 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Long-term research has become increasingly important in predicting ecological changes, although only a few studies have reported changes in amphibian communities over long time scales in urban areas. The main aim of the research was to determine the changes in the number and species composition of amphibian communities in small water bodies of two Olsztyn (NE Poland) settlements in the period between 1997 and 2015. The examined settlements are characterized by a high concentration of small water bodies and different degrees of urbanization. A decrease in the diversity index of reproducing amphibian communities was found between the years 1997 and 2015 at both settlements. Over the period of 18 years, one species – the northern crested newt – disappeared from both settlements, and another – the fire-bellied toad – disappeared from one of the two settlements. We demonstrate a significant relationship between the probability of occupancy of a water reservoir by fire-bellied toad, common frog, edible frog, and pool frog and the degree of isolation of these water reservoirs. Moreover, a decrease in the average number of species in a single water body and in the average values of diversity was also observed. The diversity of amphibian communities in water reservoirs depended mainly on the distance to the nearest water reservoir, the nearest building and the area of vegetation in the water reservoir. Available data suggests that the newly created water reservoirs play a positive role in the protection of amphibians in urban spaces, but the restoration of local amphibian communities is related to the degree of urbanization, and mostly applies to generalist species.
Introduction
Recently, there has been an increase in researchers’ interest in the transformation of the ecological landscape caused by the dynamic development and rapid expansion of urban environments. This has resulted in the remarkable development of urban ecology as a scientific discipline focused on understanding the impact of urbanization on ecological systems (Gaston Citation2010). The key task of this modern science is to answer the following questions: How do animals cope with urbanization? What are the consequences of them living in this new urban environment? The species composition, abundance, richness (the component of diversity showing the number of species) and evenness (the component of diversity showing how individuals are distributed among species) are usually radically altered in biological communities within cities (Shochat et al. Citation2006, Citation2010). The conversion of natural landscapes to urban areas is a globally observed process and it is expected that in the 21st century it will be the most important factor influencing changes in biodiversity (Sala et al. Citation2000).
Urban development is a major threat to many wildlife populations. Numerous studies have shown a negative relationship between urban land use and the species richness of various vertebrates (Czech & Krausman Citation1997; Rubbo & Kiesecker Citation2005). The most frequently encountered consequence of the increasing degree of urbanization is biotic homogenization of animal communities (McKinney & Lockwood Citation1999; McKinney Citation2006; Smart et al. Citation2006; Merckx & Van Dyck Citation2019). Homogenization refers not only to a decrease in taxonomic diversity, but also to decreased phylogenetic and functional diversity, essential for the proper functioning of ecosystems (Morelli et al. Citation2016; Hagen et al. Citation2017; La Sorte et al. Citation2018; Palacio et al. Citation2018). Dynamic anthropogenic processes, which are the main cause of biotic homogenization, reduce the number of stenobionts (which require a stable, uniform habitat) and native species, favoring the spread of eurybionts (which show wide tolerance to environmental factors), and urbanization is a major cause of this (Nowakowski Citation1996; McKinney & Lockwood Citation1999; McKinney Citation2002, Citation2006; Kuhn & Klotz Citation2006; McCleery Citation2010; Luck & Smallbone Citation2011; Morelli et al. Citation2016). Specific environmental conditions in the cities, e.g. higher annual temperature, reduced humidity, strong transformation and pollution of the area (mainly in the most urbanized part of the city), or habitat fragmentation (caused, for example by the elimination of ecological corridors), a rapidly expanding road network and the expansion of settlements, negatively affect biodiversity in highly urbanized zones (Marzluff Citation2001; Shochat et al. Citation2010; Faeth et al. Citation2011), including amphibian biodiversity (Semenov et al. Citation2000; Hamer & McDonnell Citation2008; Baillie et al. Citation2010; Nowakowski et al. Citation2010; Scheffers & Paszkowski Citation2011). Unfortunately, knowledge related to the importance of urbanization and biotic homogenization of aquatic communities is scarce (Chace & Walsh Citation2006). Small water reservoirs in cities are dynamic habitats that support significant biological diversity and provide important ecosystem services (Céréghino et al. Citation2014; Biggs et al. Citation2017; Stewart et al. Citation2017; Vad et al. Citation2017). They may play an important role as refuges and stepping stones for aquatic taxa moving between natural habitat patches in urban areas.
As a result of strong anthropogenic transformations of the environment in the 20th century, the number of natural water reservoirs in European countries drastically dropped (Oertli et al. Citation2002; Biggs et al. Citation2005; Ożgo Citation2010). The disappearance rate of these amphibian breeding sites exceeds the rate of their natural formation. In many cities, the number of water reservoirs and streams is decreasing, negatively affecting the population size of amphibians in urbanized environments (Hamer & McDonnell Citation2008). Terrestrial habitats surrounding water bodies in urban areas are also changing. Nowakowski et al. (Citation2011) found that certain amphibian species choose specific aquatic environments depending on the water bodies surroundings (agricultural areas, woods, borders of open and wooded areas, allotment gardens, open areas in industrial zone, settlements). In the ecological landscape, the diversity of amphibians depends largely on the degree of urbanization of the area (Knutson et al. Citation1999; Lehtinen et al. Citation1999; Rubbo & Kiesecker Citation2005).
Turner (Citation2005) showed that species distributions in the landscape are generally related to habitat composition and spatial habitat configuration. During the reproductive period, amphibian species have very specific preferences (size, depth, vegetation, water chemistry) regarding the reservoirs they choose as their breeding sites (Strijbosch Citation1979; Bebbee Citation1996; Hamer & McDonnell Citation2008; Nowakowski et al. Citation2011). In addition, among the factors affecting these amphibian choices are discontinuities between breeding and non-breeding habitats as well as isolation of breeding sites (Sjögren-Gulve Citation1994; Guerry & Hunter Citation2002; Ficetola & De Bernardi Citation2004; Mazerolle et al. Citation2005; Houlahan et al. Citation2006; Becker et al. Citation2007). It should be also emphasized that the dispersal distance of 56% of 53 analyzed anuran species was less than 1000 m, and the distance exceeded 10 km in only four species (Smith & Green Citation2005). Moreover, the study by Rittenhouse and Semlitsch (Citation2007) on temperate zone amphibians demonstrated that 50% of individuals stay within a 93 m radius from the breeding site. The sedentary lifestyle of most amphibian species, short-distance migrations from breeding water reservoirs (Van Gelder et al. Citation1986; Sinsch Citation1988, Citation1997; Smith & Green Citation2005; Rittenhouse & Semlitsch Citation2007; Kovar et al. Citation2009), and progressive changes in the surroundings of the water reservoirs in environments subjected to urbanization lead to a decrease in amphibian diversity. European tree frog Hyla arborea, European fire-bellied toad Bombina bombina and crested newt Triturus cristatus were reported to avoid highly urbanized zones in Olsztyn city (NE Poland) (Nowakowski et al. Citation2011).
Falaschi et al. (Citation2020) showed that the long-term dynamics of the population of many amphibian species on a regional scale depends on the processes of extinction in local habitats and colonization of new habitats, which on a spatial scale depends on the size of wetlands, their persistence, the density of water reservoirs with high amphibian richness and the occurrence of invasive species. The functioning of amphibians in a metapopulation (abandoning their existing breeding sites and settling in new ones), which is related to the dynamics of the population (Berven & Grudzien Citation1990; Sinsch & Seidel Citation1995; Skelly et al. Citation1999; Ficetola & De Bernardi Citation2004), is poorly recognized in urbanized areas, especially over the long term. The processes of amphibian species turnover in water bodies in urban landscape in long periods are little known, as well as in the situation of diverse urbanization pressure, which may cause the disappearance of water reservoirs, as well as the formation of new reservoirs.
Therefore, the aims of this study were:
To examine the species abundance and diversity of amphibian communities in small water bodies in two settlements of Olsztyn city, which differ in their urbanization rate and degree,
To evaluate the changes in amphibian communities in both settlements that took place between 1997 and 2015,
To investigate the environmental factors that influence the long-term change in amphibian communities,
To assess the role of newly created water reservoirs in the examined settlements in the protection of amphibian species diversity.
Research area
The current study was conducted in Olsztyn city (53°783’N, 20°483’E), located in the Olsztyn Lakeland, and the mesoregion of the Masurian Lakeland (Kondracki Citation2002). There are 15 lakes and three rivers (Łyna, Wadąg and Kortówka) within the city limits. The hydrological landscape of the city includes the presence of nearly 200 small water reservoirs (Śledź Citation2012). We survey the amphibian occurrence in 119 small water bodies, in two different settlements: Osiedle Gutkowo (Settlement A) and Osiedle Mazurskie (Settlement B) (). Water reservoirs of this type, with an area of up to 1 ha (), are hydrographic objects that contribute to increasing biodiversity and increase the value of the landscape. These reservoirs are also typical amphibian breeding sites. The average area of a water reservoir in 1997 and 2015 was 1693 m2 (Standard Deviation (SD) = 2517 m2, min = 23 m2, max = 13,448 m2) and 1164 m2 (SD = 1975 m2, min = 8 m2, max = 9993 m2), respectively. The chosen areas for the current study were characterized by a high concentration of small water bodies and differing degrees of intensification of the urbanization process.
Figure 1. Locations of the surveyed areas: Settlement A (Osiedle Gutkowo) and Settlement B (Osiedle Mazurskie) in the city of Olsztyn.
