Abstract
Diaphanosoma brachyurum is a dominant component of summer zooplankton in Central Europe. So far only this species has been known from Poland, and other species from this genus have been found in neighboring countries. The aim of the present study was to present the factual distribution of Diaphanosoma species in Central Europe. We investigated Diaphanosoma individuals in 109 water bodies in Central Europe (mostly from Poland) by combining morphological analyses and molecular methods (mtDNA COI gene). Our research confirmed the presence of three other species - D. orghidani, D. mongolianum, and D. cf. lacustris. We provided a detailed morphological description for every species and pointed out that each species can be easily distinguished morphologically by armament of postero-ventral valve margins. The results of phylogenetic analysis based on 27 haplotypes of mtDNA COI gene lead to the same conclusion as morphology. Our genetic and morphological analysis confirms a close affinity between D. mongolianum and D. lacustris, as well as the presence of hybrids between these species. Nevertheless, the most common was D. brachyurum - it was found in 97 water bodies, and other Diaphanosoma species begin to penetrate Central Europe via rivers.
Introduction
The genus Diaphanosoma is distributed worldwide, except in New Zealand and Antarctica (Korovchinsky Citation1992). This genus is one of the most important components of tropical–subtropical zooplankton (Han et al. Citation2011) and is sometimes called “tropical Daphnia” (Dumont Citation1994; Sarma et al. Citation2005). Diaphanosoma is also widely distributed in the temperate zone but as a thermophilous genus occurs mainly in summer (Błędzki & Rybak Citation2016). In northern Poland Diaphanosoma brachyurum was found in more than 80% of lakes (Sługocki & Czerniawski Citation2018; Karpowicz & Ejsmont-Karabin Citation2021). It often dominates the zooplankton of the warm epilimnion waters, contrary to Daphnia species that prefer lower water layers (Karpowicz & Ejsmont-Karabin Citation2021). Vertical niche separation with Daphnia may further promote the effective transfer of energy and matter in temperate lakes (Karpowicz et al. Citation2021a) because Diaphanosoma species are highly efficient bacterivores and contribute to the linkage of the classical food web with the microbial loop (Geller & Müller Citation1981).
Diaphanosoma with more than 40 species described is the most diversified group of the Ctenopoda (Korovchinsky Citation2018), and one of the cladocerans which received a lot of attention in the literature (Błędzki & Rybak Citation2016). In Europe, Diaphanosoma is represented by five species, among which the most common is D. brachyurum. Previously this species was thought to be found everywhere in temperate regions due to inadequate knowledge of the morphology of the Diaphanosoma genus (Korovchinsky Citation1987). Therefore D. brachyurum had been traditionally recorded in many lakes, but last research indicated that it cannot be located in some of those regions anymore (Lakatos et al. Citation2015). The study from Japan did not confirm the presence of D. brachyurum, and reported the occurrence of D. cf. amurensis, D. cf. dubium, D. cf. orientalis, and D. cf. macrophthalma (Lakatos et al. Citation2015). Similarly in Greece where D. brachyurum was not found, D. mongolianum, D. orghidani, and D. macedonicum were instead recorded (Alexiou et al. Citation2021).
So far only one species has been known from Poland - D. brachyurum, but other species have been found in the neighboring countries. Diaphanosoma orghidani was recorded in all neighboring countries (German, Czech, Slovakia, Ukraine, Belarus, Lithuania, and Russia) (Błędzki & Rybak Citation2016). Diaphanosoma lacustris was recorded in Czech and Slovakia, while D. mongolianum is known from Germany, Slovakia (Flößner Citation2000; Hudec Citation2010; Błędzki & Rybak Citation2016), and the Russian part of the Vistula Lagoon (Semenova & Tchougounov Citation2018). Nevertheless, the most frequently observed species in Central Europe is D. brachyurum, and other species seem to increase their range. Generally, the range of D. mongolianum is more southern, from the central and southern parts of the Palearctic to the northeast of China, and in Africa, this species was found even in the equatorial zone (Korovchinsky Citation1992). The main range of D. orghidani is south of the taiga belt, but this species recently dwells in Central and Southern Europe, Central Asia, Turkey, and Iran as well as in the south of Russia (Korovchinsky Citation1992; Lazareva & Bolotov Citation2013). Therefore, a well-established view of the presence of only one species together with inadequate knowledge of the morphology of this genus suggests that the factual distribution of Diaphanosoma species in Central Europe is not fully known.
Diaphanosoma is characterized by a relatively conservative gross morphology and the morphological diagnostic characteristics of each species are rather subtle and minute, which may cause misidentification (Dumont et al. Citation2021; Alexiou et al. Citation2021). The most informative morphological feature is the armament of postero-ventral valve margins (Korovchinsky Citation1992) which can be overlooked. Accurate morphological descriptions and identifications are cornerstone of taxonomy and genetic studies. Research that combines morphological analyses and molecular methods is thus needed to describe species and define them clearly in groups (Forró et al. Citation2008). Diaphanosoma is one of the most molecular analyzed genera of Cladocera (after Daphnia), however available genetic information about this genus in Europe is limited (Alexiou et al. Citation2021). Most deposited sequences are related to the mtDNA COI gene, which is widely used in the identification of species because it appears to be one of the most conserved genes encoding proteins in the mitochondrial animal genome (Brown Citation1985; Geller et al. Citation2013). Many research confirms that this gene is an excellent tool for the identification of Diaphanosoma species, and phylogenetic analysis leads to the same conclusion as morphology (Lakatos et al. Citation2015; Dumont et al. Citation2021; Alexiou et al. Citation2021).
