Abstract
Heavy metals contamination in environment is a global problem. Environment is polluted by different toxic metals due to industrial sources and anthropogenic activities. Variety of methodologies has been applied for the purification of waste water. Thus, article mainly focuses on the current developments and applicability of various wastewater treatment techniques, i.e., adsorption, membrane filtration, ion exchange, electrochemical treatment and electrocoagulation. Also, development of new adsorbents especially selective adsorbent, i.e., ion imprinted polymeric materials for selective removal and pre-concentration of toxic Nickel (II) and Chromium (VI) ions has been discussed in detail.
Introduction
Clean and fresh water is not accessible in the world to sustain healthy life of living organisms. Due to some natural and also anthropogenic activities currently water shortage is arising in many countries. Anthropogenic activities drain the chemical effluents into the water stream. That contaminated water contains many toxic elements including metals, which are non-biodegradable, these are between most devastating pollutants (Rashid et al., Citation2012). In developing states, water pollution is increasing day by day due to anthropogenic and industrial activities (Azmat et al., Citation2016). Mostly leather tanning, cement, mining, dyeing, fertilizer, and photography industries discharge their wastewater in river, lakes, ponds, and canals which contain a number of toxic metals, e.g., chromium, cadmium, nickel, copper, lead, and arsenic(Attia, Khedr, & Elkholy, Citation2010; Dube, Tewari, Chatterjee, & Chatterjee, Citation2003). Chromium (VI) and Nickel (II) are also present in that wastewater which causes dangerous effects on our health. Chromium (VI) is carcinogenic in nature and causes cancer in lungs, nose, and nasal sinuses (Field & Withers, Citation2012). The harmful effect of Chromium concentrations in ground and surface water cannot be predicted precisely and accurately (Waseem et al., Citation2014). The maximum permissible level of Chromium (VI) given by United States Environmental Protection Agency (US EPA) for both drinking water and wastewater is 0.05 mg/L (Acar & Malkoc, Citation2004). Beside chromium, Nickel (II) is also a heavy toxic metal and environmental pollutant which causes asthma and skin disorder (Kristiansen, Christensen, Henriksen, Nielsen, & Menné, Citation2000; Zaheer Aslam, Ramzan, Naveed, & Feroze, Citation2010). Mostly Nickel pollution is caused by nickel and sulphide ores. It is also found in the compounds of iron, copper, cobalt, and some other metals (Long, Luo, Yin, & Wu, Citation2016). When Nickel (II) comes to the environment through anthropogenic sources it contaminates water, food ingestion, inhalation, percutaneous absorption, and damage to lungs, kidneys, gastrointestinal distress (Varma, Sarode, Wakale, Bhanvase, & Deosarkar, Citation2013). The industries which expose the Nickel (II) are Tableware, electroplating, plastics manufacturing, metal finishing, nickel-cadmium batteries, fertilizers, pigments, mining, and metallurgical operation (Long et al., Citation2016; Varma et al., Citation2013). The maximum permissible level of Nickel (II) given by World Health Organization (WHO) is 0.07 mg/g (Edition, Citation2011). However, the 0.02 mg/L is the maximum limit of Nickel (II) given by National Standards for Drinking Water Quality Pakistan (NSDWQ Pak). Mostly following methods are used to decrease the harmful effects of Chromium (VI) and Nickel (II), i.e., ion exchange (Chen, Hsu, & Chang, Citation2013; Gandhi, Viswanathan, & Meenakshi, Citation2010), adsorption technique, reduction precipitation (Kato & Nagai, Citation1991; Kulkarni, Kalyani, & Mahajani, Citation2007), reverse osmosis (Schoeman, Van Staden, Saayman, & Vorster, Citation1992; Schoeman, Steyn, & Scurr, Citation1996), foam flotation, and electrolysis. The main objective of this work is to review the most widely and extensively used techniques for the removal of Chromium (VI) and Nickel (II) and to give current information in this area (Owlad, Aroua, Daud, & Baroutian, Citation2009).
Adsorption techniques
Adsorption is a highly efficient technique to decontaminate the water; this process shows very promising results when compared to other methods. In adsorption phenomenon, the molecules are adsorbed on the surface of sorbent (Owlad et al., Citation2009). The molecules of metals move from the bulk phase and get adsorbed in the pores of sorbent in a semi-liquid state (Owlad et al., Citation2009). After successful adsorption there are some models generally applied to understand the process and interaction between adsorbent and adsorbate such as Langmuir and Freundlich isotherms. Langmuir isotherm consists of a single component (monolayer) which tells about the adsorption process at a specific homogeneous binding site on the adsorbent surface which comes to an end when the entire binding site is occupied and the Freundlich isotherm is based on the adsorption of metal ions on the heterogeneous binding sites. To understate the rate of reaction between adsorbent and adsorbate kinetic models are applied, there are two important parameters, i.e., pseudo-first- and pseudo-second-order kinetics, to observe the absorption rate of Chromium (VI) and Nickel (II) on the surface of the adsorbent. Pseudo-first order tells us about the number of free binding sites present in the adsorbent (Da’na, De Silva, & Sayari, Citation2011). Whereas pseudo-second order gives the information of a number of unoccupied binding sites in the adsorbent (Ho & McKay, Citation1998).
Chromium adsorption
Chromium being a very toxic element, many researches has been conducted to remove it from water. Adsorption is more promising technique for decontamination of drinking water. In adsorption process, Chromium (VI) ion from the aqueous solutions moves towards the adsorbent surface due to the electrostatic interaction or a chemical reaction (Dinker & Kulkarni, Citation2015). Adsorption is an essential and economical process for the removal of Chromium (VI) from the water and industrial wastewater; as for its many advantages: i.e., low energy demand, availability, easy operation, high removal efficiency, less chemical investment, and reusability (Dinker & Kulkarni, Citation2015). The process of adsorption is continued till the Chromium (VI) which is present in the solution to obtain equilibrium with the adsorbent. Greater the concentration of Chromium (VI) ions, greater will be absorption (Ajouyed, Hurel, & Marmier, Citation2011; Weber, Citation1972).
Many investigations have been carried out for the adsorption and removing of Chromium (VI) using adsorbents, i.e., activated carbons (Mohan, Singh, & Singh, Citation2005), bio-sorbents, low-cost bio-sorbents (Mohan & Pittman, Citation2006), industrial waste sorbent, and chitosan. Here, we discuss it one by one.
Activated carbon used for Chromium (VI)
The activated carbon is used as the best adsorbent for the Chromium (VI) (Mohan & Pittman, Citation2006). It is the attractive source for the removal of Chromium (VI) because of the presence of internal microporosity structure (Chingombe, Saha, & Wakeman, Citation2005). For this reason, chromium containing wastewaters are mostly treated by activated carbon. There are four types of activated carbon on the basis of their size and shape: powder-activated carbon (PAC), granular-activated carbon (GAC), activated carbon fibrous (ACF), and activated carbon cloth (ACC) (Babel & Kurniawan, Citation2003). Different studies have been carried out on these types of activated carbon for the removal of Chromium (VI) from wastewater. Candela used a different form of PAC (Pérez-Candela, Martín-Martínez, & Torregrosa-Maciá, Citation1995) for the removal of Chromium (VI).The adsorption capacity obtained using PAC is 390 mg/g at pH 2. It was found that the adsorption process depends on the pretreatment of activated carbon and that the highest removal performance was obtained with those prepared by physical activation. GAC is another type of activated carbon which is studied by Shanna and Forster (Shanna & Forster, Citation1996) for the removal of Chromium (VI) from aqueous solution. The adsorption capacity was achieved using GAC is 145 mg/g at a pH range of 2.5–3.0. Chromium (VI) is also removed from wastewater by ACFs plated with copper metal. When Copper (II) is plated on ACFs surface, the adsorption capacity is increased from an aqueous solution (Park & Jung, Citation2001). The adsorption capacity of ACFs is 40 mg/g.
