Abstract
Non-mammal models are gaining popularity due to their ease of maintenance, low cost and lack of ethical restrictions. As some processes taking place in insect hemocytes are mirrored in human immunocytes, many immunological studies are now turning to the use of insect models like Galleria mellonella. G. mellonella larvae are susceptible to infection by Conidiobolus coronatus: an entomopathogenic fungus causing rhinofacial mycosis in humans. The study examines G. mellonella larvae during C. coronatus infection for the presence of certain compounds known to be involved in the human immunological response (histamine, HSF1, Cysteinyl leukotriene TLR1 and TLR2). G. mellonella larvae were exposed to fully grown fungus, and the results were determined by ELISA tests, fluorescence microscopy and flow cytometry. CysLT showed no significant changes between the control group and the infected larvae. Both histamine and HSF1 levels increased significantly after exposure to C. coronatus. Immunolocalization and flow cytometry of TLR1 and TLR2 proves that both receptors increase their levels due to the fungus infection. Our findings show that the cellular response of the G. mellonella is similar to the human response, indicating that G. mellonella larvae may be considered as a human-like immunological model in further studies.
Introduction
There is a growing need for non-mammalian research models in immunological studies (Cook & McArthur Citation2013). Using invertebrates might well be more cost-efficient in the long term due to lower maintenance requirements (Ramarao et al. Citation2012). Furthermore, as non-mammalian models are not subject to ethical restrictions and can be used in higher population sizes, it is easier to obtain statistically relevant data and pursue research using whole population (Desalermos et al. Citation2012; Huang et al. Citation2018; Rillich & Stevenson Citation2018; Coates et al. Citation2019).
Recent years have seen a growth in the interest in insect-based research, driven partly by the discovery that several insect genes are homologous to those derived from humans. One of the models used in is Galleria mellonella (Linnaeus, 1758) (Lepidoptera, Pyralidae), also known as the wax worm/moth. G. mellonella has been previously the subject of immunological, toxicological, and other biological studies (Ramarao et al. Citation2012; Jacobsen Citation2014; Binder et al. Citation2016; Coates et al. Citation2019). Its larvae are relatively large-sized (~12–20 mm), which allows almost effortless manipulation and collection of tissue/hemolymph samples for analysis. In addition, breeding G. mellonella is quite inexpensive, and many larvae can be obtained in a relatively short time (Huang et al. Citation2018). Finally, the insect maintenance does not require complicated and costly equipment (Binder et al. Citation2016). The life cycle of G. mellonella lasts 7 to 8 weeks, and can be regulated by adjusting the temperature (Bogus et al. Citation2017). G. mellonella can be handled at temperatures from 20°C to 37°C. And because the human internal temperature is about 37°C that makes G. mellonella useful in modeling humans and other mammals (Lionakis Citation2011; Ramarao et al. Citation2012; Cook & McArthur Citation2013; Jacobsen Citation2014).
G. mellonella has previously been used as a model for the infection of the fungal pathogen Conidiobolus coronatus (Entomophthorales) (Kedra & Bogus Citation2006; Wieloch et al. Citation2011; Bogus et al. Citation2017). C. coronatus is a cosmopolitan soil fungus, and it is pathogenic to both insects and humans (Bogus et al. Citation2007). In a tropical climate, C. coronatus causes rhinofacial mycosis in humans, a chronic infection of the face and nose resulting in major deformations (Fischer et al. Citation2008; Moncada et al. Citation2016). C. coronatus is known to attack G. mellonella larvae. It was found to induce 90% mortality in G. mellonella last instar larvae populations exposed to sporulating colonies (Bania et al. Citation2006; Bogus et al. Citation2017; Wrońska et al. Citation2018). Several metabolites produced by C. coronatus demonstrate insecticidal activity, e.g., coronatin-1, coronatin-2 (Wieloch et al. Citation2011; Bogus et al. Citation2017), dodecanol (Kazek et al. Citation2021), β-carboline alkaloids: harman and norharman (Wronska et al. Citation2018; Wronska & Bogus Citation2019), and two trichothecenes: HT-2 and T-2 toxin (Bogus et al. Citation2021; Piacenza et al. Citation2021). In insects surviving the infection, a deformation of the Malphigi tubules was observed (Boguś & Szczepanik Citation2000). Disintegration of epidermis, muscles and other tissues is also the result of the infection by C. coronatus. Other known damages are deformation and/or apoptosis of hemocytes, destruction of oenocytes, degranulation of granulocytes, and vacuolization of plasmatocytes (Boguś & Szczepanik Citation2000; Kedra & Bogus Citation2006; Renwick et al. Citation2006; Boguś et al. Citation2018).