The main aim of the study is to present the real distribution of Diaphanosoma species in Central Europe. For this reason, we have investigated Diaphanosoma individuals in 109 water bodies (lakes, rivers, dam reservoirs, ponds, brackish habitats) in Central Europe. We provided a detailed morphological description for each recorded species and performed a phylogenetic analysis based on sequences of the mtDNA COI gene. The combination of traditional taxonomy with genetics is particularly important in recent years when the level of taxonomic identification in developed countries has been dramatically reduced due to the well-known taxonomy crisis (Agnarsson & Kuntner Citation2007).
Table I. The list of studied water bodies (lakes, ponds, reservoirs, rivers, brackish habitats) in Central Europe with basic morphometric and trophic characteristics. The trophic state was calculated based on Secchi disc visibility (hypertrophic <1 m; eutrophic 1–3 m; mesotrophic 3–5 m; oligotrophic >5 m). The number of lakes (no.) corresponds to the map with the distribution of three Diaphanosoma species in Central Europe (). Species: B - D. brachyurum, O - D. orghidani, M - D. mongolianum.
Study sites and methods
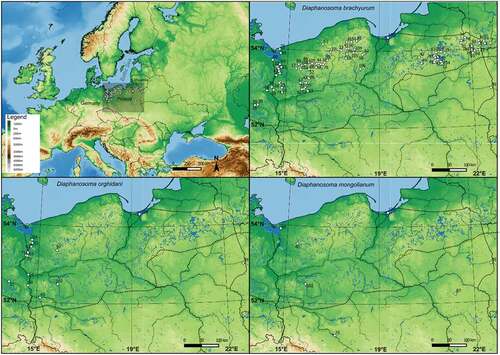
The research was conducted in 109 water bodies in Central Europe with the presence of Diaphanosoma species (). We collected samples from different types of water bodies, among which the most numerous were natural lakes (89). The lakes represent a wide trophic range from hypertrophic to oligotrophic, and dystrophic lakes (). We also sampled 10 stations along the Oder River, 3 ponds, 2 reservoirs, and 3 brackish habitats on Vistula Lagoon and Szczecin Lagoon (). Most of the studied water bodies were located in Poland (101), but we analyzed also three lakes from Germany and three from Lithuania (). The natural lakes were located within the range of the last glaciation (). Siemianówka Reservoir is located on the upper Narew River and Siedlce reservoir on the tributary of Bug River.
Figure 1. Distribution of three Diaphanosoma species (D. brachyurum, D. orghidani, D. mongolianum) in Central Europe. The dotted line shows the last glacial maximum.
The zooplankton samples were collected between July and August 2013–2021. The sampling stations in lakes were located close to the deepest point and the samples were collected by bucket or 5-L Limnos or by vertical net hauls. The water was filtered through a 50 µm plankton net and fixed with alcohol. During the sampling, we measured Secchi disc visibility (SDV) to determine trophic status. The water bodies with visibility below 1 m were classified as hypertrophic, 1–3 m as eutrophic, 3–5 m as mesotrophic, and above 5 m as oligotrophic.
We examined morphologically at least 20 individuals of Diaphanosoma from every sample (water body), but in some cases, it was even more than 100 individuals from one water body (e.g., Vistula Lagoon, Siemianówka Reservoir). The species identification was based on Korovchinsky (Citation1992), Hudec (Citation2010) and Błędzki & Rybak (Citation2016). We present the most important features to distinguishing Diaphanosoma species (armamentation of postero-ventral valve margin, body view, and postabdominal claw). Additionally, we measured the body size of Diaphanosoma species (D. brachyurum at least 10 individuals from 14 lakes with varied trophic status; D. mongolianum from 5 water bodies at least 10 individuals; D. orghidani from 4 water bodies at least 10 individuals) using an Olympus BX53 microscope with cellSens imaging software. The differences in body sizes of Diaphanosoma species were presented as box plots with basic statistical descriptions (). One-way ANOVA was followed by Tukey’s HSD (honestly significantly different) to test all pairwise differences between means, which were marked on the box plots. Statistical analyses were performed with XLSTAT Ecology (Addinsoft, New York, USA). Photographic documentation was made for each individual for genetic analysis and it is available at BOLDSystem (private project: CLAEU). Diaphanosoma individuals for genetic analysis were selected and stored in 70% ethanol at 4°C before DNA extraction. The map of the studied water bodies in Central Europe with Diaphanosoma species was created in QGIS software (version 2.18.24; www.qgis.org).