Bio-sorbents used for Chromium (VI)
Bio-sorption is a new process for the treatment of heavy metals from aqueous effluents. Adsorbent constituents are derived from agricultural wastes and can be used as an appropriate method for the removal of Chromium ions from wastewater (Volesky, Citation2003). The bio-sorption technology is effective in decreasing the concentration of heavy toxic metal ions to very low levels. The advantages of bio-sorption on the other treatment methods are low cost, highly efficient, less amount of chemical, no other nutrient requirement, and the chance of metal recovery (Owlad et al., Citation2009). Various bio-sorbents are used for the removal of Chromium (VI), i.e., wool, olive cake, sawdust, pine needles, almond shell, coal, and cactus (Dakiky, Khamis, Manassra, & Mer’eb, Citation2002).
Industrial waste sorbents used for Chromium (VI)
Industrial waste sorbents are also capable of absorbing toxic metals. It is reported that industrial waste sorbents are also used for the removal of Chromium (VI) from wastewater (Namasivayam & Ranganathan, Citation1993). Waste slurry is the industrial waste products which are produced in fertilizer plant because of liquid fuel combustion. This waste slurry is converted into a cheap carbonaceous adsorbent and used for the treatment of metal ions, i.e., Chromium (VI) and Iron (III) (Srivastava, Tyagi, & Pant, Citation1989). It has excellent absorption capacity for the removal of Chromium (VI). Blast-furnace slag is an industrial waste product generated by steel plants and also used as an adsorbent for the removal of Chromium (VI) and the maximum absorption capacity was 7.5 mg/g (Srivastava, Pant, & Pal, Citation1987). And other industrial waste bio-sorbents were also used such as Bagasse fly ash (Gupta, Mohan, Sharma, & Park, Citation1998), Red mud (Pradhan, Das, & Thakur, Citation1999), Waste slurry (Srivastava et al., Citation1989), and Blast-furnace slag (Srivastava, Gupta, & Mohan, Citation1997).
Chitosan used for Chromium (VI)
Chitin is also used as an adsorbent. Chitin is a waste product of the crab meat canning industry. Chitosan is chemically produced by chitin and it occurs naturally in some fungal cell walls. The chitosan absorbs five to six times greater amounts of metallic ions than chitin because of the presence of free amino groups which are exposed during de-acetylation (Yang & Zall, Citation1984). Chitosan is an excellent adsorbent for the treatment of heavy metals because of the presence of a large number of hydroxyl groups, a large number of primary amino groups with high activity and flexible structure of polymer chain of chitosan making an appropriate configuration for adsorption of metals. The adsorption capacity obtained is 273 mg /g at a pH of 4.0 (Udaybhaskar, Iyengar, & Rao, Citation1990). Chitosan-based polymeric surfactants (CBPS) are also used for the treatment of Chromium VI from wastewater (Lee, Hong, Kajiuchi, & Yang, Citation2005). pH, CBPS dose, and initial concentration are responsible for removal efficiency by CBPS. The highest adsorption capacity of Chromium (VI) was found to be 180 mg/g of CBPS at a final pH of 5.3.
It is reported that other chitosan-based adsorbents are also used for the removal of Chromium VI from wastewater, i.e., non-cross-linked chitosan (Schmuhl, Krieg, & Keizer, Citation2001), cross-linked chitosan (Schmuhl et al., Citation2001), Chitosan-based polymeric surfactants (Lee et al., Citation2005), chemically modified chitosan flake (Sankararamakrishnan, Dixit, Iyengar, & Sanghi, Citation2006), quaternary chitosan salt (Spinelli, Laranjeira, & Fávere, Citation2004) data compiled in Table .
Table 1. Different adsorbents are used for the removal of Chromium (VI).
Adsorption studies for Nickel (II)
The adsorption process is used to remove the pollutants from the sample. Therefore, Nickel (II) ions are also removed using adsorption process. The Nickel (II) ions are absorbed on the surface of absorbent till the equilibrium is obtained. The basic need for an economic and commercial adsorption process is the selectivity adsorbent, high adsorption capacity, and low cost. Different absorbents are used for the removal of Nickel (II) from the wastewater, i.e., activated carbon, agricultural waste, clay, synthetic materials and waste rubber tire.
Activated carbon used for Ni (II)
The properties of activated carbon (physical and chemical) depend upon its pore size, a number of active surfaces functional groups, and pores distribution. By several modifications of activated carbons, these properties can be enhanced using different activating agents (Mahvi, Naghipour, Vaezi, & Nazmara, Citation2005). Activated Carbon (AC) from almond husk shows 97.8% removal of Nickel (II) at 700 °C temperature, and pH is 5 (Hasar, Citation2003). Ni (II) can also be removed using different types of activated carbon (AC) and also has different surface characterizations (Kinhikar, Citation2012). Granular Activated carbon (GAC) has more adsorption capacity for Ni2+ ions with a greater amount of surface functional groups (Kinhikar, Citation2012). ACC is also used for the treatment of Ni (II) ions from wastewater (Kadirvelu, Faur-Brasquet, & Cloirec, Citation2000). It is commercially available, and adsorption on ACC obtained by ion exchange method and carboxylic functional groups are present on the surface. Batch adsorption experiments have been done by study of the initial metal ion concentration, altering the pH and presence of other metal ions or interfering species.
Agricultural waste adsorbents used for Nickel (II) ion
Agricultural waste is a wide source of low-cost adsorbent. Agricultural waste materials mostly contain lignin and cellulose as the important constituents. Other constituents are hemicellulose, lipids, proteins, simple sugar, starches, water, and hydrocarbons (Sud, Mahajan, & Kaur, Citation2008). The agricultural waste adsorbent is used to remove the Nickel (II) ions from aqueous solution and monitored many parameters, i.e., temperature, pH solution, adsorption capacity, thermodynamic data, adsorption isotherm, and adsorption kinetics.
Different agricultural waste material are used for the treatment of Nickel (II) ions, i.e., rice husk, wheat husk, sawdust of various plants, bark of the trees, groundnut shells, coconut shells, hazelnut shells, walnut shells, apricot stone, cotton seed hulls, waste tea leaves, maize corn cob, sugar cane bagasse, mosambi fruit, orange peels, soybean hulls, sugar beet pulp, sunflower stalks, coffee beans, neem leaves, cotton stalks, etc(Qaiser, Saleemi, & Mahmood Ahmad, Citation2007). These materials are used for the removal of Nickel (II) metal ions in their natural form as well as some physical or chemical modification(Demirbaş, Kobya, Öncel, & Şencan, Citation2002).
Orange peel used for Nickel (II)
The peel of orange fruit is used for the treatment of Nickel (II) ions from the wastewater. It has high number of cellulose, pectin, hemicelluloses, and lignin. It is an agricultural waste and used as a low-cost adsorbent for Nickel (II) metal ion (Gönen & Serin, Citation2012).