Immunological system of G. mellonella is not yet well described, but existing data show some similarities to the innate immune response in mammals. In insects, there are two defense systems acting against pathogens: the cuticle, which prevents pathogens from entering the body, and the immune system, which neutralizes pathogens that have entered the insect. In the larvae, the cuticle is analogous to the mammalian skin, while the hemolymph can be partly compared to blood. Blood and hemolymph both include immunocompetent cells, which in insects are known as hemocytes (Ashida & Brey Citation1995). Five classes of hemocytes are known to be present in the Lepidoptera: prohemocytes, plasmatocytes, granulocytes, spherulocytes and oenocytoids (Jiang et al. Citation2010). Plasmatocytes and granulocytes are the most common hemocytes, known to be involved in phagocytosis; however, the other hemocyte classes are not well described, which may indicate that they do not play such a crucial role in the immune response (Jiang et al. Citation2010; Browne et al. Citation2013; Wu et al. Citation2016; Wojda Citation2017).
The humoral immune response in insects consists of the release of AMPs (antimicrobial peptides); these are synthesized by the fat body, the IMD (immune deficiency), the JAK-STAT (Janus kinase-signal transducer and activator of transcription) and the Toll pathways (Lemaitre & Hoffmann Citation2007; Valanne et al. Citation2011). Gram-negative bacteria activate the IMD pathway, while fungi and Gram-positive bacteria induce the Toll signaling pathway (Valanne et al. Citation2011; Myllymaki & Ramet Citation2014; Kleino & Silverman Citation2014). Gram-positive bacteria are sensed through a complex of PRGP-SA (pathogen recognition peptidoglycan recognition protein-SA), PGRP-SD, and GNBP1 (Gram-negative bacteria binding protein 1); however, these proteins are not involved in the recognition of fungi (Michel et al. Citation2001). Even so, GNBP3 shows strong binding affinities to the components of the fungal cell wall, even in null Toll mutants (Gottar et al. Citation2006). Fungal infection is detected by serine protease Persephone (psh) and GNBP3 (Gottar et al. Citation2006). Their activation leads to proteolytic serine protease cascades that result in Spaetzle being split. This induces Toll-mediated AMP transcription through the nuclear translocation of Drosophila DIF, an NF-κB homolog (Gottar et al. Citation2006).
Similar regulatory pathways are described in mammals, where dendritic cells, macrophages and granulocytes provide a first line of defense against numerous pathogens (Thivierge et al. Citation2006). Molecular structures present in the pathogens are recognized by TLRs (Toll-like receptors) expressed in these cells. The major difference between mammal TLR and insect Toll is that while mammalian TLRs interact directly with the pathogen, the insect Toll only binds with Spaetzle (Wright et al. Citation1990; Park Citation2004). In mammals, TLRs have evolved to recognize conserved products unique to microbial metabolism (McCurdy et al. Citation2003).
Binding the pathogen ligand to the receptor induces a cascade of signaling pathways. These pathways are highly evolutionarily conserved from plants to insects and humans. The cascade ends with the activation of NF-κB, leading to the induction of cytokine genes and the establishment of innate immunity in mammals (Park Citation2004). As TLRs play a crucial role not only in the pro-inflammatory response but also can stimulate anti-inflammatory cytokines (Ozato et al. Citation2002), it was worth investigating whether TLRs in G. mellonella are present during C. coronatus infection.