Total genomic DNA was extracted from 40 Diaphanosoma spp. individuals using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s procedure. PCR amplification of the mitochondrial cytochrome c oxidase subunit I (COI) was performed using primers jgLCO1490 and jgHCO2198 designed for marine invertebrates by Geller et al. (Citation2013). PCR reactions were performed with ~25 ng genomic DNA, 1.7 μL Qiagen multiplex PCR Master Mix (1x), 0.3 μL mix of species-specific primers, and 1 μL Qiagen RNase-free water in a 5 μL reaction volume (Qiagen, Hilden, Germany). The PCR amplification of the COI gene fragments was performed in a Labcycler Gradient (SensoQuest, Goettingen, Germany) with the following profile: initial denaturation at 95°C for 15 min, followed by 40 cycles consisting of denaturation at 94°C for 30s, annealing at 48°C for 90s, extension at 72°C for 60s, and final extension step at 60°C for 30 min. Amplified PCR products of the mtDNA COI gene were purified with the EPPiC fast enzymatic reaction following the manufacturer’s protocol (A&A Biotechnology, Gdynia, Poland) and then sequenced with the BigDye™ Terminator Cycle Sequencing Ready Reaction Kit v 3.1 (Applied Biosystems Foster City, CA, USA) using the jgLCO1490 amplification primer. The cycling protocol for the sequencing reaction was as follows: 25 cycles consisting of 95°C for 20s, 50°C for 15s and 60°C for 1 min. Unincorporated dideoxynucleotides were eliminated from the sequencing reaction using the ExTerminator Kit (A&A Biotechnology). The sequencing products were then run on an automated capillary sequencer ABI 3130 (Applied Biosystems).
The resulting of 537-bp-long sequences of the mtDNA COI gene were manually revised and aligned using the sequence-editing program, BioEdit, version 7.0.5.3 (Hall Citation1999). The sequences of the mtDNA COI haplotypes were submitted to GenBank. We calculated the haplotype diversity (h), nucleotide diversity (π, in percent), number of segregating sites, and mean number of pairwise differences (PD) for COI marker for each Diaphanosoma studied species using the software packages ARLEQUIN v3.5.1.2 (Excoffier & Lischer Citation2010).
To test the phylogenetic relationships among the obtained haplotypes of the mtDNA COI gene and sequences downloaded from GenBank (Supplementary Information, Table S1), we constructed phylogenetic trees using a maximum-likelihood (ML) algorithm in Mega v6.06 (Tamura et al. Citation2013) with 1,000 bootstrap replicates to assess the tree node support. In the phylogenetic analyses, we used a nucleotide substitution model determined under the Akaike information criterion (Akaike Citation1973) implemented in jModelTest v0.1.1 (Posada Citation2008). The GTR+I+G model was selected as the best-fitting model for the mtDNA COI gene sequences.
Results
Based on the morphology we identified three species: Diaphanosoma brachyurum (Liévin, 1848), Diaphanosoma orghidani Negrea, 1982, and Diaphanosoma mongolianum Ueno, 1938 (). However, genetic results indicated that one individual was closely related to Diaphanosoma lacustris Kořínek, 1981 (). It had intermediate features between D. mongolianum and D. lacustris.
Figure 2. General view of the body and postabdominal claws Diaphanosoma brachyurum (a, b), D. mongolianum (c, d), and D. orghidani (e, f). Scale bars are 100 µm.
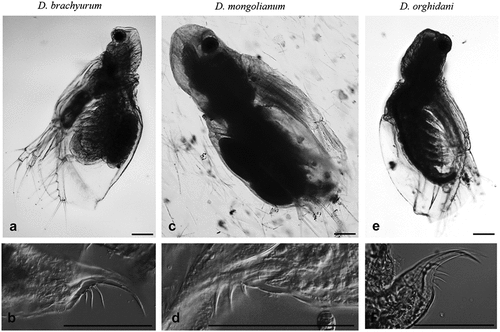
Figure 3. Armament of postero-ventral valve margins of D. brachyurum (a – Boczne; b – Kierzlińskie; c - Buwełno; d – Siemianówka), D. mongolianum (e, f - Siemianówka), and D. orghidani (g – Siemianówka; h - Oder River-warm canal). Scale bars are 50 µm.
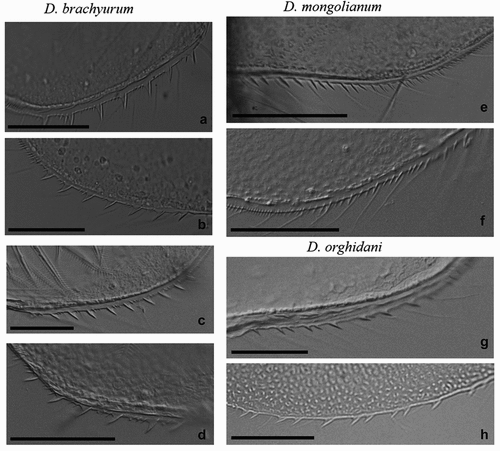
The most common was D. brachyurum and it was found in 97 water bodies ( and ). This species most often occurred in natural lakes with wide trophic gradients (90 lakes), but it was also found in three ponds, one reservoir (Siemianówka), one river station in Lower Oder, and two brackish habitats (Vistula Lagoon and Szczecin Lagoon) (). There were large differences in body size of D. brachyurum from 590 µm to 1120 µm with a mean size (± SD) of 838 ± 131 µm ()). There was a significant difference in body size of D. brachyurum found in different trophic conditions ()). Larger D. brachyurum occurred in mesotrophic and oligotrophic conditions and generally smaller body size in higher trophic conditions ()). However, in the eutrophic conditions, there was a large variety of body sizes ()). The head of D. brachyurum is generally small and rectangular ()) sometimes roundish. Basal spines of terminal postabdominal claw are large with proximal spine a bit smaller ()). Armamentation of postero-ventral valve margin with 6–10 large, sharp denticles, often much wider at the base. Between these large denticles are 1 to 5 small denticles and each group of denticles is divided by a thin setula ()). However, there were differences in the number and shape of denticles between populations ()).