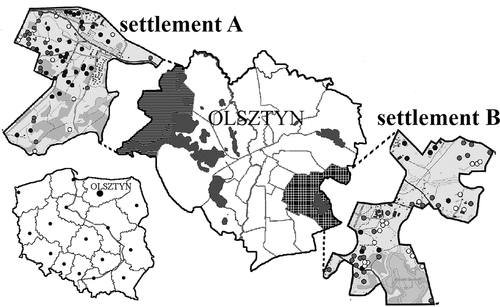
Settlement A covers an area of about 7.19 km2 and is located in the north-western part of the city. The area has been subjected to strong anthropogenic forces, which led to a doubling in built-up area between 1995 and 2009 and to a 13.2% increase in human population between 2008 and 2011 (Śledź Citation2012). The number of water reservoirs decreased from 56 in 1997 to 33 in 2015 (). The built-up area within a 200 m radius of the water reservoirs has significantly increased ( and )), from ca. 6400 m2 to 12,900 m2 (Wilcoxon test: T = 144.0, Z = 5.714, p < .0001). The average length of roads (Wilcoxon test: T = 23.0, Z = 3.215, p = .0013) and asphalt roads (Wilcoxon test: T = 1.0, Z = 2.547, p = .011) have also significantly increased within a 200 m radius from the water reservoirs (). In contrast, the average distance between the water body and the nearest road (Mann-Whitney test: Z = 2.956; p = .003) and the distance to the nearest building (Mann-Whitney test: Z = 2.298; p = .022) significantly decreased during the analyzed period. The average distance between the closest adjacent water bodies (Mann-Whitney test: Z = −0.144; p = .887), as well as the number of adjacent water bodies (Mann-Whitney test: Z = 0.280; p = .779) within a 200 m radius of the water reservoir, did not change in the studied period ().
Table I. Characteristics of the study area and the small water bodies in the settlements of Olsztyn city in 1997 and 2015.
Figure 3. Changes in the surroundings of water reservoirs in settlements A and B between 1997 and 2015 (blue – reservoirs functioning in 1997 and 2015; red – reservoirs that were destroyed; green – reservoirs that were created).
Settlement B covers an area of about 5.95 km2 and is located in the south-eastern part of the city. Anthropogenic changes in the settlement were much slower; the human population increased between 2008 and 2011 by 4.7% (Śledź Citation2012). The number of small water reservoirs increased from 34 in 1997 to 43 in 2015 (). The built-up area around the water reservoirs within a 200 m radius of water bodies also significantly increased, from ca. 7500 m2 to 9150 m2 ( and ); Wilcoxon test: T = 11.0, Z = 4.731, p < .0001). There was also an increase in the average length of roads within a 200 m radius of the water reservoirs (Wilcoxon test: T = 42.0, Z = 3.086, p = .002), and in the average length of asphalt roads in this area (Wilcoxon test: T = 19.0, Z = 3.354, p = .0008) (). The average distance between the water reservoir and the nearest road also decreased significantly during the analyzed period (Mann-Whitney test: Z = 2.959; p = .003) (). Changes in other characteristics (distance to the nearest building: Mann-Whitney test: Z = 1.888; p = .059; distance between the nearest neighboring water bodies: Mann-Whitney test: Z = −0.436; p = .663; number of water bodies within a 200 m radius around the water reservoir (Mann-Whitney test: Z = −0.280; p = .400) were statistically nonsignificant ().
Methods
Amphibian sampling methods
The research was carried out in 1997 and 2015. The transect stripe method was used to estimate the number of amphibians in the shore zone of small water reservoirs (Mazgajska Citation1996; Manley et al. Citation2006; Dodd Citation2010; Makomaska-Juchiewicz & Baran Citation2012), which, due to the size of the reservoirs, allowed for a fairly precise count. The presence of amphibian species was assessed by visual searching for adults and egg clutches (Dodd Citation2010; Nowakowski et al. Citation2010) and by identifying their mating voices. In both years, each water reservoir was monitored three or four times a day between 20 March and 5 June, and two times after dusk and during the night in May. The latter approach is especially important for detection of European green toad Bufotes viridis, natterjack toad Epidalea calamita and European tree frog Hyla arborea, which frequently sing after dusk. Newts were recorded by searching for animals and eggs in water vegetation during the day and by shining a torch into the water at night. The selection of dates of subsequent surveys took into account the weather conditions of the phenological seasons. The dates were selected to coincide with periods of the highest mating activity of the examined species. The population size was estimated in each water reservoir using the following rank scale: 0 – lack, 1 – up to 5 individuals, 2 – up to 10, 3 – up to 20, 4 – up to 40, 5 – up to 80, 6 – up to 160, 7 – up to 320, 8 – up to 640, 9 – up to 1280, 10 – above 1280. The maximum value of the rank was used as an estimator of population size in each water body.
The measurements of environmental variables
Several environmental factors affecting amphibian diversity were selected to use in examining the small water bodies in the two settlements. They include: the surface of built-up area (m2), sum of the length of all road types (m), sum of the length of asphalt roads (m), and number of adjacent water bodies (). The factors were measured separately in 1997 and 2015 within a 200 m radius of each water reservoir. The 200 m radius was chosen on the basis of the studies by Rittenhouse and Semlitsch (Citation2007) and Van Gelder et al. (Citation1986), who demonstrated that amphibians lead a rather sedentary lifestyle around the breeding site. The average distance between adjacent water reservoirs – approximately 100 m – was another important factor that determined the choice of a 200 m radius (). The distance to the nearest building (m), distance to the nearest road (m), distance to the nearest water reservoir (m), and length of the shoreline (m) were also measured in the current study (). The measurements of these variables were made based on maps of “Geoportal 2” (https://mapy.geoportal.gov.pl/) using “QGIS 3.2.3” program. In addition, the coverage of the surface of a water body by water plants (%) was also noted during field work ().
Statistical analyses
The alpha diversity of the amphibian communities, following Whittaker (Citation1975), was characterized by the number of species (S) and the Shannon species diversity index (H’) (Magurran Citation1996), assessed for each water body in 1997 and 2015. The H’ index values were calculated from the mean range rank of species population size. The number of species inhabiting an individual water body (S) and the Shannon index (H’) were compared between the two study years and between settlements A and B. The differences in the number of amphibian species (S) were tested using the non-parametric Mann-Whitney test (Sokal & Rohlf Citation1994). The Shannon indices (H’) were compared using the Hutcheson test (Hutcheson Citation1970).
The similarity of the ecological dominance structure of amphibian communities in the compared years was described by the Renkonen index (Re) (Renkonen Citation1938). To describe the relationships between the target variable (number of species (S), Shannon index – H’) and the explanatory variables (environmental factors – characteristics of the water body and its surroundings), in the first step we conduct a factor analysis on the explanatory variables. As the tested characteristics of water reservoirs were not completely independent, a reduction of the correlation matrix of the examined traits to the principal factors was performed using the factor analysis method (Manly Citation1986; Ferguson & Takane Citation1989). The reduction of the variables was done to distinguish the principal factors, being a linear combination of Cartesian coordinates of the examined features characterizing the water reservoirs in the space of a rectangular coordinate system. The number of factors identified was based on the criteria from the analyses of Kaiser (Citation1958) and Cattell (Citation1966), combined. The Kaiser criterion and the Cattell scree test suggested the inclusion of four principal factors (F-1–F-4) in the model. The extracted factors () explained 71.1% of the variance. The first factor (F-1) was strongly related to built-up area within a radius of 200 m around the water reservoir (factor coordinates – 0.803), sum of the length of asphalt roads (0.807), and sum of the length of all roads (0.871). The second factor (F-2) was related to the distance to the nearest water body (−0.798) and the number of water bodies within a 200 m radius of the water reservoir (0.771). F-3 was related to the vegetation surface area in the water reservoir (0.819), and partially to the length of shoreline (0.451), while F-4 was related to distance to the nearest building (0.636).
Table II. Factor coordinates (F-1 to F-4) of the studied variables in factor analysis model.
Reducing the number of variables to the most important main factors (F-1 to F-4) allowed us to distinguish conceptual variables synthetically describing the characteristics of water reservoirs and the surrounding environment, and to analyze their relationship with the diversity of amphibians. The relationship was tested using a generalized linear mixed model (GLMM) (McCulloch & Searle Citation2001), and the search for the best fitted models was made based on the corrected Akaike information criterion (AICc). The Shannon index (H’) was transformed as H’ + 1, to distribution without zero values, and the gamma distribution was assumed in the models. The main factors (F-1-F-4) were used as the constant explanatory variables, and as random factors we used the year of research and the location (id: settlement). Therefore, our models have the following form:
where the first line in the models describes the species diversity of the amphibian communities (H’ – Shannon index), Si (number of species in community), and the second line describes the fixed environmental factors (F-1–F-4 – main factors) and random factors.
The probability of occurrence of amphibian species (occurrence: 1; not present: 0) depending on the characteristics of the water reservoir and its surroundings (main factors F-1–F-4) was tested in the logistic regression models (Cramer Citation2002). The Rosenbrock and quasi-Newton methods of searching for the best fitted function were used, and the error was calculated asymptotically (Rosenbrock Citation1960; Dennis & Schnabel Citation1983; TIBCO Software Inc Citation2020).
The level of species turnover of amphibian groups (STI – species turnover index) in each water reservoir between 1997 and 2015 was assessed, using measures of dissimilarity between amphibian communities – Jaccard’s index – βj (Jaccard Citation1912), Sorensen’s index – βs (Sorensen Citation1948), and Simpson’s index – βsim (Simpson Citation1943; Lennon et al. Citation2001). The values of the STI indices were calculated as follows:
where a is the number of amphibian species occurring in the water reservoir only in 1997, b is the number only in 2015; and c is the number of species permanently present in the water reservoir.