It is also possible that other factors that regulate the immune system of mammals may also be present in insects. Many stress factors, such as hypoxia, virus infections or harsh temperature, are known to cause the activation of HS genes (heat shock genes), also known as stress genes (Shamovsky & Nudler Citation2008). One product of those genes is HSPs (heat shock proteins); in mammals, these perform multiple functions in order to protect cells, for example, by preventing denatured proteins from aggregating (Pockley & Henderson Citation2018). HSPs also act as ligands for TLRs (Vabulas et al. Citation2002). Interestingly, both bacterial and mammalian HSPs interact with TLRs, demonstrating that there is no exclusive association between TLRs with microbial ligands (Vabulas et al. Citation2002). The first examples of non-pathogen derived TLR ligands are Human HSP60 and Gp96 (Vabulas et al. Citation2002). Since the presence of HSP-like proteins has been confirmed in G. mellonella larvae (Wronska & Bogus Citation2020), the next step was to investigate the presence of HSF1 (Heat Shock Factor 1). In humans, HSF1, encoded by the HSF1 gene, is highly conserved in eukaryote cells and is the primary mediator of transcriptional responses to proteotoxic stress, with important roles in non-stress regulations such as development and metabolism (Rabindran et al. Citation1991; Vihervaara & Sistonen Citation2014). HSF1 regulates the HSR (heat shock response) pathway in humans (Vihervaara & Sistonen Citation2014). It also plays a crucial role in proper folding and distribution of proteins within cells (Dayalan Naidu & Dinkova-Kostova Citation2017).
Histamine is synthesized in the mast cells by decarboxylation of the amino acid histidine (Uvnas et al. Citation1970). After the synthesis, histamine is accumulated in secretory granules in complexes with heparin and/or granular protein (Uvnas et al. Citation1970). When allergy occurs, histidine is released and binds with specific allergens to IgE antibodies on mast cells and basophils, initiating a Ca2+-dependent degranulation reaction (Carswell & Young Citation1985). After continuity is established between the secretory granule and the plasma membrane of the mast cell, histamine dissociates from the partially solubilized granular matrix by exchanging with sodium ions in the extracellular environment and is extruded into tissue spaces (Uvnas Citation1964). As early as 1983, histamine was also described in insects (Bai & Suzuki Citation2022). In mammals and Drosophila flies, it functions to promote wakefulness and circadian rhythm regulation (Elias & Evans Citation1983a). The highest levels of histamine were detected in the retina and in the lamina neuropil of the optic lobe, with lower levels of histamine being found throughout the nervous system (Elias & Evans Citation1983b). Histamine is known to be the main neurotransmitter released by Drosophila photoreceptors (Elias & Evans Citation1983b; Denno et al. Citation2016; Bai & Suzuki Citation2022)
In mammals, histamine production is commonly connected with the expression of the Cysteinyl leukotrienes (CysLTs) (White Citation1999). CysLTs, namely LTC4, LTD4, and LTE4, were originally described as the SRS-A (slow-reacting substance of anaphylaxis) (Singh et al. Citation2010). In mammals, CysLTs act as multi-functional mediators in allergic airway diseases, dermatological diseases, cardiovascular diseases, liver injury, atherosclerosis and colon cancer (Bisgaard Citation2001; Singh et al. Citation2010). In addition, CysLT receptors are located in the nasal tissue, making them a valuable option for study (Peters-Golden et al. Citation2006; Gusach et al. Citation2019). Moreover, some data suggest that selective TLR agonists can modulate human MoDC (monocyte-derived dendritic cell) expression of CysLT receptors and cysLT-mediated functions.
The main aim of the present work was to examine the presence of histamine, HSF1, Cysteinyl leukotriene, TLR1 and TLR2 on G. mellonella larvae during C. coronatus infection. The testable hypothesis was that exposure of G. mellonella larvae to C. coronatus affects the presence of histamine, HSF1, Cysteinyl leukotriene, TLR1 and TLR2, which are crucial elements of the immune system in mammals.