Batch adsorption studies will be obtained by the study of the effect of variation in pH, temperature, and initial concentration of metal ions. The absorption capacity of Ni (II) metal ions is 96% obtained at pH 6 at 250 °C temperature (Ajmal, Rao, Ahmad, & Ahmad, Citation2000).
Mosambi fruit peels used for Nickel (II)
The mosambi fruit peelings (MFP) powder is a low-cost adsorbent in aqueous solution for the treatment of Nickel (II)(Krishna & Swamy, Citation2011).Batch adsorption parameters are pH, adsorbent dosage, agitation time, and metal ion concentration. The maximum adsorption obtained using mosambi fruit is 96.5% at pH 4. The optimum values observed that initial metal ion concentration was 50 mg/L, agitation time was 90 min, pH 4, particle size was 0.6 mm, and adsorbent dosage was 125 mg/50 mL.
Sawdust used for Nickel (II)
Sawdust is manufactured by saw mills in larger amounts as a solid waste. Sawdust is a cost-effective adsorbent used for the removal of Nickel (II) metal ions. Oak (QuercuscocciFera) tree sawdust reacted with HCl and used as an effective removal of Nickel metal ions (Argun, Dursun, Ozdemir, & Karatas, Citation2007). Using sawdust maximum adsorption of Nickel (II) metal ions has been obtained as 82% at pH 8.
Neem leaves used for Nickel (II)
Neem leaves are the novel adsorbent used for the treatment of Ni (II), Cu (II), and Zn (II) metal ions (Oboh, Aluyor, & Audu, Citation2009). The 68.75% of Ni2+ ions are removed with 1.0 g of adsorbent dosage at pH 7. The powder of neem leaf is also used for the treatment of Ni (II) metal ions as a bio-sorbent and shows 92.6% of adsorption at the pH 5 for the 4 g/L of adsorbent dosage (Bhattacharyya, Sarma, & Sarma, Citation2009). The neem powder has large adsorption capacity because of the presence of certain functional groups on its surface, i.e., carboxyl, hydroxyl, carbonyl, etc.
Maize cob used for Nickel (II)
Maize cob is a waste material used as a adsorbent for the treatment of Nickel (II) from aqueous solution(Muthusamy, Murugan, & Manothi, Citation2012). Batch adsorption study parameters are adsorbent dosage, metal–ion concentration, agitation speed, and contact time. To examine the concentration of metals they used atomic adsorption spectroscopy technique. Other Adsorbents are Cashew Nut Shell, Almond Husk, Hazelnut AC, Digested raw bark (RB), Zeolite A, Zeolite X, Waste rubber tire are used for the removal of Nickel (II) and are listed below in the Table .
Table 2. Different adsorbents are used for the removal of Nickel (II).
Membrane filtration technique
For the treatment of heavy metals, membrane filtration technique is mainly considered. This technique is used for the removal of inorganic effluent. It is not only employed for the removal of suspended solid and organic compounds but also for inorganic effluent such as toxic metals. These are accomplished to remove the toxic metals from aqueous solution, i.e., Chromium VI. There are several types of membrane filtration, i.e., inorganic, polymeric, and liquid membrane used for the removal of Chromium VI. These techniques are discussed in the following.
Inorganic membrane used for Chromium VI
The inorganic membrane is made up of a porous material due to their high chemical and great thermal stability. The inorganic membrane is used for the removal of Cr VI (Pugazhenthi, Sachan, Kishore, & Kumar, Citation2005). The membrane was constructed by supported non-interpenetrating and modified ultrafiltration carbon by gas phase nitration NOx and used hydrazine hydrate for animation. The porous radius of nitrated, unmodified and animated carbon are found to be 2.8, 2.0, 3.3 nm is observed. It was found that modified membrane has a tendency to increase two times water flux than the unmodified membrane. The removal of Cr VI was found using nitrated (giving 84% rejection), by unmodified (giving 96% rejection), and by animated carbon membrane (giving 88% rejection).
Polymeric membrane used for Chromium (VI)
The polymeric membrane is used as an important wastewater treatment technology for the removal and recovery of pollutants and solvent, i.e., water. Polymer-enhanced ultrafiltration (PEUF) process is used for the removal of Cr (VI) from aqueous solutions (Aroua, Zuki, & Sulaiman, Citation2007). Different nanofiltration composite polyamide membranes are used for the treatment for Cr (VI) for the concentration. Another non-matted polyacrylonitrile fibre (PAN) membrane was also used for the removal of Cr (VI) from underground water (Sthiannopkao & Sreesai, Citation2009). It was observed that membranes of a 17.5% polymer retain 98% of the Chromium (VI). This result shows that ultrafiltration process is effective for the treatment of Cr (VI) from aqueous system.
Membrane separation for Nickel II
Several membranes are employed in membrane filtration techniques for the separation of different toxic metals with great efficiency. Ultrafiltration and reverse osmosis membranes are mostly used for the removal of heavy metals.
Ultrafiltration
The ultrafiltration is the process in which low trans-membrane pressures is used for the treatment of dissolved and colloidal material. For obtaining high removal efficiency of toxic metals two types of an ultrafiltration are used i.e. micellar-enhanced ultrafiltration (MEUF) and polymer-enhanced ultrafiltration (PEUF).
Firstly, Scamehorn et al. in the 1980s used MUEF for the treatment of toxic metals from wastewater and dissolved organic compounds (Landaburu-Aguirre, García, Pongrácz, & Keiski, Citation2009). MEUF has been shown that it is an active technique for the separation of toxic metal ions from wastewater. In this separation, surfactant is added to water.
PEUF (polymer-enhanced ultrafiltration) is also a reliable technique for the removal of toxic metal ions. Water-soluble polymer is applied to make complex with metal ions and form macromolecule which have higher molecular weight than the molecular weight of the membrane. Both membranes are used for the removal of Nickel (II) from wastewater. MEUF was used for the removal of Ni (II) from wastewater and has removal efficiency of 96.8% at the pH 7 and PEUF was also used for the removal which has adsorption capacity of 100% at the pH 8 (Molinari, Poerio, & Argurio, Citation2008).
Reverse osmosis (R.O)
In reverse osmosis, R.O semi-permeable membrane is used, which allows the fluid that is being cleaned to pass through it. Reverse osmosis is a technique which is capable of separating the extensive range of toxic metals from an aqueous system. Now R.O is increasing day by day for the treatment of water in chemical and environmental engineering. R.O technique is used for the treatment of metals as well as for Ni (II) with the removal efficiency of 99.5% (Mohsen-Nia, Montazeri, & Modarress, Citation2007).
Ion-exchange techniques for Chromium (VI)
In last few years, ion-exchange technique is a popular and widespread method used for the treatment of Cr (VI) from wastewater. In several studies, different ion-exchange resins were used for the removal of Cr (VI). Synthetic Dowex 2-X4 ion-exchange resin was used to examine the uptake rate of hexavalent chromium from real plating wastewater (Bohdziewicz, Citation2000) The anionic-based resin was used in hydroxide form in the column and 100% removal of chromium (VI) was obtained. Other ion exchange resin, Ambersep 132 was used to recover chromic acid from synthetic plating solution in four steps (Landaburu-Aguirre et al., Citation2009) In the first step, ion-exchange resin captures the chromic acid, and in the second step, it is reacted with sodium hydroxide solution and transformed into sodium chromate In the third step, cationic ion-exchange resin was used to again transfer the sodium chromate into chromic acid, and in the final fourth step, the ion-exchange resin reacts with hydrochloric acid (HCL) to regenerate it again This fourth step is shown to be effective and also accomplished to provide continuous chromic acid. Solvent-impregnated resin (SIR) with aliquot 336 is prepared by (Kabay) (Kabay, Arda, Saha, & Streat, Citation2003) and batch adsorption studies will be done. Batch adsorption studies show that solvent-impregnated resins comprising of Aliquat 336 can be effectively and efficiently used for the treatment of hexavalent chromium from an aqueous solutions.