These STI indicators provide information about the degree of dissimilarity between communities and thus the degree of exchange of species in individual reservoirs over time. The Jaccard index (βj) and Sørensen index (βs) are the most used measures due to their dependence on the proportion of species shared between two communities and its linear relationship with Whittaker’s beta diversity (Diserud & Ødegaard Citation2007). It is well known that this measure incorporates both true spatial turnover and differences in species richness (Koleff et al. Citation2003). To describe spatial turnover without the influence of species richness gradients, the Simpson dissimilarity index (βsim) was used.
GLMMs (McCulloch & Searle Citation2001) were used also to the assess the relationship between the value of the species turnover of the amphibian communities in water reservoirs (STIj – βj; STIs – βs) depending on the main factor (Factor-1 to Factor-4), the numbers of species (S) and the random factor (id of water reservoir). The relationship between the value of STIsim – βsim) was tested in models depending on the main factors (Factor-1 to Factor-4) and the random factor (id of water reservoir). The target variable distribution of the data was transformed as STI + 1, to a distribution without zero values, and the gamma distribution was assumed in the models. As the linking function in the models for a best model fit, we used the log function. Therefore, our models have the following form:
where the first line in the models describes the species turnover of the amphibian communities in a given water reservoir (STI), and the second line describes the fixed environmental factors (F-1 to F-4 – main factors; S – number of species) and the random factor.
The degree of freedom in the models was estimated according to the Kenward-Roger method, which is useful for restricted maximum likelihood (REML) models, especially for small sample sizes (Kenward & Roger Citation1997; IBM Citation2020). The relationship between the value of the species turnover of the amphibian communities in water reservoirs (STIj – βj; STIs – βs) and the Shannon index (H’) was also described using the Spearman correlation and simple linear regression. All calculations were made using the statistical package Statistica 13.3 (TIBCO Software Inc. Citation2017) and SPSS 27 (IBM Citation2020).
Results
Species richness and its relationship with habitat characteristics
Ten amphibian species were identified in all water reservoirs of the studied area. Nine and eight (excluding fire-bellied toad Bombina bombina (Linnaeus, 1761)) breeding species were found in Settlement A in 1997 and 2015, respectively (). Ten species were recorded in both years in Settlement B; in 2015, European tree frog Hyla arborea (Linnaeus, 1758), unlike northern crested newt Triturus cristatus (Laurenti, 1768) was identified in this settlement (). The majority of water reservoirs in Settlement A were inhabited by common frog Rana temporaria (Linnaeus, 1758), edible frog Pelophylax kl. esculentus (Linnaeus, 1758) and moor frog Rana arvalis (Nilsson, 1842). In turn, common toad Bufo bufo (Linnaeus, 1758); common frog, edible frog and pool frog Pelophylax lessonae (Camerano, 1882) inhabited Settlement B ().
Table III. Distribution and species richness of amphibian communities in the settlements of Olsztyn city in 1997 and 2015.
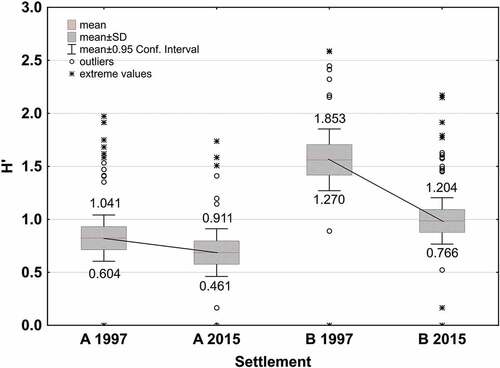
The mean amphibian diversity in the examined water reservoirs was significantly higher in Settlement B (H’ = 1.561) than in Settlement A in 1997 (H’ = 0.822) (Hutcheson test: t = 15.531, p < .001), and in 2015 (H’ = 0.985 vs H’ = 0.686; Hutcheson test: t = 68.835, p < .001) ( and ). A decrease in the diversity index of breeding amphibian communities was demonstrated between 1997 and 2015 in both settlements. The decrease was more pronounced in the Settlement B (Hutcheson test: t = 33.082, p < .0001) than in Settlement A (Hutcheson test: t = 10.098, p < .0001) (). The number of species inhabiting individual water reservoirs was positively related to the variability of the main factor F-2 (associated with the distance to the nearest water reservoir and the number of other reservoirs within a 200 m radius from a given water reservoir) and negatively with the main factor F-3 values (associated with the size of plant surface in water reservoir) (). The value of the Shannon (H’) index of the amphibian community inhabiting individual water reservoir was negatively related with the main factor F-4 values (associated with the distance to the nearest building) and positively with the F-3 values ().
Table IV. Model of the relationship between the number of amphibian species in a water reservoir and the environmental factors (main factors - F-1 to F-4).
Table V. Model of the relationship between species richness (H’) of amphibian communities and environmental factors (main factors - F-1 to F-4).
Figure 4. Variability of amphibian species diversity coefficients (H’) between settlements A and B and between 1997 and 2015.
For four species (fire-bellied toad, common frog, pool frog and edible frog), a significant relationship was observed between the probability of a species’ occupancy of a water reservoir and the features of the water reservoirs and of its surroundings (). The logistic regression model showed that the occurence of fire-bellied toad (the projected probability of occurrence, ) was favored by a high degree of isolation of water reservoirs, but these reservoirs were located closer to built-up areas – (). The lack of common frog and pool frog in the reservoirs (the projected probability of non-occurrence, ) was also associated with the high degree of isolation of the water reservoirs, but the two frog species differed in their dependence on the degree of vegetation coverage of the water reservoir surface (). Common frog occurred more often in water reservoirs with large vegetation area. In contrast, there was no relationship between pool frog occurence and this variable (F-3) (). The likelihood of the edible frog avoiding a water reservoir was associated negatively with the main factor F-3 and positively with the main factor F-4 (). Edible frog more often inhabited water reservoirs located at a great distance from buildings and with a high percentage of vegetation surface in a water reservoir.
Table VI. Models of logistic regression showing that the probability of occupancy of a water reservoir by particular amphibian species (target value = 1) depends on the water reservoirs and surrounding characteristics (main factors F-1–F-4). The models with main factors statistically significant at the accepted confidence level (for p<0.05) are indicated in bold writing.
Table VII. Models of logistic regression showing that the probability of occupancy of a water reservoir by a particular amphibian species (target value = 0) depends on the water reservoirs and surrounding characteristics (main factors F-1–F-4). The models with main factors statistically significant at the accepted confidence level (for p<0.05) are indicated in bold writing.
Turnover of amphibian species in communities
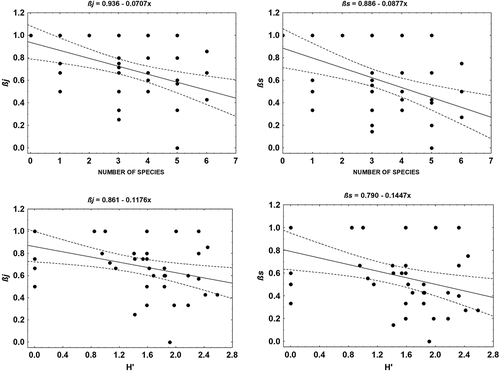
All three indices describing the turnover of amphibian species over time in water reservoirs clearly and in a similar manner indicate a greater species exchange in Settlement A than B (). Two of the indices showed a negative relationship only with the number of species in the water reservoir (GLMMs: STIj: fixed coefficient = −0.040; p = .004; STIs: fixed coefficient = −0.052; p = .002). The turnover indices are also negatively correlated with H’ index values (STIj: rs = –0.420; p = .004; STIs: rs = –0.420; p = .004). The univariate relationship between these variables is presented in . No significant relationship was demonstrated between STIsim values and characteristics of a habitat.
Figure 5. Relationship of coefficients of species turnover (βj, βs) with amphibian species richness (S) and the diversity index (H’).
The amphibian communities on the two settlements showed a similar structure of domination in 1997 and 2015 (Settlement A: Re = 62.02%; Settlement B: Re = 55.40%).
In both settlements, a decrease was noted in the number of water reservoirs inhabited by the common frog, edible frog, pool frog, fire-bellied toad, common toad and northern crested newt (). We recorded an increase in the number of water reservoirs inhabited by moor frog, smooth newt, European tree frog and common spadefoot toad. The latter two species settled new water reservoirs only in Settlement B (). The highest index of the amphibian species diversity and the highest number of species were recorded in water reservoirs that persisted through the studied period (H’ = 1.164 ± 0.741; S: Median = 3, Interquartile Range - IQR = 2.25). In the water reservoirs that disappeared during the analyzed period, the species diversity in 1997 was average (H’ = 0.827 ± 0.734). These reservoirs were usually inhabited by a single species (Median = 1, IQR = 3.00). In new reservoirs (those created between the two study dates) the diversity of amphibian communities originated at a low level (H’ = 0.730 ± 0.736). These reservoirs were also most often inhabited by a single species (Median = 1, IQR = 2.00). Moor frog (17/29 – 58.6% of all new reservoirs), common frog (12/29 – 41.4%), edible frog (9/29 – 31.0%), smooth newt (8/29 – 27.6%, and common toad (7/29 – 24.1%) were the most common species in new reservoirs. In addition, pool frog (5/29 – 17.2%), common spadefoot toad (3/29 – 10.3%) and green toad (1/29 – 3.4%) were found. European tree frog, fire-bellied toad and northern crested newt were not reported in the new reservoirs.