Figure 4. Body sizes of three Diaphanosoma species (a), and body sizes of D. brachyurum in different trophic conditions (b). The lower and upper limits of the boxes are the first and third quartiles, respectively. The crosses correspond to the means and the central horizontal bars are the medians. Points above or below the whiskers are the outliers and extreme values. The different letters (a, b) above the box plots denote significantly different values at p < 0.05; the same letters denote no statistically significant differences.
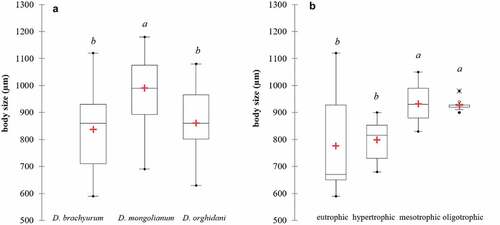
Diaphanosoma orghidani was found in 14 water bodies, half of them were on the Odra River - 7 stations from Krosno Odrzańskie to Szczecin (). It was also collected from four natural lakes, as well as from Siemianówka Reservoir, Vistula and Szczecin lagoons (). The body size of D. orghidani was similar to D. brachyurum with a mean size of 861 ± 109 µm ()). The head is quadrangular and small ()), but slightly larger than that of D. brachyurum. Basal spines of terminal postabdominal claw of D. orghidani are small and each of similar length ()). The postero-ventral valve margin has a row of 10–14 large and widely spaced denticles. This row is subdivided into poorly delineated groups of mostly 1–2 denticles, each divided by a thin setula ().
Diaphanosoma mongolianum was recorded in 7 water bodies: Vistula Lagoon, Szczecin Lagoon, Siemianówka Reservoir, Siedlce Reservoir, Oder River (Wrocław), Lake Kamienny Most, and Lake Wędromierz ( and ). D. mongolianum had a larger body size than the other two species ()), with a mean size of 990 ± 110 µm. The head is large and massive somewhat roundish with a projecting dorsal part ()). Basal spines of terminal postabdominal claw with a much smaller proximal spine ()). The posterior-ventral margin of the carapace valve with 20–28 small, thin and sharp denticles which are grouped into 2–4 denticles - a thin setule between these groups ().
However, many specimens of D. mongolianum have intermediate features between D. lacustris (especially smaller head and eye, basal spines of terminal postabdominal claw quite large with proximal spine a bit smaller). Nevertheless, the smaller number of denticles on postero-ventral valve margins (mostly about 20) rather characterized them as D. mongolianum. While one individual from Vistula Lagoon who was selected for genetic analysis (haplotype H26) resembled more D. lacustris due to the small head, slightly larger number of denticles on postero-ventral valve margins (about 25–28) (), but the postabdominal claws with a much smaller proximal spine resembled D. mongolianum (). This could indicate the existence of hybrids between these species.
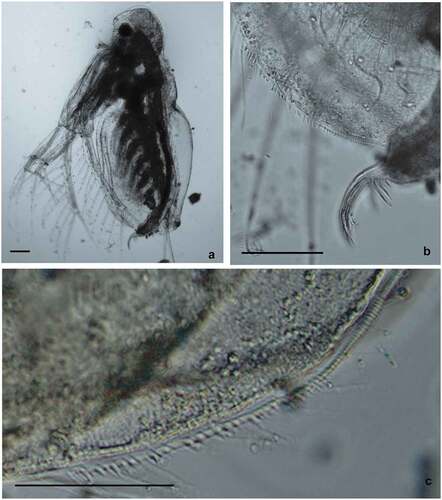
Figure 5. Diaphanosoma cf. lacustris from Vistula Lagoon. Specimen selected for the genetic analysis – haplotype H27. General view of the body (a), postabdominal claw (b), and armament of postero-ventral valve margins (c). Scale bars are 100 μm (a and b) and 50 μm (c).
The derived sequences of the mtDNA COI gene of Diaphanosoma species were submitted to the GenBank database. The maximum-likelihood phylogenetic reconstructions produced a strong topology (). Among 40 individuals of Diaphanosoma spp. we found 20 haplotypes of D. brachyurum (haplotypes H1 – H20; GenBank accession numbers: ON479214–ON479233), 5 haplotypes of D. mongolianum (H21 – H25; GenBank accession no.: ON479234–ON479238), 1 haplotype of D. lacustris (H26; GenBank accession no. ON479239), and also 1 haplotype of D. orghidani (H27; GenBank accession no. ON479240). 20 haplotypes of D. brachyurum described among 22 individuals were defined by 61 polymorphic sites, 46 transitions, and 15 transversions. The haplotype (h) and nucleotide diversity (π), as well as the pairwise difference (PD) values for the mtDNA COI gene of D. brachyurum were very high (1.00 ± 0.02, 1.77% ± 0.94%, and 9.49 ± 4.54, respectively). Our haplotypes created a phylogenetic branch together with haplotypes from Germany (GenBank accession no. EF189666, Richter et al. Citation2007), and haplotypes from Italy (H31 – H42 Supplementary Information, Table S1; ). Haplotype H11 showed 95.34% similarity with H29 and H30 found in China by Liu et al. (Citation2018; GenBank accession no. KY788790, KY788792, respectively).