Ion-exchange technique for Nickel (II)
Ceralite IR 120 cationic exchange resin as an adsorbent for the treatment of Ni (II) from the industrial wastewater (Kumar, Ramakrishnan, & Gayathri, Citation2010). Batch adsorption studies show that percentage of an adsorption decreases with the increase in initial concentration of Nickel (II) metal ions. The maximum removal of Ni (II) ions observed pH 5. It is also reported that anion-exchange resins are used for the removal of Ni (II) from electroplating wastewater (Dave, Dave, & Mishra, Citation2011). They saw the effect of initial metal ion concentration and pH on capacities of ion-exchange resins. According to them, adsorption process was very sensitive and gives maximum removal at pH 4-6 and initial concentration of Ni (II) ions was 5–30 mg/L.
Electrochemical Treatment Techniques for Chromium VI
Electrochemical method is also used for treatment of industrial wastewater containing high contamination of metal ions which are not easily biodegradable and their physical pretreatment is also very expensive. For the removal of Chromium (VI) two electrochemical techniques are discussed below. For the removal of Chromium (VI) two electrochemical techniques are discussed below.
Membrane electrolysis
Membrane electrolysis is the type of electrochemical in which treatment of Cr (VI) from wastewater takes place. It is a chemical process run by an electrolytic potential. Two types of cathodes are used in conventional metal cathode (electrowinning) and a high surface area cathode (Janssen & Koene, Citation2002). The reduction and oxidation reactions take place at the anode and cathode. Carbon aerogel electrodes are also used for the treatment of hexavalent chromium from the industrial wastewater (Rana, Mohan, & Rajagopal, Citation2004). The removal of Cr(VI) was enhanced at low pH and high charge. It was reported that the concentration of hexavalent chromium in the wastewater can be reduced by 98.5% at a high charge (0.8 Ah) and low pH.
Electrochemical precipitation
It is a chemical precipitation method, where electrical potential has been applied to improve the efficiency of Cr (VI) metal ions from the industrial wastewater (Kurniawan, Chan, Lo, & Babel, Citation2006).
Several studies has been reported for application of electrochemical precipitation reaction (ECP) for the removal of hexavalent chromium from the electroplating wastewater (Kongsricharoern & Polprasert, Citation1995). The ECP is made up of an electrolytic cell which consists of cathode and anode. Cr (VI) ranges from 215 to 3.860 mg/L at pH 1.5. The change in parameters throughout the ECP process was electrical potential, hydraulic retention time, Cr (VI) concentration, conductivity, and initial pH and the optimum conditions of ECP are 75 V electrical potential, 4.8A current, 50 min hydraulic retention time, and 3.2 initial pH. At optimum conditions, the concentration of Cr (VI) in the remaining effluent was 0.2 mg/L.
Electrocoagulation for Nickel (II)
It is reported that Kundra et al. used electrocoagulation process for the treatment of heavy metal ions from industrial processes (Kundra, Sachdeva, Attar, & Parande, Citation2012). In this process, titanium is used as a working electrode, which is stable and can treat a number of contaminants. Electrocoagulation process is used for the removal of toxic metals, i.e., Cr, Cd, Hg, Ni, etc.
When aluminium electrodes are used in electrocoagulation process, the toxic metals, i.e., nickel, cadmium, mercury, chromium removed simultaneously from the aqueous solutions. Another study was carried out on electroplating wastewater for the removal of heavy metals (Dermentzis, Christoforidis, & Valsamidou, Citation2011). Author reported the greatest removal capacity of all studied metals in the pH range of 4–8.
The mixed solutions contain the equal concentrations of all metals, i.e., 75, 150, and 300 mg/L. The same rate of heavy metals, i.e., nickel, copper, and zinc were obtained. The time taken for their total removal was 20, 40, and 50 min, respectively, whereas the chromium takes 40, 60, and 80 min for their complete removal. The new study raveled that electrochemical treatment is highly beneficial for industrial lead frame nickel-plating waste-water. In electrochemical treatment the high flow rate of electrolyte between the anode and cathode, which allows the recovery of nickel after its complete deposition at the surface of the cathode. It was observed that the pH is dropped during the electrochemical treatment of wastewater. The reduction in pH could attribute to the production of H+ ions at anode surface and lower current density is obtained with higher current efficiency.
Molecular imprinting technology
Molecular imprinted technology (MIT) is a versatile technique of imprinting molecules, in which complexation occurs between the template molecule (analyte) and functional monomer (Cheong, Yang, & Ali, Citation2013). Then for cross-linking, cross-linker is used in a large amount. After the process of polymerization, the template molecule removed from the polymer matrix leaves specific binding sites, shape, and functional monomer. Now it is only selective for that particular template molecule. In a polymer, generally intermolecular interactions, dipole–dipole, and ionic interactions are formed between the template molecule and functional monomer. MIT is used for the recognition of biological and chemical compounds (Scorrano, Mergola, Del Sole, & Vasapollo, Citation2011), food and drugs (Pichon & Chapuis-Hugon, Citation2008), pollutants, separation and purification (Korytkowska-Wałach, Citation2013; Sadeghi, Aboufazeli, Zhad, Karimi, & Najafi, Citation2013), and chemical sensors (Morelli et al., Citation2010). There are two types of imprinting technology, i.e., molecular imprinting polymer (MIP) and ion imprinting polymer (IIP).
Molecular imprinting polymer
In MIP the template (analyte) is a molecule. MIP is prepared using a template, a functional monomer, a cross-linker, initiator, and a solvent. In a polymerization process, complex formation occurs between the template and functional monomer and complex is enclosed by cross-linker which yields three-dimensional network in which the template (analyte) is stuck after the completion of the polymerization process. A template molecule is removed by washing, which left certain cavities of that particular template in size, shape, and molecular interactions (Cheong et al., Citation2013).
