, syfB and cemA genes, which were found to be specific to the Synurophyceae (Kim et al. Citation2019), are missing from P. malhamensis.
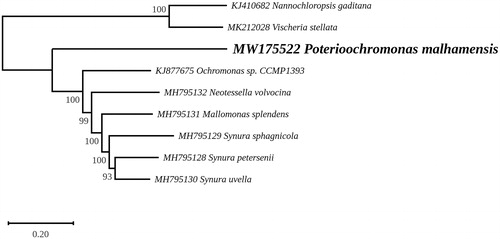
A maximum likelihood phylogeny was inferred from 107 chloroplast protein-coding genes of 9 taxa, including the two representatives of the Eustigmatophyceae (Nanochloropsis gaditana and Vischeria stellata) that were used as outgroup (). Sequence alignments were performed using MAFFT (Katoh and Standley Citation2013) and variable regions were removed with trimAl (Capella-Gutierrez et al. Citation2009). The phylogenetic analysis was carried out using RAxML 8.0 (Stamatakis Citation2014) under the GTR + I + G model, with the best tree out of 100 being computed for 1000 bootstrap replicates. In this tree, P. malhamensis represents the deepest branching lineage in the strongly supported clade (100% bootstrap support) containing the chrysophytes sensu lato. The relationships observed for the Synurales and Chromulinales are in agreement with the chloroplast phylogenomic analysis reported by Kim et al. (Citation2019).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are openly available in GenBank of NCBI at (https://www.ncbi.nlm.nih.gov/) under the accession no. MW175522. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA681133, SRR13155188, and SAMN16933430 respectively. The genome sequence is also available on Zenodo at: http://doi.org/10.5281/zenodo.4233311.
Additional information
Funding
References
- Bankevich A, Nurk S, Antipov D, Gurevich A, Dvorkin M, Kulikov AS, Lesin V, Nikolenko S, Pham S, Prjibelski A, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19(5):455–477.
- Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. 2009. trimAl: a tool for automated alignment trimming in largescale phylogenetic analyses. Bioinformatics. 25(15):1972–1973.
- Stamatakis A. 2014. RAxML Version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30:1312–1313.
- Dorrell RG, Azuma T, Nomura M, Audren de Kerdrel G, Paoli L, Yang S, Bowler C, Ishii K-I, Miyashita H, Gile GH, et al. 2019. Principles of plastid reductive evolution illuminated by nonphotosynthetic chrysophytes. Proc Natl Acad Sci USA. 116(14):6914–6923.
- Doyle JJ, Doyle JL. 1990. Isolation of plant DNA from fresh tissue. Focus. 12:13–15.
- Gordon D, Green P. 2013. Consed: a graphical editor for next-generation sequencing. Bioinformatics. 29(22):2936–2937.
- Graupner N, Jensen M, Bock C, Marks S, Rahmann S, Beisser D, Boenigk J. 2018. Evolution of heterotrophy in chrysophytes as reflected by comparative transcriptomics. FEMS Microbiol Ecol. 94(4):fiy039.
- Holen DA. 1999. Effects of prey abundance and light intensity on the mixotrophic chrysophyte Poterioochromonas malhamensis from a mesotrophic lake. Freshw Biol. 42(3):445–455.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Kauss H. 1974. Osmoregulation in Ochromonas. In: Zimmermann U, Dainty J, editors. Membrane transport in plants. Berlin, Heidelberg: Springer.
- Kim JI, Shin H, Škaloud P, Jung J, Yoon HS, Archibald JM, Shin W. 2019. Comparative plastid genomics of Synurophyceae: inverted repeat dynamics and gene content variation. BMC Evol Biol. 19(1):20.
- Ma M, Gong Y, Hu Q. 2018. Identification and feeding characteristics of the mixotrophic flagellate Poterioochromonas malhamensis, a microalgal predator isolated from outdoor massive Chlorella culture. Algal Res. 29:142–153.
- Ma M, Yuan D, He Y, Park M, Gong Y, Hu Q. 2017. Effective control of Poterioochromonas malhamensis in pilot-scale culture of Chlorella sorokiniana GT-1 by maintaining CO2-mediated low culture pH. Algal Res. 26:436–444.
- Pringsheim EG. 1952. On the nutrition of Ochromonas. Q J Microsc Soc. 93:71–96.
- Ševčíková T, Horák A, Klimeš V, Zbránková V, Demir-Hilton E, Sudek S, Jenkins J, Schmutz J, Přibyl P, Fousek J, et al. 2015. Updating algal evolutionary relationships through plastid genome sequencing: did alveolate plastids emerge through endosymbiosis of an ochrophyte? Sci Rep. 5:10134.
- Turmel M, Lemieux C. 2018. Evolution of the plastid genome in green algae. In: Chaw S-M, Jansen RJ, editors. Advances in botanical research. Plastid genome evolution. Vol. 85. London, England: Academic Press; p. 157–193.
- Turmel M, Otis C, Lemieux C. 2017. Divergent copies of the large inverted repeat in the chloroplast genomes of ulvophycean green algae. Sci Rep. 7:994.
- Zhang X, Watanabe MM. 2001. Grazing and growth of the mixotrophic chrysomonad Poterioochromonas malhamensis (Chrysophyceae) feeding on algae. J Phycol. 37(5):738–743.
- Zhang X, Watanabe MM, Inouye I. 1996. Light and electron microscopy of grazing by Poterioochromonas malhamensis (Chrysophyceae) on a range of phytoplankton taxa. J Phycol. 32(1):37–46.