Discussion
There have been few long-term studies of amphibian populations in urban environments (Gibbs et al. Citation2005; Ogielska & Kierzkowski Citation2010; Vershinin et al. Citation2015; Konowalik et al. Citation2020), and recent studies on the ecology of urban amphibians (Hamer & McDonnell Citation2008; Scheffers & Paszkowski Citation2013; Sievers et al. Citation2019) do not fill the information gap regarding some key areas of amphibian ecology (e.g. terrestrial habitat availability, habitat use and selection, species-specific responses to urbanization, amphibian movements and spread in the urban landscape). Attempts to fill this gap led us to the discovery that various amphibian species respond differently to changes in the city environment. In the current study, we found that the features of the habitat surrounding a water reservoir as well as the features of the water reservoir itself affect the likelihood of colonization of the reservoir by fire-bellied toad, common frog, pool frog and, possibly, northern crested newt. On the other hand, the habitat features did not affect the probability of occurrence of smooth newt, common toad or European green toad. Such a phenomenon associated with the plasticity of species has been previously observed in other animals. Species requiring specific ecological niches (i.e. specialists with a narrow niche) quickly disappear from a changing environment, and plastic species (i.e. generalists with a wide niche) may be successful in settling that environment (e.g. Pretelli et al. Citation2018). Responses to changes in the city environment have been reported for animal biota (e.g. McKinney & Lockwood Citation1999; McKinney Citation2002; McCleery Citation2010), and also described in birds of Olsztyn city (Dulisz & Nowakowski Citation1996; Nowakowski Citation1996; Nowakowski et al. Citation2006).
In the current study, fire-bellied toad was demonstrated to disappear from Settlement A, where the urbanization process was much stronger than in Settlement B. This indicates a significant negative reaction of this species to the changes in the water reservoirs and their surroundings. It is a point of interest that the species preferred reservoirs located farther from other reservoirs, regardless of buildings’ proximity. This phenomenon may result from the species’ preference for its original habitat despite the construction of new housing estates. Fire-bellied toad is widespread in Central and Eastern Europe (Gasc et al. Citation2004; Kwet Citation2009; Sillero et al. Citation2014), and is strongly associated with open grasslands – extensively used meadows, pastures, grasslands and floodplains in river valleys, and agricultural areas with numerous permanent ponds covered by plants (Juszczyk Citation1987; Arnold & Ovenden Citation2004; Kwet Citation2009). In our earlier study (Nowakowski et al. Citation2010), concerning the amphibian distribution in Olsztyn in the 1990s, we found that the disappearance of waterlogged areas, a drop in the water level, the degradation of meadows and the creation of artificial banks of urban water reservoirs were the main anthropogenic factors responsible for the extinction of fire-bellied toad habitat in the city.
In contrast to the fire-bellied toad, the likelihood of the northern crested newt occurring in a given habitat was associated with a low degree of isolation of water reservoirs, which were located peripherally in settlements and away from buildings. In Olsztyn, the species occurs only in a few, clearly isolated groups of water reservoirs. The highest value of the Jacobs index, characterizing the preferences of the species for particular habitats, was recorded in water reservoirs located in agricultural areas (fields) (Nowakowski et al. Citation2011). The northern crested newt also preferred water reservoirs located in forest and forest-open landscape ecotone (~150 m from a forest), but avoided water reservoirs in other environments, especially those located in settlements and industrial city areas (Nowakowski et al. Citation2008, Citation2011; Knozowski, unpublished data). The presently identified northern crested newt preferences concerning water reservoirs are in agreement with its habitat requirements as described by Juszczyk (Citation1987) and Skei et al. (Citation2006). The isolated occurrence of northern crested newt in Olsztyn and the decline in newt numbers between the 1990s and the present day suggest that the current distribution of the newt is just a remnant of the altered system of its earlier breeding sites. As in Olsztyn, the number of northern crested newt in the Warsaw agglomeration also decreased dramatically in the 1990s (Mazgajska Citation1996), and now the newt is a rare species. The rapid decline of northern crested newt in urban environments may be associated with a low dispersion distance, so characteristic of this species. The dispersion distance of the northern crested newt ranges from 400 m (Baker & Halliday Citation1999) to 1000–1290 m (Laan & Verboom Citation1990; Kupfer Citation1998).
The decline in the colonization of water reservoirs by common frog and pool frog, similarly to fire-bellied toad and northern crested newt, indicates the sensitivity of this species to urbanization-driven changes. In the present study, the two frog species were usually found in reservoirs distant from other small water bodies. It is possible that in water-rich areas only a few reservoirs are selected for breeding sites, but in isolated water bodies, the lack of choice forces the frogs to colonize these isolated reservoirs. Our results indicate that areas with high density of water reservoirs are one of the most important factors positively affecting the number of species inhabiting individual reservoirs. Although some (fire-bellied toad, pool frog, common frog) have been recorded more frequently in isolated reservoirs, the amphibian community in general appears to function better when water reservoir density is higher. High reservoir densities increase choice and reduce competition from other species. In turn, the scattered and less numerous reservoirs remaining in developing cities are inhabited by various species of amphibians that cannot colonize other reservoirs (because they have disappeared). In the latter situation, there is increased interspecies competition – a problem that is poorly researched for many European amphibian species. Results of most experiments performed in laboratory and artificial ponds indicated strong asymmetric intra- and interspecies effects of competitor density on the fitness-related parameters of the examined species (Morin & Johnson Citation1988; Parris & Semlitsch Citation1998). Alford and Wilbur (Citation1985) reported that the competition between the genera Bufo and Rana depended on the reproductive time of each species. Thurman and Garcia (Citation2019) suggested that asymmetric competition shapes the reaction of North American amphibian species to environmental changes and that ignoring biotic reactions may lead to incomplete conclusions. Also, some competitive biotic interactions between amphibian species and other species (fish) cannot be ruled out, as pointed out by Falaschi et al. (Citation2019, Citation2020).
Regardless of the above findings, the high density of water reservoirs favors the abundance of amphibians. This phenomenon is probably the result of migration between reservoirs or the functioning in the metapopulation model. The classical metapopulation theory (Levins Citation1969; Hanski Citation1989, Citation1991) assumes that species extinction, colonization and recolonization are natural components of dynamics of local species population. Hanski and colleagues (Hanski et al. Citation1995; Hanski Citation1999) defined four conditions necessary to demonstrate the existence of the metapopulation effect: (i) habitat patches support local populations, (ii) no single population is large enough to ensure long-term survival, (iii) patches are not too isolated for possible recolonization and (iv) the simultaneous extinction of all local populations is unlikely. Many studies have shown that amphibians usually live in natural environments as metapopulations (e.g. Sjögren Citation1991; Ebisuno & Gentilli Citation2002; Bonato & Fracasso Citation2003; Bradford et al. Citation2003; Griffiths et al. Citation2010). However, only one experimental study, performed on an amphibian population in Melbourne (Australia), supported this observation for urban areas (Parris Citation2006).
Isolation of water reservoirs is a typical feature of urban environments. Many studies have demonstrated that isolation of water reservoirs is influenced by road density (Lehtinen et al. Citation1999; Löfvenhaft et al. Citation2004; Kruger et al. Citation2015). Minton (Citation1968), in turn, argued that the low abundance of amphibian species in municipal parks was due to the parks’ small size and their isolation. Also, Sjögren (Citation1991) showed that isolation of habitats was responsible for the decline in the number of pool frog. Amphibians are very sensistive to adverse changes in the environment, during both the aquatic phase and the terrestrial phase of their life stages. Following metamorphosis, juveniles are often dispersed and distant from their natal ponds (Sinsch Citation1992) and adults of many species migrate seasonally between terrestrial and aquatic habitats (Vos & Stumpel Citation1996; Vos et al. Citation2001; Semlitsch & Bodie Citation2003). Many amphibian species live in networks of local populations associated with dispersed individuals and move between these populations, where long-term survival depends on extinction and colonization processes. Fragmentation and destruction of habitats in urban landscapes disturb connections between these habitats, connections that are necessary for the proper functioning of amphibian populations (Lehtinen et al. Citation1999; Ficetola & De Bernardi Citation2004). The stability of amphibian communities is closely associated with the stability of individual local habitats (water reservoir and breeding sites) as well as with the spatial distribution of the water reservoirs. This exchange was stronger in settlements characterized by a high pace of urbanization and depended on the size of the amphibian community. The turnover of species was greater in reservoirs with lower species diversity and a smaller population, which appears to occur in reservoirs of lower quality. Werner et al. (Citation2007) reported that both local and regional factors play a role in the complex exchange of species within amphibian communities. The local dynamics of the water level in the reservoir was the most important factor influencing successful reproduction: the more stable the water level in the reservoir during the breeding season, the smaller the species exchange. Similarly, the larger the surface of the reservoir, the smaller the exchange of species. Falaschi et al. (Citation2020) showed that the duration and colonization of local populations were determined jointly by factors operating on different scales. The size of the wetland and the number of sites with numerous amphibian species had a positive effect, whereas the presence of invasive competing species had a negative effect.