Ion imprinting polymer
Firstly, Nishide et al in 1976 used IIP by cross-linking of poly(4-vinyl pyridine) with 1,4-dibromobutane in the existence of a metal ion (Morelli et al., Citation2010). The concept of MIP and IIP is same by altering the template. IIP preparation requires a ligand which is used to form a complex and particular metal ion which produces selective and specific binding sites after the escape of metal. This is the schematic diagram of IIP.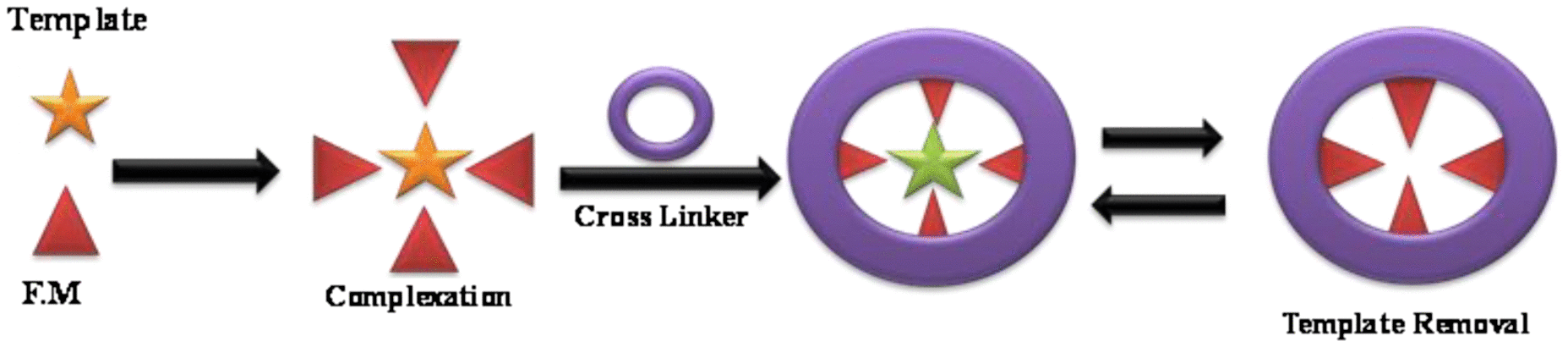
IIP preparation consists of a ligand–metal complex which is formed by copolymerization with a cross-linker in order to form a three-dimensional polymer network.
There are three steps in a polymerization process (Saatçilar, Satiroglu, Say, Bektas & Denizli, Citation2006).
Step 1: Polymerizable ligand form complexation with a metal ion.
Step 2: Polymerization of this complex.
Step 3: After polymerization process, the removal of template takes place.
The choice of ligand to form interactions between the polymer network and polymer network depends on coordinative bonds from some electron donating atoms to unfilled orbitals of the metal ions of the outer sphere (Pinheiro, Descalzo, Raimundo, Orellana, & Moreno-Bondi, Citation2012).
IIP used for Chromium (VI)
IIP is a useful technique which comprises of separation, selective extraction, and sensing of metal ions in various type of samples, i.e., water, wastewater, soil, and food samples. Chromium (VI) is carcinogenic and toxic in nature because of its variable oxidation states which may cause lung cancer and liver damage. Mostly, Cr (VI) is soluble in water; therefore, it is necessary to remove it from the aqueous solution. The removal of Cr (VI) using IIP is reported by many authors. Firstly IIP is synthesized by Pakade, Cukrowska, Darkwa, Torto, & Chimuka, Citation2011 for the selective treatment of Cr (VI) with 37.58 mg/g adsorption capacity (Pakade et al., Citation2011). The synthesis of IIP for the removal of Cr (VI) is prepared by Bayramoglu and Arica in 2011 and their maximum adsorption capacity was 170 mg/g (Bayramoglu & Arica, Citation2011). IIP-polyethyleneimine/silicon dioxide is synthesized for the removal of Cr (VI) and observed 80% maximum adsorption capacity at pH 3 (Li, Gao, & Du, Citation2011) . Zh ongqi Ren, Kong, Wang, & Zhang, Citation2014 prepared the IIP for the removal of Cr (VI) using bulk polymerization and examine eight functional monomers, among them five were basic, one acidic and two were neutral. The maximum adsorption capacity was obtained using 4-VP in which basic functional monomer was 338.73 mg/g (Ren et al., Citation2014).
IIP used for Nickel (II)
As Nickel (II) is a carcinogenic environmental contaminant so its removal is necessary. Therefore a numbers of research has been carried out by synthesizing Ni (II) ion-imprinted polymer for the removal of Ni by using various methods i.e. bulk polymerization(Demiralay, Andac, Say, Alsancak, & Denizli, Citation2010), precipitation polymerization(Otero-Romaní, Moreda-Piñeiro, Bermejo-Barrera, & Martin-Esteban, Citation2009) and dispersion polymerization (Ersöz, Say, & Denizli, Citation2004). In many articles, the initiator AIBN is used, and for the leaching of template acidic media was used (Rammika, Darko, & Torto, Citation2012), EDTA is employed as a leacher(Ersöz et al., Citation2004). Several researchers employed 4-VP as a monomer (Rammika et al., Citation2012) and EGDMA as cross-linker (Singh & Mishra, Citation2010).
The instruments AAS (Singh & Mishra, Citation2010), spectrophotometry (Demiralay et al., Citation2010), and ICP-OES (Rammika et al., Citation2012) were used to determine Ni (II). Various aqueous samples were used for the determination of Nickel (II), i.e., river water (Rammika et al., Citation2012), sea water (Otero-Romaní et al., Citation2009) and wastewater (Saraji & Yousefi, Citation2009). In the end, some advantages and disadvantages of reported methods have been compiled in Table .
Table 3. Advantages and disadvantages of various techniques.
Conclusion
Chromium (VI) and Nickel (II) are the toxic metals contaminants present in the aqueous system. Both these metals are carcinogenic and cause different types of cancer. Due to its toxicity, it is considered as a threat to our health and environment. Several techniques were studied for the removal of Cr (VI) and Ni (II) in this review.
The separation of Cr (VI) and Ni (II) depends on various parameters i.e., pH, temperature, initial concentration.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
This work was partially supported by the National Centre of Excellence in Analytical Chemistry, University of Sindh and Pakistan Science foundation Pakistan under research grant PSF/Res/S-SU/Chem (465).
References
- Acar, F., & Malkoc, E. (2004). The removal of chromium (VI) from aqueous solutions by Fagus orientalis L. Bioresource Technology, 94, 13–15. doi:10.1016/j.biortech.2003.10.03210.1016/j.biortech.2003.10.032
- Ajmal, M., Rao, R.A.K., Ahmad, R., & Ahmad, J. (2000). Adsorption studies on Citrus reticulata (fruit peel of orange): Removal and recovery of Ni(II) from electroplating wastewater. Journal of Hazardous Materials, 79, 117–131.10.1016/S0304-3894(00)00234-X
- Ajouyed, O., Hurel, C., & Marmier, N. (2011). Evaluation of the adsorption of hexavalent chromium on kaolinite and illite. Journal of Environmental Protection, 02, 1347.10.4236/jep.2011.210155
- Argun, M. E., & Dursun, S. (2008). A new approach to modification of natural adsorbent for heavy metal adsorption. Bioresource Technology, 99, 2516–2527.10.1016/j.biortech.2007.04.037
- Argun, M.E., Dursun, S., Ozdemir, C., & Karatas, M. (2007). Heavy metal adsorption by modified oak sawdust: Thermodynamics and kinetics. Journal of Hazardous Materials, 141, 77–85.10.1016/j.jhazmat.2006.06.095
- Aroua, M.K., Zuki, F.M., & Sulaiman, N.M. (2007). Removal of chromium ions from aqueous solutions by polymer-enhanced ultrafiltration. Journal of Hazardous Materials, 147, 752–758.10.1016/j.jhazmat.2007.01.120
- Attia, A., Khedr, S., & Elkholy, S. (2010). Adsorption of chromium ion (VI) by acid activated carbon. Brazilian Journal of Chemical Engineering, 27, 183–193.10.1590/S0104-66322010000100016
- Azmat, H., Ali, W., Javid, A., Hussain, A., Hussain, S.M., Saeed, Z., & Bukhari, S.S.H. (2016). Chromium contamination in water, sediment and its bioaccumulation in Indian major carps in river Chenab, Pakistan. Punjab Univ. J. Zool, 31, 83–86.