Figure 6. Maximum-likelihood tree computed with the GTR+I+G model of sequence evolution, representing phylogenetic relationships among the sequences of mitochondrial cytochrome c oxidase subunit I (COI) of Diaphanosoma species found in our study: D. brachyurum (H1 – H20, marked with green color); D. mongolianum (H21 – H25, marked with blue color); D. lacustris (H26 marked with red color); D. orghidani (H27 marked with a purple color) and downloaded (H28 – H77) from GenBank. Detailed information in Supplementary Information – Table S1. Numbers listed at nodes represent percent support for that node from 1,000 bootstrap replicates.
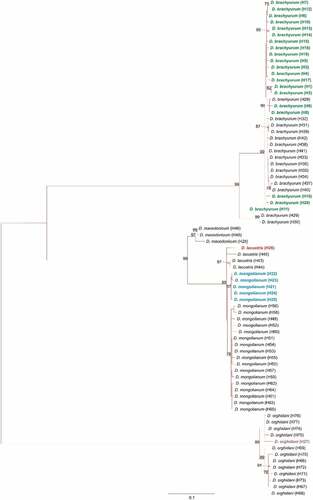
The phylogenetic lineage of D. mongolianum is represented in our study by 5 haplotypes found among 14 different individuals. These haplotypes differed by 4 substitutions, all being transitions and grouped together on the phylogenetic tree with haplotypes found by Alexiou et al. (Citation2021; haplotypes H49 – H65 Supplementary Information, Table S1; ). The haplotype (h) and nucleotide diversity (π), as well as the pairwise difference (PD) values for the mtDNA COI gene of D. mongolianum were high and medium (1.00 ± 0.13, 0.34% ± 0.27%, and 1.80 ± 1.24, respectively). The most common D. mongolianum haplotype 21 (H21) was found in 9 individuals (5 from Vistula Lagoon, 3 from Siemianówka Reservoir, and 1 from Odra River – Wrocław). This haplotype matched with BOLD System to D. mongolianum with 99.81% similarity. The haplotype H26 of D. lacustris found in one individual share 98.14% similarity with D. lacustris described by Liu et al. (Citation2018) in Hungary (GenBank accession no. KY788934, KY788936, KY788937; haplotypes H43 – H45 Supplementary Information, Table S1). BOLD System does not contain the species D. lacustris, and the nearest match was with D. mongolianum with 97.93%.
The haplotype H27 represents in our survey three different D. orghidani individuals grouped together with haplotypes described in Greece by Alexiou et al. (Citation2021; GenBank accession no. MW259011, MW259038, MW259042, MW259043; haplotypes H74 – H77 Supplementary Information, Table S1), showed with them at least 98.7% similarity ().
Discussion
So far only D. brachyurum was known from Poland (Błędzki & Rybak Citation2016), while our research confirmed the presence of three other species - D. orghidani, D. mongolianum, and D. cf. lacustris. Diaphanosoma brachyurum was previously considered cosmopolitan and most recorded worldwide due to inadequate knowledge of the morphology (Korovchinsky Citation2018). However last research confirms that it cannot be located in many of those regions anymore (Lakatos et al. Citation2015). In Europe D. brachyurum name has been erroneously used to refer to other species, such as D. orghidani, D. mongolianum, and D. lacustris (Korovchinsky Citation2018; Alexiou et al. Citation2021). Misidentification could be attributed to conservative gross morphology and diagnostic characters are rather subtle and minute (Lakatos et al. Citation2015; Alexiou et al. Citation2021). We showed that each species could be easily distinguishable morphologically by armament of postero-ventral valve margins (). The differences are subtle only in the case of two closely related species, D. mongolianum and D. lacustris which differ in inconspicuous morphological details (Korovchinsky Citation1987; Korovchinsky & Petkovski Citation2014). We found many specimens which have intermediate features between D. mongolianum and D. lacustris (number of denticles on postero-ventral valve margins, size and shape of head, and postabdomen). Similarly, intermediate features between both species were observed in East Africa by Korovchinsky et al. (Citation2017). These species are also genetically similar (Liu et al. Citation2018; Alexiou et al. Citation2021). Some molecular data suggest that D. mongolianum may be a hybrid between D. lacustris and D. dubium (Liu et al. Citation2018). Others suggest a close affinity between D. dubium and D. orghidani (Dumont et al. Citation2021). Our genetic and morphological analysis confirms a close affinity between D. mongolianum and D. lacustris. Nevertheless, further analysis of nuclear genes could be useful to resolve the taxonomic status of both species.