In the present study, we found that the high value of the Shannon (H’) index was negatively related with the distance to the nearest building and positively related to the plant surface percentage in the water reservoir and the size of the reservoir. These results are similar to those of other authors who indicated that decreased habitat quality (Hamer & McDonnell Citation2008), habitat fragmentation (Vos & Chardon Citation1998; Beninde et al. Citation2015), and microhabitat loss in urban areas are the primary causes of biodiversity loss in general, and amphibian loss in particular (Silvano & Segalla Citation2005). The high level of urbanization causes the impoverishment of aquatic invertebrates and macrophytes, and often leads to their complete disappearance. It also has a negative impact on complex biota and the composition of biocenosis of urban ponds (Hill et al. Citation2017) and streams (Roy et al. Citation2003; Walsh et al. Citation2005).
In addition, we demonstrated a strong negative impact on amphibian diversity of the density of water reservoirs and the distance between the reservoirs; such a relationship was not found for the road density. These findings are in agreement with results presented by Gledhill et al. (Citation2008), who reported that the density of ponds is a determinant of aquatic species richness in the urban landscape. It should be noted that the impact of ecological factors may vary depending on place, habitat type and time. Löfvenhaft et al. (Citation2004) described the negative effects of increased road density in Stockholm. They found that the increase in the associated traffic was delayed, with the effects observed after several decades.
The species–area relationship is one of the main topics debated in the theory of ecology (Rosenzweig Citation1995; Lomolino Citation2000; Rybicki & Hanski Citation2013), and in amphibian ecology in particular (Motta-Tavares et al. Citation2020). The changes in the environment and the conversion of habitats by humans appear to be the reasons for the decline in animal biodiversity. Changes in the habitat structure of urban environments can be very intense, and the degree of environmental disturbance and habitat heterogeneity have a negative impact on the use of resources by specialists, thus favoring generalists. It is often assumed that there is a trade-off between the width of the niche and the ability to use resources: in an optimal resource environment specialists can function well and even outnumber generalists (Futuyma & Moreno Citation1988; Van Tienderen Citation1991; Devictor et al. Citation2010). We observed a decrease in colonization of Olsztyn water reservoirs by northern crested newt, fire-bellied toad and pool frog, or rare abundance (European tree frog, European green toad, spadefoot toad), which seem to be very sensitive to changes in the urban space (specialist species). On the other hand, we have not found such a strong influence of the environmental changes on the occurrence of other species, especially common toad and edible frog, which in our opinion can be considered generalists. The edible frog seems to be the most plastic species because it is an egzopolyploid and a relatively young species, evolutionarily speaking; it is also adaptable to new environments. Moor frog and common frog are species that show large inter-annual changes in abundance in the northeastern Poland region (Gotkiewicz et al. Citation2020); thus in some years of peak dynamics they may definitely inhabit water reservoirs, including those located on the outskirts of cities. Scheffers and Paszkowski (Citation2011) found that North American amphibians responded negatively to urbanization – 69 species were reported to respond negatively, 35 species showed no effect and only 6 responded positively. Similarly, the responses of some species to urbanization pressures were found to be different in amphibians of Central Europe (Ogielska & Kierzkowski Citation2010; Nowakowski et al. Citation2011; Mazgajska & Mazgajski Citation2020).
The planning of urban settlements includes both removal and creation of water reservoirs. The removal of the reservoirs is random and contributes to the loss of habitat of local species. In turn, the newly created water reservoirs are able to provide new breeding sites. Most often, the new artificial ponds act as retention or recreational reservoirs. According to many authors, such reservoirs may constitute alternative breeding habitat for amphibians (Simon et al. Citation2009; Clevenot et al. Citation2018). We found that the restoration of amphibian diversity in the newly created reservoirs fell within the average number of species and within the lower ranges of the diversity coefficient compared to the the diversity of communities in older reservoirs that survived in the studied environment. This suggests that the newly created reservoirs play a positive role in the preservation of amphibians on the spatial scale of the city space, although the reconstruction of local amphibian communities in urbanized areas may mainly concern species that are plastic with respect to environmental requirements. The result is amphibian communities made up of generalists and consisting of a smaller number of species. Similar results were obtained for plants and other groups of animals, showing that urbanization leads to taxonomic and functional homogenization (McKinney Citation2002, Citation2006; Kuhn & Klotz Citation2006; Luck & Smallbone Citation2011). Homogenization of amphibian communities in the newly created water reservoirs may also result from a more regulated nature of the water reservoir structure, its coastal zone and immediate surroundings.
The problem of species exchange and the reconstruction of amphibian communities in the newly created urban water reservoirs is undoubtedly closely related to the fluctuations in the population size over the years (Green Citation2003; Semlitsch & Bodie Citation2003). Only long-term multiannual monitoring can reveal the actual trends in changes in the amphibian population over time. As we compared the communities at only two time points, further monitoring studies, extended with additional environmental characteristics, are needed. For example, the chemical composition of water should be controlled, as some authors indicate that the main purpose of the newly created water reservoirs in the urban environment is to collect pollutants from runoff rainwater so that reservoirs with polluted water may constitute an ecological trap for amphibians (Snodgrass et al. Citation2008).
In addition, as our research shows, we should strive to create ecological corridors connecting natural environments, because preserving valuable natural water reservoirs in the city in the long run is not possible without special protection programs (Hill et al. Citation2017) and without taking into account the shaping of these habitats in the city landscape (Semlitsch & Skelly Citation2007).
Acknowledgements
Sincere thanks to Prof. R. Ciereszko for valuable comments that contributed to the revision of the original version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alford RA, Wilbur HM. 1985. Priority effects in experimental pond communities: Competition between Bufo and Rana. Ecology 66(4):1097–1105. DOI: 10.2307/1939161.
- Arnold N, Ovenden D. 2004. A field guide to the reptiles and amphibians of Britain and Europe. London: HarperCollins Publisher.
- Baillie JEM, Griffiths J, Turvey ST, Loh J, Collen B. 2010. Evolution lost: Status and trends of the world’s vertebrates. UK: Zoological Society of London.
- Baker JMR, Halliday TR. 1999. Amphibian colonization of new ponds in an agricultural landscape. Herpetological Journal 9:55–63.
- Bebbee TJC. 1996. Ecology and conservation of amphibians. London: Chapman & Hall. pp. 214.
- Becker CG, Fonseca CF, Haddad CFB, Batista RF, Prado PI. 2007. Habitat split and the global decline of amphibians. Science 318(5857):1775–1777. DOI: 10.1126/science.1149374.
- Beninde J, Veith M, Hochkirch A. 2015. Biodiversity in cities needs space: A meta-analysis of factors determining intra-urban biodiversity variation. Ecology Letters 8:581–592. DOI: 10.1111/ele.12427.
- Berven KA, Grudzien TA. 1990. Dispersal in the wood frog (Rana sylvatica): Implications for genetic population structure. Evolution 44(8):2047–2056. DOI: 10.1111/j.1558-5646.1990.tb04310.x.
- Biggs J, Williams P, Whitfield M, Nicolet P, Weatherby A. 2005. 15 years of pond assessment in Britain: Results and lessons learned from the work of pond conservation. Aquatic Conservation: Marine and Freshwater Ecosystems 15(6):693–714. DOI: 10.1002/aqc.745.
- Biggs J, von Fumetti S, Kelly-Quinn M. 2017. The importance of small waterbodies for biodiversity and ecosystem services: Implications for policy makers. Hydrobiologia 793(1):3–39. DOI: 10.1007/s10750-016-3007-0.
- Bonato L, Fracasso G. 2003. Movements, distribution pattern and density in a population of Salamandra atra aurorae (Caudata: Salamandridae). Amphibia–Reptilia 24(3):251–260. DOI: 10.1163/156853803322440736.
- Bradford DF, Neale AC, Nash MS, Sada DW, Jaeger JR. 2003. Habitat patch occupancy by toads (Bufo punctatus) in a naturally fragmented desert landscape. Ecology 84(4):1012–1023. DOI: 10.1890/0012-9658(2003)084[1012:hpobtb]2.0.co;2.
- Cattell RB. 1966. The screen test for the number of factors. Multivariate Behavioral Research 1(2):245–276. DOI: 10.1207/s15327906mbr0102_10.
- Céréghino R, Boix D, Cauchie HM, Martens K, Oertli B. 2014. The ecological role of ponds in a changing world. Hydrobiologia 723(1):1–6. DOI: 10.1007/s10750-013-1719-y.
- Chace JF, Walsh JJ. 2006. Urban effects on native avifauna: A review. Landscape and Urban Planning 74(1):46–69. DOI: 10.1016/j.landurbplan.2004.08.007.
- Clevenot L, Carre C, Pech P. 2018. A review of the factors that determine whether stormwater ponds are ecological traps and/or high-quality breeding sites for amphibians. Frontiers in Ecology and Evolution 6(40). DOI: 10.3389/fevo.2018.00040.
- Cramer JS. 2002. The origins of logistic regression. Tinbergen Institute Working Paper No. 2002 119(4):1–15. DOI: 10.2139/ssrn.360300.
- Czech B, Krausman PR. 1997. Distribution and causation of species endangerment in the United States. Science 277(5329):1116–1117. DOI: 10.1126/science.277.5329.1116.
- Dennis JE, Schnabel RB. 1983. Numerical methods for unconstrained optimization and nonlinear equations. Englewood Cliffs, NJ: Prentice Hall.
- Devictor V, Clavel J, Julliard R, Lavergne S, Mouillot D, Thuiller W, Venail P, Villéger S, Mouquet N. 2010. Defining and measuring ecological specialization. Journal of Applied Ecology 47(1):15–25/. DOI: 10.1111/j.1365-2664.2009.01744.x.