- Babel, S., & Kurniawan, T.A. (2003). Low-cost adsorbents for heavy metals uptake from contaminated water: A review. Journal of Hazardous Materials, 97, 219–243.10.1016/S0304-3894(02)00263-7
- Bayramoglu, G., & Arica, M.Y. (2011). Synthesis of Cr(VI)-imprinted poly(4-vinyl pyridine-co-hydroxyethyl methacrylate) particles: Its adsorption propensity to Cr(VI). Journal of Hazardous Materials, 187, 213–221.10.1016/j.jhazmat.2011.01.022
- Bhattacharyya, K.G., & Sarma, A. (2003). Adsorption characteristics of the dye, brilliant green, on neem leaf powder. Dyes and Pigments, 57, 211–222.10.1016/S0143-7208(03)00009-3
- Bhattacharyya, K.G., Sarma, J., & Sarma, A. (2009). Azadirachta indica leaf powder as a biosorbent for Ni(II) in aqueous medium. Journal of Hazardous Materials, 165, 271–278.10.1016/j.jhazmat.2008.09.109
- Bohdziewicz, J. (2000). Removal of chromium ions (VI) from underground water in the hybrid complexation-ultrafiltration process. Desalination, 129, 227–235.10.1016/S0011-9164(00)00063-1
- Chen, J.-H., Hsu, K.-C., & Chang, Y.-M. (2013). Surface modification of hydrophobic resin with tricaprylmethylammonium chloride for the removal of trace hexavalent chromium. Industrial & Engineering Chemistry Research, 52, 11685–11694.10.1021/ie401233r
- Cheong, W.J., Yang, S.H., & Ali, F. (2013). Molecular imprinted polymers for separation science: A review of reviews. Journal of Separation Science, 36, 609–628.10.1002/jssc.v36.3
- Chingombe, P., Saha, B., & Wakeman, R. (2005). Surface modification and characterisation of a coal-based activated carbon. Carbon, 43, 3132–3143.10.1016/j.carbon.2005.06.021
- Da’na, E., De Silva, N., & Sayari, A (2011). Adsorption of copper on amine-functionalized SBA-15 prepared by co-condensation: Kinetics properties. Chemical Engineering Journal, 166, 454–459.10.1016/j.cej.2010.11.017
- Dakiky, M., Khamis, M., Manassra, A., & Mer’eb, M. (2002). Selective adsorption of chromium(VI) in industrial wastewater using low-cost abundantly available adsorbents. Advances in Environmental Research, 6, 533–540.10.1016/S1093-0191(01)00079-X
- Dave, R., Dave, G., & Mishra, V. (2011). Removal of nickel from eletroplating wastewater by weakly basic chelating anion exchange resins: Dowex 50x4, Dowex 50x2 and Dowex M-4195. Journal of Applied Sciences in Environmental Sanitation, 6, 39–44.
- Demiralay, E. Ç., Andac, M., Say, R., Alsancak, G., & Denizli, A. (2010). Nickel (II)-imprinted monolithic columns for selective nickel recognition. Journal of Applied Polymer Science, 117, 3704–3714.
- Demirbaş, E., Kobya, M., Öncel, S., & Şencan, S. (2002). Removal of Ni(II) from aqueous solution by adsorption onto hazelnut shell activated carbon: Equilibrium studies. Bioresource Technology, 84, 291–293.10.1016/S0960-8524(02)00052-4
- Dermentzis, K., Christoforidis, A., & Valsamidou, E. (2011). Removal of nickel, copper, zinc and chromium from synthetic and industrial wastewater by electrocoagulation. International Journal of Environmental Sciences, 1, 697.
- Dinker, M.K., & Kulkarni, P.S. (2015). Recent advances in silica-based materials for the removal of hexavalent chromium: A review. Journal of Chemical & Engineering Data, 60, 2521–2540.10.1021/acs.jced.5b00292
- Dube, B., Tewari, K., Chatterjee, J., & Chatterjee, C. (2003). Excess chromium alters uptake and translocation of certain nutrients in citrullus. Chemosphere, 53, 1147–1153.10.1016/S0045-6535(03)00570-8
- Edition, F. (2011). Guidelines for drinking-water quality. WHO Chronicle, 38, 104–108.
- Ersöz, A., Say, R., & Denizli, A. (2004). Ni(II) ion-imprinted solid-phase extraction and preconcentration in aqueous solutions by packed-bed columns. Analytica Chimica Acta, 502, 91–97.10.1016/j.aca.2003.09.059
- Field, R.W., & Withers, B.L. (2012). Occupational and environmental causes of lung cancer. Clinics in Chest Medicine, 33, 681–703.10.1016/j.ccm.2012.07.001
- Gandhi, M.R., Viswanathan, N., & Meenakshi, S. (2010). Adsorption mechanism of hexavalent chromium removal using Amberlite IRA 743 resin. Ion Exchange Letters, 3, 25–35.
- Gönen, F., & Serin, D.S. (2012). Adsorption study on orange peel: Removal of Ni (II) ions from aqueous solution. African Journal of Biotechnology, 11, 1250–1258.
- Gupta, V.K., Ganjali, M., Nayak, A., Bhushan, B., & Agarwal, S. (2012). Enhanced heavy metals removal and recovery by mesoporous adsorbent prepared from waste rubber tire. Chemical Engineering Journal, 197, 330–342.10.1016/j.cej.2012.04.104
- Gupta, V.K., Mohan, D., Sharma, S., & Park, K.T. (1998). Removal of chromium (VI) from electroplating industry wastewater using bagasse fly ash – A sugar industry waste material. The Environmentalist, 19, 129–136.10.1023/A:1006693017711
- Güzel, F., Yakut, H., & Topal, G. (2008). Determination of kinetic and equilibrium parameters of the batch adsorption of Mn(II), Co(II), Ni(II) and Cu(II) from aqueous solution by black carrot (Daucus carota L.) residues. Journal of Hazardous Materials, 153, 1275–1287.10.1016/j.jhazmat.2007.09.087
- Hasar, H. (2003). Adsorption of nickel(II) from aqueous solution onto activated carbon prepared from almond husk. Journal of Hazardous Materials, 97, 49–57.10.1016/S0304-3894(02)00237-6
- Ho, Y., & McKay, G. (1998). Kinetic model for lead (II) sorption on to peat. Adsorption Science & Technology, 16, 243–255.
- Jamil, T.S., Ibrahim, H.S., El-Maksoud, I.A., & El-Wakeel, S. (2010). Application of zeolite prepared from Egyptian kaolin for removal of heavy metals: I. Optimum conditions. Desalination, 258, 34–40.10.1016/j.desal.2010.03.052
- Janssen, L., & Koene, L. (2002). The role of electrochemistry and electrochemical technology in environmental protection. Chemical Engineering Journal, 85, 137–146.10.1016/S1385-8947(01)00218-2
- Kabay, N., Arda, M., Saha, B., & Streat, M. (2003). Removal of Cr(VI) by solvent impregnated resins (SIR) containing aliquat 336. Reactive and Functional Polymers, 54, 103–115.10.1016/S1381-5148(02)00186-4
- Kadirvelu, K., Faur-Brasquet, C., & Cloirec, P.L. (2000). Removal of Cu(II), Pb(II), and Ni(II) by adsorption onto activated carbon cloths. Langmuir, 16, 8404–8409.10.1021/la0004810
- Kato, I., & Nagai, S. (1991). Treatment of chromium containing wastewater. Japan Kokai Tokkyo Koho, 224–691.