Furthermore, the molecular and morphological results of our study suggested the common presence of hybrids between D. mongolianum and D. lacustris. The occurrence of hybrids in Daphnia is a widespread phenomenon (Schwenk & Spaak Citation1995; Xu et al. Citation2013), especially in the parental species (Spaak & Hoekstra Citation1997; Hebert & Finston Citation2001; Keller & Spaak Citation2004; Wolinska et al. Citation2006). The intermediate morphology traits make also hard to distinguish cryptic species among Daphnia longispina group (Kotov Citation2015). The research on hybridization in Diaphanosoma performed by Liu et al. (Citation2018) provides surprising results, where hybridization was observed only between non-closely related species, and no hybridization events within species complexes. We found the possibility of hybrids between closely related D. mongolianum and D. lacustris, and we did not find any molecular or morphological evidence for hybridization between non-closely related species. The results of our study also show large molecular and morphological variability of D. brachyurum, but the morphological traits are stable and clear. Nevertheless, Diaphanosoma seems to need taxonomic revision to understand the actual level of species diversity in the genus (Liu et al. Citation2018; Dumont et al. Citation2021). Our results integrating morphological and genetic approaches provide some new insight into the taxonomic status of Diaphanosoma species in Central Europe. Finally, we confirm that the mtDNA COI gene is an excellent tool for the identification of Diaphanosoma species, and phylogenetic analysis leads to the same conclusion as morphology (Lakatos et al. Citation2015; Dumont et al. Citation2021; Alexiou et al. Citation2021). This gene is a widely-adopted taxonomic marker across the animal tree of life (Pentinsaari et al. Citation2016). However, the COI gene was insufficient to evaluate the taxonomic status of the genus Bythotrephes (Diplostraca: Cercopagididae) where the same haplotype gives different morphological traits (Karpowicz et al. Citation2021b).
The results of our study indicated that D. brachyurum was the most common in Central Europe. It occurred in 97 water bodies, especially in natural lakes. This is in accordance with the real geographical range of D. brachyurum s. str. which mainly covers Europe up to the Subarctic in the north, but it was also found in Central Asia and Siberia (Korovchinsky Citation2018). Our study suggests that D. brachyurum is tolerant of brackish water as well as different trophic conditions, from oligotrophic to hypertrophic and even in dystrophic lakes. Nevertheless, we have observed a large difference in body size related to trophic conditions, where bigger individuals were found in lower trophic conditions (oligotrophic and mesotrophic). This is consistent with our previous findings of other dominant cladocerans – Daphnia cucullata which grows larger in a better resource environment, and body size decreased in more eutrophic lakes (Karpowicz et al. Citation2020).
The presence of newly confirmed Diaphanosoma species in Poland was likely because they were found in neighboring countries, however, Central Europe is rather limit of their range. Generally, the range of D. mongolianum and D. lacustris is more southern, while the main range of D. orghidani is south of the taiga belt to Eastern Europe. Recently these species penetrate even equatorial African lakes along with the Nile system (Korovchinsky et al. Citation2017). Furthermore, Diaphanosoma orghidani is considered an invasive species in Europe (Lazareva & Bolotov Citation2013). Firstly, Korovchinsky characterizes this species as relatively rare (Korovchinsky Citation1986), but its abundance increases permanently, especially via large rivers. Diaphanosoma orghidani appearance and population growth were observed from the middle ’90s in the Danube River (Illyova & Nemethova Citation2005), Rhine River (Weiler Citation1997), Volga River (Lazareva & Bolotov Citation2013, Citation2014). In the reservoirs located on the Volga River D. orghidani prefers lotic habitats, while D. brachyurum prefers lentic parts of reservoirs (Lazareva & Bolotov Citation2013). The results of our study seem to confirm the preference of D. orghidani for large rivers because we observed it at 7 stations along the Oder River and in Siemianówka Reservoir on Narew River. We found this species only in four natural lakes (one in Germany and three in NW Poland), and in both studied brackish habitats (Vistula Lagoon and Szczecin Lagoon). Similarly, D. mongolianum was found in both studied brackish habitats, one station on the Oder River, two reservoirs, and one lake (). Thus D. brachyurum dominates in Polish lakes and other Diaphanosoma species begin to penetrate the area of Central Europe via rivers. However, Diaphanosoma species showed the largest population reduction in the river below the dam among all planktonic crustacean species (Napiórkowski et al. Citation2006; Karpowicz Citation2014, Citation2017).
Supplemental Material
Download MS Word (32.1 KB)Acknowledgements
We thank Piotr Rode for his help in preparing . We also thank Agnieszka Ochocka for sharing a few samples.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2022.2120106.
Additional information
Funding
References
- Agnarsson I, Kuntner M. 2007. Taxonomy in a changing world: Seeking solutions for a science in crisis. Systematics Biology 56:531–539. DOI: 10.1080/10635150701424546.
- Akaike H. 1973. Maximum likelihood identification of Gaussian autoregressive moving average models. Biometrika 60:255–265. DOI: 10.1093/biomet/60.2.255.
- Alexiou R, Stamou G, Minoudi S, Tourli F, Tsartsianidou V, Triantafyllidis A, Michaloudi E. 2021. The genus Diaphanosoma (Diplostraca: Sididae) in Greece: Morphological and molecular assessment. Zootaxa 5082:572–582. DOI: 10.11646/zootaxa.5082.6.4.
- Błędzki LA, Rybak JI. 2016. Freshwater Crustacean zooplankton of Europe. Switzerland: Springer Nature.
- Brown WM. 1985. The mitochondrial genome of animals. In: MacIntyre RJ, editor. Molecular evolutionary genetics. New York: Plenum. pp. 399–411.
- Dumont HJ. 1994. On the diversity of the Cladocera in the tropics. Hydrobiologia 272:27–38. DOI: 10.1007/BF00006510.