- Diserud OH, Ødegaard F. 2007. A multiple-site similarity measure. Biology Letters 3(1):20–22. DOI: 10.1098/rsbl.2006.0553.
- Dodd CK Jr, editor. 2010. Amphibian ecology and conservation, A handbook of techniques. Oxford, New York: Oxford University Press Inc.
- Dulisz B, Nowakowski JJ. 1996. The species diversity of the avifauna in built-up areas in the city of Olsztyn (NE Poland). Acta Ornithologica 31(1):33–38.
- Ebisuno M, Gentilli A. 2002. Reproductive site selection and characteristics of sources and sinks in an Italian tree frog metapopulation (Hyla intermedia, Boulenger 1882). Reveu d’ecologie-la Terre Et la Vie 57:263–278.
- Faeth SH, Bang C, Saari S. 2011. Urban biodiversity: Patterns and mechanisms. Annals of the New York Academy of Sciences 1223(1):69–81. DOI: 10.1111/j.1749-6632.2010.05925.x.
- Falaschi M, Manenti R, Thuiller W, Ficetola GF. 2019. Continental‐scale determinants of population trends in European amphibians and reptiles. Global Change Biology 25(10):3504–3515. DOI: 10.1111/gcb.14739.
- Falaschi M, Giachello S, Lo Parrino E, Muraro M, Manenti R, Ficetola GF. 2020. Long-term drivers of persistence and colonization dynamics in spatially structured amphibian populations. Conservation Biology 35:1530–1539. DOI: 10.1111/cobi.13686.
- Ferguson GA, Takane Y. 1989. Statistical analysis in psychology and education. 6th ed. New York: McGraw-Hill, Inc.
- Ficetola GF, De Bernardi F. 2004. Amphibians in a human-dominated landscape: The community structure is related to habitat features and isolation. Biological Conservation 119(2):219–230. DOI: 10.1016/j.biocon.2003.11.004.
- Futuyma DJ, Moreno G. 1988. The evolution of ecological specialization. Annual Review of Ecology and Systematics 19(20):207–233. DOI: 10.1146/annurev.es.19.110188.001231.
- Gasc JP, Cabela A, Crnobrnja-Isailovic J, Dolmen D, Grossenbacher K, Haffner P, Lescure J, Martens H, Martinez Rica JP, Maurin H, Oliveira ME, Sofianidou TS, Veith M, Zuiderwijk A. 2004. Atlas of amphibians and reptiles in Europe. Patrimoines naturels 29. Paris: Muséum National d’Histoire Naturelle.
- Gaston KJ, editor. 2010. Urban ecology. (Ecological Reviews). Cambridge: Cambridge University Press.
- Gibbs JP, Whiteleather KK, Schueler FW. 2005. Changes in frog and, toad populations over 30 years in New York State. Ecological Applications 15(4):1148–1157. DOI: 10.1890/03-5408.
- Gledhill, DG, James, P, and Davies, DH 2008. Pond density as a determinant of aquatic species richness in an urban landscape. Landscape Ecology 23(10):1219–1230. DOI:10.1007/s10980-008-9292-x.
- Gotkiewicz W, Wittbrodt K, Dragańska E. 2020. The dynamics of changes in the amphibian (Amphibia) population size in the masurian landscape park monitoring results of Spring migration monitoring from the years 2011–2019. Environmental Protection and Natural Resources 31(4):8–16. DOI: 10.2478/oszn-2020-0013.
- Green DM. 2003. The ecology of extinction: Population fluctuation and decline in amphibians. Biological Conservation 111(3):331–343. DOI: 10.1016/S0006-3207(02)00302-6.
- Griffiths RA, Sewell D, McCrea RS. 2010. Dynamics of a declining amphibian metapopulation: Survival, dispersal and the impact of climate. Biological Conservation 143(2):485–491. DOI: 10.1016/j.biocon.2009.11.017.
- Guerry AD, Hunter ML. 2002. Amphibian distributions in a landscape of forests and agriculture: An examination of habitat composition and configuration. Conservation Biology 16(3):745–754. DOI: 10.1046/j.1523-1739.2002.00557.x.
- Hagen EO, Hagen O, Ibáñez-Álamo JD, Petchey OL, Evans KL. 2017. Impacts of urban areas and their characteristics on avian functional diversity. Frontiers in Ecology and Evolution 5(84):1–16. DOI: 10.3389/fevo.2017.00084.
- Hamer AJ, McDonnell MJ. 2008. Amphibian ecology and conservation in the urbanising world: A review. Biological Conservation 141(10):2432–2449. DOI: 10.1016/j.biocon.2008.07.020.
- Hanski I. 1989. Metapopulation dynamics: Does it help to have more of the same? Trends in Ecology & Evolution 4(4):17–38. DOI: 10.1016/0169-5347(89)90061-X.
- Hanski I. 1991. Single-species metapopulation dynamics: Concepts, models and observations. Biological Journal of the Linnean Society 42(1–2):17–38. DOI: 10.1111/j.1095-8312.1991.tb00549.x.
- Hanski I, Pakkala T, Kuussaari M, Lei G. 1995. Metapopulation persistence of an endangered butterfly in a fragmented landscape. Oikos 72(1):21–28. DOI: 10.2307/3546033.
- Hanski I. 1999. Metapopulation ecology. New York: Oxford University Press.
- Hill MJ, Biggs J, Thornhill I, Briers RA, Gledhill DG, White JC, Wood PJ, Hassall C. 2017. Urban ponds as an aquatic biodiversity resource in modified landscapes. Global Change Biology 23(3):986–999. DOI: 10.1111/gcb.13401.
- Houlahan JE, Keddy PA, Makkay K, Findlay CS. 2006. The effects of adjacent land use on wetland species richness and community composition. Wetlands 26(1):79–96. DOI: 10.1672/0277-5212(2006)26[79:TEOALU]2.0.CO;2.
- Hutcheson K. 1970. A test for comparing diversities based on the Shannon formula. Journal of Theoretical Biology 29(1):151–154. DOI: 10.1016/0022-5193(70)90124-4.
- IBM. 2020. IBM manuals. IBM® SPSS® statistics algorithms. Armonk, NY: IBM Corporation.
- Jaccard P. 1912. The distribution of the flora of the Alpine zone. New Phytologist 11(2):37–50. DOI: 10.1111/j.1469-8137.1912.tb05611.x.
- Juszczyk W. 1987. Płazy i gady krajowe. Vol. 2. Warszawa: PWN. [ in Polish].
- Kaiser HF. 1958. The varimax criterion for analytic rotation in factor analysis. Psychometrika 23(3):187–200. DOI: 10.1007/BF02289233.
- Kenward MG, Roger JH. 1997. Small sample inference for fixed effects estimator from restricted maximum likelihood. Biometrics 53(3):983–997. DOI: 10.2307/2533558.
- Knutson M, Sauer J, Olsen D, Mossman M, Hemesath L, Lannoo M. 1999. Effects of Landscape Composition and Wetland Fragmentation on Frog and Toad Abundance and Species Richness in Iowa and Wisconsin, U.S.A. Conservation Biology 13(6):1437–1446. DOI: 10.1046/j.1523-1739.1999.98445.x.
- Koleff P, Gaston KJ, Lennon JJ. 2003. Measuring beta diversity for presence-absence data. Journal of Animal Ecology 72(3):367–382. DOI: 10.1046/j.1365-2656.2003.00710.x.
- Kondracki J. 2002. Regional geography of Poland. Warszawa: Wydawnictwo Naukowe PWN.
- Konowalik A, Najbar A, Konowalik K, Dylewski Ł, Frydlewicz M, Kisiel P, Starzecka A, Zaleśna A, Kolenda K. 2020. Amphibians in an urban environment: A case study from a central European city (Wrocław, Poland). Urban Ecosystems 23(2):235–243. DOI: 10.1007/s11252-019-00912-3.
- Kovar R, Brabec M, Bocek R, Vita R. 2009. Spring migration distances of some Central European amphibian species. Amphibia-Reptilia 30(3):367–378. DOI: 10.1163/156853809788795236.
- Kruger DJD, Andrew J, Hamer AJ, Du Preez LH. 2015. Urbanization affects frog communities at multiple scales in a rapidly developing African city. Urban Ecosystems 18(4):1333–1352. DOI: 10.1007/s11252-015-0443-y.
- Kuhn I, Klotz S. 2006. Urbanization and homogenization – Comparing the floras of urban and rural areas in Germany. Biological Conservation 127(3):292–300. DOI: 10.1016/j.biocon.2005.06.033.
- Kupfer A. 1998. Wanderstrecken einzelner Kammolche (Triturus cristatus) in einem Agrarlebensraum. Zeitschrift Für Feldherpetologie 5:238–242.
- Kwet A. 2009. New Holland European reptile and amphibian guide. London: New Holland Publishers.
- La Sorte FA, Lepczyk CA, Aronson MFJ, Goddard MA, Hedblom M, Katti M, MacGregor‐Fors I, Mörtberg U, Nilon CH, Warren PS, Williams NSG, Yang J. 2018. The phylogenetic and functional diversity of regional breeding bird assemblages is reduced and constricted through urbanization. Diversity & Distributions 24(7):928–938. DOI: 10.1111/ddi.12738.
- Laan R, Verboom B. 1990. Effects of pool size and isolation on amphibian communities. Biological Conservation 54(3):51–262. DOI: 10.1016/0006-3207(90)90055-T.