- Kinhikar, V. (2012). Removal of nickel (II) from aqueous solutions by adsorption with granular activated carbon (GAC). Research Journal of Chemical Sciences, 2, 6–11.
- Kongsricharoern, N., & Polprasert, C. (1995). Electrochemical precipitation of chromium (Cr) from an electroplating wastewater. Water Science and Technology, 31, 109–117.10.1016/0273-1223(95)00412-G
- Korytkowska-Wałach, A. (2013). Molecularly imprinted hydrogels for application in aqueous environment. Polymer Bulletin, 70, 1647–1657.10.1007/s00289-012-0869-9
- Krishna, R.H., & Swamy, A. (2011). Studies on the removal of Ni (II) from aqueous solutions using powder of mosambi fruit peelings as a low cost sorbent. Chemical Sciences Journal,.1–13
- Kristiansen, J., Christensen, J.M., Henriksen, T., Nielsen, N.H., & Menné, T. (2000). Determination of nickel in fingernails and forearm skin (stratum corneum). Analytica Chimica Acta, 403, 265–272.10.1016/S0003-2670(99)00568-1
- Kulkarni, P.S., Kalyani, V., & Mahajani, V.V. (2007). Removal of hexavalent chromium by membrane-based hybrid processes. Industrial & Engineering Chemistry Research, 46, 8176–8182.10.1021/ie070592v
- Kumar, P.S., Ramakrishnan, K., & Gayathri, R. (2010). Removal of nickel (II) from aqueous solutions by ceralite IR 120 cationic exchange resins. Journal of Engineering Science and Technology, 5, 232–243.
- Kundra, R., Sachdeva, R., Attar, S., & Parande, M. (2012). Studies on the removal of heavy metal ions from industrial waste water by using titanium electrodes. Journal of Current Chemical and Pharmaceutical Sciences, 2.
- Kurniawan, T.A., Chan, G.Y., Lo, W.-H., & Babel, S. (2006). Physico–chemical treatment techniques for wastewater laden with heavy metals. Chemical Engineering Journal, 118, 83–98.10.1016/j.cej.2006.01.015
- Landaburu-Aguirre, J., García, V., Pongrácz, E., & Keiski, R.L. (2009). The removal of zinc from synthetic wastewaters by micellar-enhanced ultrafiltration: Statistical design of experiments. Desalination, 240, 262–269.10.1016/j.desal.2007.11.077
- Lee, M.-Y., Hong, K.-J., Kajiuchi, T., & Yang, J.-W. (2005). Synthesis of chitosan-based polymeric surfactants and their adsorption properties for heavy metals and fatty acids. International Journal of Biological Macromolecules, 36, 152–158.10.1016/j.ijbiomac.2005.05.004
- Li, Y., Gao, B., & Du, R. (2011). Studies on preparation and recognition characteristic of surface-ion imprinting material IIP-PEI/SiO 2 of chromate anion. Separation Science and Technology, 46, 1472–1481.10.1080/01496395.2011.561821
- Long, J., Luo, X., Yin, X., & Wu, X. (2016). An ion-imprinted polymer based on the novel functional monomer for selective removal of Ni(II) from aqueous solution. Journal of Environmental Chemical Engineering, 4, 4776–4785.10.1016/j.jece.2016.11.004
- Mahvi, A.H., Naghipour, D., Vaezi, F., & Nazmara, S. (2005). Tea waste as an adsorbent for heavy metal removal from industrial wastewaters. Am. J. Appl. Sci, 2, 372–375
- Mohan, D., & Pittman, C.U. (2006). Activated carbons and low cost adsorbents for remediation of tri-and hexavalent chromium from water. Journal of Hazardous Materials, 137, 762–811.10.1016/j.jhazmat.2006.06.060
- Mohan, D., Singh, K.P., & Singh, V.K. (2005). Removal of hexavalent chromium from aqueous solution using low-cost activated carbons derived from agricultural waste materials and activated carbon fabric cloth. Industrial & Engineering Chemistry Research, 44, 1027–1042.10.1021/ie0400898
- Mohsen-Nia, M., Montazeri, P., & Modarress, H. (2007). Removal of Cu2+ and Ni2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination, 217, 276–281.10.1016/j.desal.2006.01.043
- Molinari, R., Poerio, T., & Argurio, P. (2008). Selective separation of copper(II) and nickel(II) from aqueous media using the complexation–ultrafiltration process. Chemosphere, 70, 341–348.10.1016/j.chemosphere.2007.07.041
- Morelli, I., Chiono, V., Vozzi, G., Ciardelli, G., Silvestri, D., & Giusti, P. (2010). Molecularly imprinted submicronspheres for applications in a novel model biosensor-film. Sensors and Actuators B: Chemical, 150, 394–401.10.1016/j.snb.2010.06.046
- Muthusamy, P., Murugan, S., & Manothi, S. (2012). Removal of Nickel ion from industrial waste water using Maize cob. ISCA Journal of Biological Sciences, 1, 7–11.
- Namasivayam, C., & Ranganathan, K. (1993). Waste Fe(III)/Cr(III) hydroxide as adsorbent for the removal of Cr(VI) from aqueous solution and chromium plating industry wastewater. Environmental Pollution, 82, 255–261.10.1016/0269-7491(93)90127-A
- Oboh, I., Aluyor, E., & Audu, T. (2009). 58 Biosorption of heavy metal ions from aqueous solutions using a biomaterial. Leonardo Journal of Sciences, 14, 58–65
- Otero-Romaní, J., Moreda-Piñeiro, A., Bermejo-Barrera, P., & Martin-Esteban, A. (2009). Ionic imprinted polymer for nickel recognition by using the bi-functionalized 5-vinyl-8-hydroxyquinoline as a monomer: Application as a new solid phase extraction support. Microchemical Journal, 93, 225–231.10.1016/j.microc.2009.07.011
- Owlad, M., Aroua, M.K., Daud, W.A.W., & Baroutian, S. (2009). Removal of hexavalent chromium-contaminated water and wastewater: A review. Water, Air, and Soil Pollution, 200, 59–77.10.1007/s11270-008-9893-7
- Pakade, V., Cukrowska, E., Darkwa, J., Torto, N., & Chimuka, L. (2011). Selective removal of chromium (VI) from sulphates and other metal anions using an ion-imprinted polymer. Water Sa, 37, 529–538.
- Park, S.-J., & Jung, W.-Y. (2001). Removal of chromium by activated carbon fibers plated with copper metal. Carbon Letters, 2, 15–21.