- Dumont HJ, Han BP, Guo F et al. 2021. Toward a phylogeny and biogeography of Diaphanosoma (Crustacea: Cladocera). Aquatic Ecology 55:1207–1222. DOI: 10.1007/s10452-020-09819-0.
- Excoffier L, Lischer HEL. 2010. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources 10:564–567. DOI: 10.1111/j.1755-0998.2010.02847.x.
- Flößner D. 2000. Die Haplopoda und Cladocera (ohne Bosminidae) Mitteleuropas. Leiden: Backhuys.
- Forró L, Korovchinsky NM, Kotov AA, Petrusek A. 2008. Global diversity of cladocerans (Cladocera; Crustacea) in freshwater. Hydrobiologia 595:177–184. DOI: 10.1007/s10750-007-9013-5.
- Geller J, Meyer C, Parker M, Hawk H. 2013. Redesign of PCR primers for mitochondrial cytochrome c oxidase subunit I for marine invertebrates and application in all-taxa biotic surveys. Molecular Ecology Resources 13:851–861. DOI: 10.1111/1755-0998.12138.
- Geller W, Müller H. 1981. The filtration apparatus of Cladocera: Filter mesh-sizes and their implications on food selectivity. Oecologia 49:316–321. DOI: 10.1007/BF00347591.
- Hall TA. 1999. BioEdit: A user‐friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41:95–98.
- Han B-P, Yin J, Lin X, Dumont HJ. 2011. Why is Diaphanosoma (Crustacea: Ctenopoda) so common in the tropics? Influence of temperature and food on the population parameters of Diaphnaosoma dubium and a hypothesis on the nature of tropical cladocerans. Hydrobiologia 668:109–115. DOI: 10.1007/s10750-010-0501-7.
- Hebert PDN, Finston TL. 2001. Macrogeographic patterns of breeding system diversity in the Daphnia pulex group from the United States and Mexico. Heredity 87:153–161. DOI: 10.1046/j.1365-2540.2001.00885.x.
- Hudec I. 2010. Fauna Slovenska III. Anomopoda, Ctenopoda, Haplopoda, Onychopoda (Crustacea: Branchiopoda). Vol III. Bratislava: VEDA, Vydavatel stvo Slovenskej adadémie vied.
- Illyova M, Nemethova D. 2005. Long-term changes in cladoceran assemblages in the Danube floodplain area (SlovakHungarian stretch). Limnologica 35:274–282. DOI: 10.1016/j.limno.2005.08.004.
- Karpowicz M. 2014. Influence of eutrophic lowland reservoir on crustacean zooplankton assemblages in river valley oxbow lakes. Polish Journal of Environmental Studies 23:2055–2061.
- Karpowicz M. 2017. Biodiversity of microcrustaceans (Cladocera, Copepoda) in a lowland river ecosystem. Journal of Limnology 76:15–22. DOI: 10.4081/jlimnol.2016.1449.
- Karpowicz M, Ejsmont-Karabin J. 2021. Diversity and structure of pelagic zooplankton (Crustacea, Rotifera) in NE Poland. Water 13:456. DOI: 10.3390/w13040456.
- Karpowicz M, Feniova I, Gladyshev MI, Ejsmont-Karabin J, Górniak A, Sushchik NN, Anishchenko OV, Dzialowski AR. 2021a. Transfer efficiency of carbon, nutrients, and polyunsaturated fatty acids in planktonic food webs under different environmental conditions. Ecology and Evolution 11:8201–8214. DOI: 10.1002/ece3.7651.
- Karpowicz M, Sługocki Ł, Kozłowska J, Ochocka A, López C. 2020. Body size of Daphnia cucullata as an indicator of the ecological status of temperate lakes. Ecological Indicators 17:106585. DOI: 10.1016/j.ecolind.2020.106585.
- Karpowicz M, Świsłocka M, Moroz J, Sługocki Ł. 2021b. New insight into the taxonomic resolution of the genus Bythotrephes Leydig (Crustacea: Cladocera) based on molecular data from Central Europe. Scientific Reports 11:23158. DOI: 10.1038/s41598-021-02648-7.
- Keller B, Spaak P. 2004. Non-Random sexual reproduction and diapausing egg production in a Daphnia hybrid species complex. Limnology and Oceanography 49:1393–1400. DOI: 10.4319/lo.2004.49.4_part_2.1393.
- Korovchinsky NM. 1986. Variability, taxonomy, distribution of Diaphanosoma orghidani (Cladocera, Sididae) and description of D. orientalis sp.n. Zoologicheskii Zhurnal 2:208–220.
- Korovchinsky NM. 1987. A study of Diaphanosoma species (Crustacea: Cladocera) of the “mongolianum” group. Internationale Revue der gesamten Hydrobioligie 72:727–758. DOI: 10.1002/iroh.19870720609.
- Korovchinsky NM. 1992. Sididae & Holopediidae (Crustacea: Daphniiformes). In: F DHJ, editor. Guides to the identification of the microinvertebrates of the continental waters of the world. Vol. 3. The Hague: SPB Academic Publishing. 82 pp.