- Lehtinen RM, Galatowitsch SM, Tester JR. 1999. Consequences of habitat loss and fragmentation for wetland amphibian assemblages. Wetlands 19(1):1–12. DOI: 10.1007/BF03161728.
- Lennon JJ, Koleff P, Greenwood JJD, Gaston KJ. 2001. The geographical structure of British bird distributions: Diversity, spatial turnover and scale. Journal of Animal Ecology 70(6):966–979. DOI: 10.1046/j.0021-8790.2001.00563.x.
- Levins R. 1969. Some genetic and demographic consequences of environmental heterogeneity for biological control. Bulletin of the Entomological Society of America 15(3):237–240. DOI: 10.1093/besa/15.3.237.
- Löfvenhaft K, Runborg S, Sjögren-Gulve P. 2004. Biotope patterns and amphibian distribution as assessment tools in urban landscape planning. Landscape and Urban Planning 68(4):403–427. DOI: 10.1016/S0169-2046(03)00154-3.
- Lomolino MV. 2000. Ecology’s most general, yet protean 1 pattern: The species-area relationship. Journal of Biogeography 27(1):17–26. DOI: 10.1046/j.1365-2699.2000.00377.x.
- Luck G, Smallbone L. 2011. The impact of urbanization on taxonomic and functional similarity among bird communities. Journal of Biogeography 38(5):894–906. DOI: 10.1111/j.1365-2699.2010.02449.x.
- Magurran A. 1996. Ecological diversity and its measurement. Cambridge: Chapman & Hall
- Makomaska-Juchiewicz M, Baran P. 2012. Monitoring of animal species. Methodological guide. Part three. Warszawa: GIOŚ.
- Manley PN, Van Horne B, Roth JK, Zielinski WJ, McKenzie MM, Weller TJ, Weckerly FW, Vojta C. 2006. Multiple species inventory and monitoring technical guide. Gen. Tech. Rep. WO-73. Washington, DC: U.S. Department of Agriculture, Forest Service. p. 204.
- Manly BFJ. 1986. Multivariate statistical methods. A primer. London: Chapman and Hall.
- Marzluff JM. 2001. Worldwide urbanization and its effects on birds. In: Marzluff JM, Bowman R, Donnelly R, editors. Avian ecology and conservation in an urbanizing world. Kluwer. pp. 19–47. DOI: 10.1007/978-1-4615-1531-9_2.
- Mazerolle MJ, Desrochers A, Rochefort L. 2005. Landscape characteristics influence pond occupancy by frogs after accounting for detectability. Ecological Applications 15(3):824–834. DOI: 10.1890/04-0502.
- Mazgajska J. 1996. Distribution of amphibians in urban water bodies (Warsaw agglomeration, Poland). Ekologia Polska 44:245–257.
- Mazgajska J, Mazgajski TD. 2020. Two amphibian species in the urban environment: Changes in the occurrence, spawning phenology and adult condition of common and green toads. The European Zoological Journal 87(1):170–179. DOI: 10.1080/24750263.2020.1744743.
- McCleery RA. 2010. Urban mammals. In: Aitkenhead-Peterson J, Volder A, editors. Urban ecosystem ecology. American Society of Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America, Inc. DOI: 10.2134/agronmonogr55.c5
- McCulloch CE, Searle SR. 2001. Generalized, linear, and mixed models. New York: John Wiley and Sons.
- McKinney ML, Lockwood JL. 1999. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends in Ecology & Evolution 14(11):450–453. DOI: 10.1016/S0169-5347(99)01679-1.
- McKinney ML. 2002. Urbanization, biodiversity, and conservation. BioScience 52(10):883–890. DOI: 10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2.
- McKinney ML. 2006. Urbanization as a major cause of biotic homogenization. Biological Conservation 127(3):247–260. DOI: 10.1016/j.biocon.2005.09.005.
- Merckx T, Van Dyck H. 2019. Urbanization-driven homogenization is more pronounced and happens at wider spatial scales in nocturnal and mobile flying insects. Global Ecology and Biogeography 28(10):1440–1455. DOI: 10.1111/geb.12969.
- Minton SA Jr. 1968. The fate of amphibians and reptiles in a suburban area. Journal of Herpetology 2(3/4):113–116. DOI: 10.2307/1563109.
- Morelli F, Benedetti Y, Ibáñez-Álamo JD, Jokimäki J, Mänd R, Tryjanowski P, Møller AP. 2016. Evidence of evolutionary homogenization of bird communities in urban environments across Europe. Global Ecology and Biogeography 25(11):1284–1293. DOI: 10.1111/geb.12486.
- Morin PJ, Johnson EA. 1988. Experimental studies of asymmetric competition among anurans. Oikos 53(3):398–407. DOI: 10.2307/3565542.
- Motta-Tavares T, Rocha C, Anjos L. 2020. The influence of matrix type in the relationship between patch size and amphibia richness: A global meta-analysis. Acta Oecologica 105:103577. DOI: 10.1016/j.actao.2020.103577.
- Nowakowski JJ. 1996. Changes in the breeding avifauna of Olsztyn (NE Poland) in the years 1968-1993. Acta Ornithologica 31(1):39–44.
- Nowakowski JJ, Dulisz B, Lewandowski K. 2006. Birds of Olsztyn city. Olsztyn: Elset.
- Nowakowski JJ, Górski A, Lewandowski K, Dulisz B. 2008. Płazy i gady Olsztyna [Amphibian and reptiles of Olsztyn city]. In: Indykiewicz P, Jerzak L, and Barczak T, editors. Fauna Miast – Ochronić różnorodność biotyczną w miastach. SAR „Pomorze”, Bydgoszcz. pp. 151–167.
- Nowakowski JJ, Górski A, Lewandowski K. 2010. Amphibian communities in small water bodies in the city of Olsztyn. Fragmenta Faunistica 53(2):213–231. DOI: 10.3161/00159301FF2010.53.2.213.
- Nowakowski JJ, Górski A, Lewandowski K, Dulisz B. 2011. Habitat selection of amphibians in water bodies in Olsztyn city. In: Indykiewicz P, Jerzak L, Böhner J, and Kavanagh B, editors. Studies of animal biology, ecology and conservation in European cities. Bydgoszcz: UTP. pp. 333–344.
- Oertli B, Joye DA, Castella E, Juge R, Cambin D, Lachavanne J. 2002. Does size matter? The relationship between pond area and biodiversity. Biological Conservation 104(1):59–70. DOI: 10.1016/S0006-3207(01)00154-9.
- Ogielska M, Kierzkowski P. 2010. Long term data on the amphibians of Wrocław. Fragmenta Faunistica 53(2):195–212. DOI: 10.3161/00159301FF2010.53.2.195.
- Ożgo M. 2010. The role of small water bodies in the protection of biodiversity. National Parks and Nature Reserves 29(3):117–124. DOI: 10.1007/s10750-016-3007-0.
- Palacio FX, Ibañez LM, Maragliano RE, Montalti D. 2018. Urbanization as a driver of taxonomic, functional, and phylogenetic diversity losses in bird communities. Canadian Journal of Zoology 96(10):1114–1121. DOI: 10.1139/cjz-2018-0008.
- Parris MJ, Semlitsch RD. 1998. Asymmetric competition in larval amphibian communities: Conservation implications for the northern crawfish frog, Rana areolata circulosa. Oecologia 116(1–2):219–226. DOI: 10.1007/PL00013822.
- Parris KM. 2006. Urban amphibian assemblages as metacommunities. Journal of Animal Ecology 75(3):757–764. DOI: 10.1111/j.1365-2656.2006.01096.x.
- Pretelli MG, Isacch JP, Cardoni DA. 2018. Species-area relationships of specialist versus opportunistic pampas grassland birds depend on the surrounding landscape matrix. Ardeola 65(1):3–23. DOI: 10.13157/arla.65.1.2018.ra1.
- Renkonen O. 1938. Statisch-ökologische Untersuchungen über die terrestrische Käferwelt der finnischen Bruchmoore. Annales Botanici Societatis Zoologicae Botanicae Fennicae 6:1–231.
- Rittenhouse TAG, Semlitsch RD. 2007. Distribution of amphibians in terrestrial habitat surrounding wetlands. Wetlands 27(1):153–161. DOI: 10.1672/0277-5212(2007)27[153:DOAITH]2.0.CO;2.
- Rosenbrock HH. 1960. An automatic method for finding the greatest or least value of a function. The Computer Journal 3(3):175–184. DOI: 10.1093/comjnl/3.3.175.
- Rosenzweig ML. 1995. Species diversity in space and time. Cambridge: Cambridge University Press.
- Roy AH, Rosemond AD, Paul MJ, Leigh DS, Wallace JB. 2003. Stream macroinvertebrate response to catchment urbanisation (Georgia, U.S.A.). Freshwater Biology 48(2):329–346. DOI: 10.1046/j.1365-2427.2003.00979.x.
- Rubbo MJ, Kiesecker JM. 2005. Amphibian breeding distribution in an urbanized landscape. Conservation Biology 19(2):504–511. DOI: 10.1111/j.1523-1739.2005.000101.x.
- Rybicki J, Hanski I. 2013. Species-area relationships and extinctions caused by habitat loss and fragmentation. Ecology Letters 16:27–38. DOI: 10.1111/ele.12065.
- Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH. 2000. Biodiversity: Global biodiversity scenarios for the year 2100. Science 270:1770–1774. DOI: 10.1126/science.287.5459.1770.