- Pérez-Candela, M., Martín-Martínez, J., & Torregrosa-Maciá, R. (1995). Chromium(VI) removal with activated carbons. Water Research, 29, 2174–2180.10.1016/0043-1354(95)00035-J
- Pichon, V., & Chapuis-Hugon, F. (2008). Role of molecularly imprinted polymers for selective determination of environmental pollutants – A review. Analytica Chimica Acta, 622, 48–61.10.1016/j.aca.2008.05.057
- Pinheiro, S.C.L., Descalzo, A.B., Raimundo, I.M., Orellana, G., & Moreno-Bondi, M.C. (2012). Fluorescent ion-imprinted polymers for selective Cu(II) optosensing. Analytical and Bioanalytical Chemistry, 402, 3253–3260.10.1007/s00216-011-5620-0
- Pradhan, J., Das, S.N., & Thakur, R.S. (1999). Adsorption of hexavalent chromium from aqueous solution by using activated red mud. Journal of Colloid and Interface Science, 217, 137–141.10.1006/jcis.1999.6288
- Pugazhenthi, G., Sachan, S., Kishore, N., & Kumar, A. (2005). Separation of chromium (VI) using modified ultrafiltration charged carbon membrane and its mathematical modeling. Journal of Membrane Science, 254, 229–239.10.1016/j.memsci.2005.01.011
- Qaiser, S., Saleemi, A.R., & Mahmood Ahmad, M. (2007). Heavy metal uptake by agro based waste materials. Electronic Journal of Biotechnology, 10, 409–416.
- Rammika, M., Darko, G., & Torto, N. (2012). Optimal synthesis of a Ni (II)-dimethylglyoxime ion-imprinted polymer for the enrichment of Ni (II) ions in water, soil and mine tailing samples. Water Sa, 38, 261–268.
- Rana, P., Mohan, N., & Rajagopal, C. (2004). Electrochemical removal of chromium from wastewater by using carbon aerogel electrodes. Water Research, 38, 2811–2820.10.1016/j.watres.2004.02.029
- Rashid, H., Hasan, M.N., Tanu, M.B., Parveen, R., Sukhan, Z.P., Rahman, M.S., & Mahmud, Y. (2012). Heavy metal pollution and chemical profile of Khiru river, Bangladesh. International Journal of Environment, 2, 57–63.
- Ren, Z., Kong, D., Wang, K., & Zhang, W. (2014). Preparation and adsorption characteristics of an imprinted polymer for selective removal of Cr( vi ) ions from aqueous solutions. Journal of Materials Chemistry A, 2, 17952–17961.10.1039/C4TA03024A
- Saatçılar, Ö., Şatıroğlu, N., Say, R., Bektas, S., & Denizli, A. (2006). Binding behavior of Fe3+ ions on ion-imprinted polymeric beads for analytical applications. Journal of Applied Polymer Science, 101, 3520–3528.10.1002/(ISSN)1097-4628
- Sadeghi, O., Aboufazeli, F., Zhad, H.R.L.Z., Karimi, M., & Najafi, E. (2013). Determination of Pb(II) ions using novel ion-imprinted polymer magnetic nanoparticles: Investigation of the relation between Pb(II) ions in cow’s milk and their nutrition. Food Analytical Methods, 6, 753–760.10.1007/s12161-012-9481-8
- Sankararamakrishnan, N., Dixit, A., Iyengar, L., & Sanghi, R. (2006). Removal of hexavalent chromium using a novel cross linked xanthated chitosan. Bioresource Technology, 97, 2377–2382.10.1016/j.biortech.2005.10.024
- Saraji, M., & Yousefi, H. (2009). Selective solid-phase extraction of Ni(II) by an ion-imprinted polymer from water samples. Journal of Hazardous Materials, 167, 1152–1157.10.1016/j.jhazmat.2009.01.111
- Schmuhl, R., Krieg, H., & Keizer, K. (2001). Adsorption of Cu (II) and Cr (VI) ions by chitosan: Kinetics and equilibrium studies. Water Sa, 27, 1–8.
- Schoeman, J., Van Staden, J., Saayman, H., & Vorster, W. (1992). Evaluation of reverse osmosis for electroplating effluent treatment. Water Science and Technology, 25, 79–93.
- Schoeman, J.J., Steyn, A., & Scurr, P.J. (1996). Treatment using reverse osmosis of an effluent from stainless steel manufacture. Water Research, 30, 1979–1984.10.1016/0043-1354(96)00014-0
- Scorrano, S., Mergola, L., Del Sole, R., & Vasapollo, G. (2011). Synthesis of molecularly imprinted polymers for amino acid derivates by using different functional monomers. International Journal of Molecular Sciences, 12, 1735–1743.10.3390/ijms12031735
- Shanna, D., & Forster, C. (1996). Removal of hexavalent chromium from aqueous solutions by granular activated carbon. Water SA, 22, 153–160.
- Sharma, S. (2014). A brief study of various adsorbents for removal of Ni metal ions from waste water. Research Cell : An International Journal of Engineering Sciences, 3.
- Singh, D., & Mishra, S. (2010). Synthesis, characterization and analytical applications of Ni(II)-ion imprinted polymer. Applied Surface Science, 256, 7632–7637.10.1016/j.apsusc.2010.06.018
- Spinelli, V.A., Laranjeira, M.C., & Fávere, V.T. (2004). Preparation and characterization of quaternary chitosan salt: Adsorption equilibrium of chromium(VI) ion. Reactive and Functional Polymers, 61, 347–352.10.1016/j.reactfunctpolym.2004.06.010
- Srivastava, S., Gupta, V., & Mohan, D. (1997). Removal of lead and chromium by activated slag – A blast-furnace waste. Journal of Environmental Engineering, 123, 461–468.10.1061/(ASCE)0733-9372(1997)123:5(461)
- Srivastava, S., Pant, N., & Pal, N. (1987). Studies on the efficiency of a local fertilizer waste as a low cost adsorbent. Water Research, 21, 1389–1394.10.1016/0043-1354(87)90014-5
- Srivastava, S., Tyagi, R., & Pant, N. (1989). Adsorption of heavy metal ions on carbonaceous material developed from the waste slurry generated in local fertilizer plants. Water Research, 23, 1161–1165.10.1016/0043-1354(89)90160-7
- Sthiannopkao, S., & Sreesai, S. (2009). Utilization of pulp and paper industrial wastes to remove heavy metals from metal finishing wastewater. Journal of Environmental Management, 90, 3283–3289.10.1016/j.jenvman.2009.05.006
- Sud, D., Mahajan, G., & Kaur, M. (2008). Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions – A review. Bioresource Technology, 99, 6017–6027.10.1016/j.biortech.2007.11.064
- Udaybhaskar, P., Iyengar, L., & Rao, A. (1990). Hexavalent chromium interaction with chitosan. Journal of Applied Polymer Science, 39, 739–747.10.1002/app.1990.070390322
- Varma, S., Sarode, D., Wakale, S., Bhanvase, B., & Deosarkar, M. (2013). Removal of nickel from waste water using graphene nanocomposite. International Journal of Chemical and Physical Sciences, 2, 132–139.
- Volesky, B. (2003). Sorption and biosorption. Montreal: BV Sorbex.
- Waseem, A., Arshad, J., Iqbal, F., Sajjad, A., Mehmood, Z., & Murtaza, G. (2014). Pollution status of Pakistan: A retrospective review on heavy metal contamination of water, soil, and vegetables. BioMed Research International, 2014.
- Weber, W.J. (1972). Physicochemical processes for water quality control. Wiley Interscience.
- Yang, T.C., & Zall, R.R. (1984). Absorption of metals by natural polymers generated from seafood processing wastes. Industrial & Engineering Chemistry Product Research and Development, 23, 168–172.10.1021/i300013a033
- Zaheer Aslam, M., Ramzan, N., Naveed, S., & Feroze, N. (2010). Ni (II) removal by biosorption using Ficus religiosa (peepal) leaves. Journal of the Chilean Chemical Society, 55, 81–84.