- Korovchinsky NM. 2018. Cladocera: Ctenopoda: Families Sididae, Holopediidae & Pseudopenilidae (Branchiopoda: Cladocera). In: Dumont HJF, editor. Identification guides to the plankton and benthos of inland waters 27. Weikersheim: Backhuys Publishers and Margraf Publishers. 204 pp.
- Korovchinsky NM, Petkovski TK. 2014. The ancient Balkan lakes harbor a new endemic species of Diaphanosoma Fischer, 1850 (Crustacea: Branchiopoda: Cladocera). Zootaxa 3784:539–549. DOI: 10.11646/zootaxa.3784.5.3.
- Korovchinsky NM, Walsh EJ, Smolak R. 2017. Diaphanosoma Fischer, 1850 (Crustacea: Cladocera: Sididae) of Lake Turkana (East Africa), with the description of a new species of the genus. Zootaxa 4250:77–89. DOI: 10.11646/zootaxa.4250.1.6.
- Kotov AA. 2015. A critical review of the current taxonomy of the genus Daphnia OF Müller, 1785 (Anomopoda, Cladocera). Zootaxa 3911:184–200. DOI: 10.11646/zootaxa.3911.2.2.
- Lakatos C, Urabe J, Makino W. 2015. Cryptic diversity of Japanese Diaphanosoma (Crustacea: Cladocera) revealed by morphological and molecular assessments. Inland Waters 5:253–262. DOI: 10.5268/IW-5.3.847.
- Lazareva VI, Bolotov SE. 2013. Analysis of coexistence of the recent invader Diaphanosoma orghidani Negrea with the aboriginal species D. brachyurum (Lievin) (Crustacea, Cladocera) in the Rybinsk Reservoir. Russian Journal of Biological Invasions 4:161–173. DOI: 10.1134/S2075111713030077.
- Lazareva VI, Bolotov SE. 2014. Peculiarities of the biology of two Diaphanosoma species (Crustacea, Cladocera) in Rybinsk Reservoir. Inland Water Biology 7:108–116. DOI: 10.1134/S1995082914020096.
- Liu P, Xu L, Xu S-L, Martínez A, Chen H, Cheng D, Dumont HJ, Han B-P, Fontaneto D. 2018. Species and hybrids in the genus Diaphanosoma Fischer, 1850 (Crustacea: Branchiopoda: Cladocera). Molecular Phylogenetics and Evolution 118:369–378. DOI: 10.1016/j.ympev.2017.10.016.
- Napiórkowski P, Kentzer A, Dembowska E. 2006. Zooplankton of the lower Vistula River: The effect of Włocławek dam reservoir (Poland) on community structure. SIL Proceedings 29:2109–2114.
- Pentinsaari M, Salmela H, Mutanen M, Roslin T. 2016. Molecular evolution of a widely-adopted taxonomic marker (COI) across the animal tree of life. Scientific Reports 6:35275. DOI: 10.1038/srep35275.
- Posada D. 2008. jModelTest: Phylogenetic model averaging. Molecular Biology and Evolution 25:1253–1256. DOI: 10.1093/molbev/msn083.
- Richter S, Olesen J, Wheeler WC. 2007. Phylogeny of Branchiopoda (Crustacea) based on a combined analysis of morphological data and six molecular loci. Cladistics 23:301–336. DOI: 10.1111/j.1096-0031.2007.00148.x.
- Sarma SSS, Nandini S, Gulati RD. 2005. Life history strategies of cladocerans: Comparisons of tropical and temperate taxa. Hydrobiologia 542:315–333. DOI: 10.1007/s10750-004-3247-2.
- Schwenk K, Spaak P. 1995. Evolutionary and ecological consequences of interspecific hybridization in cladocerans. Experientia 51:465–481. DOI: 10.1007/BF02143199.
- Semenova AS, Tchougounov VK. 2018. The distribution of Moina micrura Kurz, 1875 (Crustacea: Moinidae) in the Russian part of the Vistula Lagoon (Baltic Sea). Russian Journal of Biological Invasions 9:175–183. DOI: 10.1134/S207511171802011X.
- Sługocki Ł, Czerniawski R. 2018. Trophic state (TSISD) and mixing type significantly influence pelagic zooplankton biodiversity in temperate lakes (NW Poland). PeerJ 6:e5731. DOI: 10.7717/peerj.5731.
- Spaak P, Hoekstra JR. 1997. Fish predation on a Daphnia hybrid species complex: A factor explaining species coexistence? Limnology and Oceanography 42:753–762. DOI: 10.4319/lo.1997.42.4.0753.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30:2725–2729. DOI: 10.1093/molbev/mst197.
- Weiler W. 1997. Erstfund von Diaphanosoma orghidani Negrea 1982 (Crustacea: Sididae) für Deutschland und ihre Begleitarten [First record of Diaphanosoma orghidani Negrea 1982 (Crustacea: Sididae) in Germany and its companions]. Lauterbornia H 32:73–77.
- Wolinska J, Bittner K, Ebert D, Spaak P. 2006. The coexistence of hybrid and parental Daphnia: The role of parasites. Proceedings of the Royal Society B: Biological Sciences 273:1977–1983. DOI: 10.1098/rspb.2006.3523.
- Xu S, Innes DJ, Lynch M, Cristescu ME. 2013. The role of hybridization in the origin and spread of asexuality in Daphnia. Molecular Ecology 22:4549–4561. DOI: 10.1111/mec.12407.