- Scheffers B, Paszkowski C. 2011. The effects of urbanization on North American amphibian species: Identifying new directions for urban conservation. Urban Ecosystems 15(1):133–147. DOI: 10.1007/s11252-011-0199-y.
- Scheffers B, Paszkowski C. 2013. Amphibian use of urban stormwater wetlands: The role of natural habitat features. Landscape and Urban Planning 113:139–149. DOI: 10.1016/j.landurbplan.2013.01.001.
- Semenov DV, Leontyeva OA, Pavlinov IJ. 2000. Analysis of the environmental determinants of the amphibian (Vertebrata: Amphibia) distribution on the urbanized territories in Moscow City. Bulletin of Moscow Society of Naturalists. Biological Series 105(2):3–9.
- Semlitsch RD, Bodie JR. 2003. Biological criteria for buffer zones around wetlands and riparian habitats for amphibians and reptiles. Conservation Biology 17(5):1219–1228. DOI: 10.1046/j.1523-1739.2003.02177.x.
- Semlitsch RD, Skelly D. 2007. Ecology and conservation of pool-breeding amphibians. In: Calhoun AJK, and De Maynadier PG, editors. Science and conservation of vernal pools in Northeastern North America: Ecology and conservation of seasonal wetlands in Northeastern North America. Boca Raton, FL: CRC Press. pp. 127–148.
- Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D. 2006. From patterns to emerging processes in mechanistic urban ecology. Trends in Ecology & Evolution 21(4):186–191. DOI: 10.1016/j.tree.2005.11.019.
- Shochat E, Lerman SB, Anderies JM, Warren PS, Faeth SH, Nilon CH. 2010. Invasion, competition, and biodiversity loss in urban ecosystems. Bioscience 60(3):199–208. DOI: 10.1525/bio.2010.60.3.6.
- Sievers M, Hale R, Parris KM. 2019. Frog occupancy of polluted wetlands in urban landscapes. Conservation Biology 33(2):389–402. DOI: 10.1111/cobi.13210.
- Sillero N, Campos J, Bonardi A, Corti C, Creemers R, Crochet PA, Isailović JC, Denoël M, Ficetola GF, Gonçalves J, Kuzmin S. 2014. Updated distribution and biogeography of amphibians and reptiles of Europe. Amphibia-Reptilia 35(1):1–31. DOI: 10.1163/15685381-00002935.
- Silvano DL, Segalla MV. 2005. Conservation of Brazilian Amphibians. Conservation Biology 19(3):653–658. DOI: 10.1111/j.1523-1739.2005.00681.x.
- Simon JA, Snodgrass JW, Casey RE, Sparling DW. 2009. Spatial correlates of amphibian use of constructed wetlands in an urban landscape. Landscape Ecology 24(3):361–373. DOI: 10.1007/s10980-008-9311-y.
- Simpson GG. 1943. Mammals and the nature of continents. American Journal of Science 241(1):1–31. DOI: 10.2475/ajs.241.1.1.
- Sinsch U. 1988. Temporal spacing of breeding activity in the natterjack toad, Bufo calamita. Oecologia 76(3):399–407. DOI: 10.1007/BF00377035.
- Sinsch U. 1992. Structure and dynamic of a natterjack toad metapopulation (Bufo calamita). Oecologia 90(4):489–499. DOI: 10.1007/BF01875442.
- Sinsch U, Seidel D. 1995. Dynamics of local and temporal breeding assemblages in a Bufo calamita metapopulation. Australian Journal of Ecology 20:351–361. DOI: 10.1111/J.1442-9993.1995.TB00550.X.
- Sinsch U. 1997. Postmetamorphic dispersal and recruitment of first breeders in a Bufo calamita metapopulation. Oecologia 112(1):42–47. DOI: 10.1007/s004420050281.
- Sjögren P. 1991. Extinction and isolation gradients in metapopulations: The case of the pool frog (Rana lessonae). Biological Journal of the Linnean Society 42(1–2):135–147. DOI: 10.1111/j.1095-8312.1991.tb00556.x.
- Sjögren-Gulve P. 1994. Distribution and extinction patterns within a northern metapopulation of the pool frog Rana lessonae Ecology 75:1357–1367.
- Skei JK, Dolmen D, Rønning L, Ringsby TH. 2006. Habitat use during the aquatic phase of the newts Triturus vulgaris (L.) and T. cristatus (Laurenti) in central Norway: Proposition for a conservation and monitoring area. Amphibia-Reptilia 27:309–324.
- Skelly DK, Werner EE, Cortwright SA. 1999. Long-term distributional dynamics of a Michigan amphibian assemblage. Ecology 80(7):2326–2337. DOI: 10.1890/0012-9658(1999)080[2326:LTDDOA]2.0.CO;2.
- Śledź D. 2012. Report on the state of the city of Olsztyn for the years 2010, 2011. Olsztyn: Institute of Research and Analysis, Olsztyn School of Business
- Smart SM, Thompson K, Marrs RH, Le Duc MG, Maskell LC, Firbank LG. 2006. Biotic homogenization and changes in species diversity across human-modified ecosystems. Philosophical Transactions of the Royal Society B: Biological Sciences 273:2659–2665. DOI: 10.1098/rspb.2006.3630.
- Smith MA, Green DM. 2005. Dispersal and the metapopulation paradigm in amphibian ecology and conservation: Are all amphibian populations metapopulations? Ecography 28(1):110–128. DOI: 10.1111/j.0906-7590.2005.04042.x.
- Snodgrass JW, Casey RE, Joseph D, Simon JA. 2008. Microcosm investigations of stormwater pond sediment toxicity to embryonic and larval amphibians: Variation in sensitivity among species. Environmental Pollution 154(2):291–297. DOI: 10.1016/j.envpol.2007.10.003.
- Sokal RR, Rohlf FJ. 1994. Biometry: The principles and practices of statistics in biological research. 3rd ed. New York: W.H. Freeman and Company.
- Sorensen T. 1948. A method of establishing groups of equal amplitudes in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish Commons. Kongelige Danske Videnskabernes Selskab, Biologiske Skrifter 5(4):1–34.
- Stewart RIA, Andersson GKS, Brönmark C, Klatt BK, Hansson L-A, Zülsdorff V, Smith HG. 2017. Ecosystem services across the aquatic–terrestrial boundary: Linking ponds to pollination. Basic and Applied Ecology 18:13–20. DOI: 10.1016/j.baae.2016.09.006.
- Strijbosch H. 1979. Habitat selection of amphibians during their aquatic phase. Oikos 33(3):363–372. DOI: 10.2307/3544324.
- Thurman LL, Garcia TS. 2019. Asymmetric competition shapes amphibian response to rapid environmental change. Ecosphere 10(12):e02950. DOI: 10.1002/ecs2.2950.
- TIBCO Software Inc. 2017. Statistica (Data analysis software system), version 13. http://statistica.io.
- TIBCO Software Inc. 2020. Data science textbook. https://docs.tibco.com/data-science/textbook.
- Turner MG. 2005. Landscape ecology: What is the state of the science? Annual Review of Ecology, Evolution, and Systematics 36(1):319–344. DOI: 10.1146/annurev.ecolsys.36.102003.152614.
- Vad CF, Péntek AL, Cozma NJ, Földi A, Tóth A, Tóth B, Böde NA, Móra A, Ptacnik R, Ács É, Zsuga K, Horvath Z. 2017. Wartime scars or reservoirs of biodiversity? The value of bomb crater ponds in aquatic conservation. Biological Conservation 209:253–262. DOI: 10.1016/j.biocon.2017.02.025.
- Van Gelder JJ, Aarts HMJ, H-JWM S. 1986. Routes and speed of migrating toads (Bufo bufo L.): A telemetric study. Herpetological Journal 1:111–114.
- Van Tienderen PH. 1991. Evolution of generalists and specialist in spatially heterogeneous environments. Evolution 45(6):1317–1331. DOI: 10.1111/j.1558-5646.1991.tb02638.x.
- Vershinin VL, Vershinina SD, Berzin DL, Zmeeva DV, Kinev AV. 2015. Long-term observation of amphibian populations inhabiting urban and forested areas in Yekaterinburg, Russia. Scientific Data 2(1):150018. DOI: 10.1038/sdata.2015.18.
- Vos CC, Stumpel AHP. 1996. Comparison of habitat-isolation parameters in relation to fragmented distribution patterns in the tree frog (Hyla arborea). Landscape Ecology 11(4):203–214. DOI: 10.1007/BF02071811.
- Vos CC, Chardon JP. 1998. Effects of habitat fragmentation and road density on the distribution pattern of the moor frog Rana arvalis. Journal of Applied Ecology 35(1):44–56. DOI: 10.1046/j.1365-2664.1998.00284.x.
- Vos CC, Verboom J, Opdam PFM, Ter Braak CJF. 2001. Toward ecologically scaled landscape indices. The American Naturalist 157(1):24–41. DOI: 10.2307/3079086.
- Walsh CJ, Roy AH, Feminella JW, Cottingham PD. 2005. The urban stream syndrome: Current knowledge and the search for a cure. Journal of the North American Benthological Society 24:706–723.
- Werner EE, Yurewicz KL, Skelly DK, Relyea RA. 2007. Turnover in an amphibian metacommunity: The role of local and regional factors. Oikos 116(10):1713–1725. DOI: 10.1111/j.0030-1299.2007.16039.x.
- Whittaker RH. 1975. Communities and ecosystems. New York: Macmillan.