Materials and methods
Insects
G. mellonella was maintained and reared in temperature and humidity-controlled chambers (30°C, 70% r.h.) in constant darkness on an artificial diet (Kwadha et al. Citation2017). Fully grown larvae were collected before pupation, surface-sterilized and homogenized, and then used as a supplement in the fungal cultures. Five-day-old last instar larvae were used for the study. Breeding of G. mellonella was based on Wrońska et al. (Citation2018).
Fungus
C. coronatus (isolate number 3491), originally isolated from Dendrolaelaps spp., was obtained from the collection of Prof. Bałazy (Polish Academy of Sciences, Research Center for Agricultural and Forest Environment, Poznań). It was routinely maintained in 90 mm Petri dishes at 20°C with cyclic changes of light (L:D 12:12) on SAB-G medium. SAB-G medium consists of Sabouraud agar medium with the addition of homogenized G. mellonella larvae to a final concentration of 10% wet weight. The addition of the homogenate enhances the sporulation and virulence of the C. coronatus. This protocol was based on Włóka et al. (Citation2022).
Infection of insects with C. coronatus
G. mellonella larvae were exposed for 24 h at a temperature of 20°C to fully grown and sporulating C. coronatus colonies. Ten individuals were maintained in each Petri dish, each experimental group consisted of 100 animals. Following this time, one group of insects was collected immediately for examination (F24 group). The second group (F48) was transferred to new, clean Petri dishes with appropriate food (an artificial diet (Sehnal Citation1966)) and kept at 20°C for the next 24 h: 20°C is an optimal temperature for C. coronatus and does not affect G. mellonella larvae significantly (Lionakis Citation2011). After this time, the insects were used for experiments. A control group was formed of larvae exposed for 24 h to a sterile SAB-G medium. This method was based on Wronska et al. (Citation2021).
Larval hemolymph collection
G. mellonella hemolymph was collected from controlled and infected (F24 and F48) larvae. Before bleeding, larvae were washed in 70% (v/v) ethanol to sterilize their surfaces, and then in ultrapure water, thus reducing any possible contamination of hemolymph samples. Hemolymph was taken from the larvae through an incision made in the last proleg. The hemolymph was prepared in various ways depending on the planned method.
For hemocyte culture, 100 μl of fresh hemolymph collected from 10 larvae was suspended in 500 μl of supplemented Grace’s Insect Medium (GIM; Invitrogen) with added gentamicin (10 mg/ml; Gibco), amphotericin B (250 µg/ml; Gibco) and phenylothiourea (PTU; 0.1 mM; Sigma-Aldrich). Following this, hemolymph in GIM+PTU was transferred to a six-channel μ-Slide IV 0.4 (IBIDI)- 100 μl for each channel. The slides were incubated in 30°C for 24 h. Each test was conducted in three repetitions.
For ELISA (enzyme-linked immunosorbent assay) 100 μl of fresh hemolymph collected from 10 larvae was suspended in 100 μl of supplemented GIM. Samples were sonicated (20 kHz, 1 min; room temperature) for cell lysis. Probes were centrifuged at 13,000 × g for 10 min in 4°C to pellet debris. Supernatants were transferred to a new microcentrifuge tube and stored at −80°C. Larval hemolymph collection methods were based on Wronska & Bogus (Citation2020). Each test was conducted in three repetitions.
Immunocytochemical analysis
Immunolocalization of TLR 1 and TLR2 was performed in all hemocyte cultures (controls, F24 and F48) obtained from 100 insects each. The cells were fixed in 4% Paraformaldehyde (Sigma-Aldrich; PFA) in phosphate-buffered saline (PBS) and permeabilized in 0.1% Triton X-100 (Sigma-Aldrich) in PBS. The cells were incubated overnight at 4°C with monoclonal primary antibody antiTLR1 or antiTLR2 (Enzo Life Sciences). The antibodies were diluted 1:40 in PBS with 1% bovine serum albumin (BSA, Sigma-Aldrich). The cells were then incubated in 4% BSA-PBS for 2 h to prevent non-specific antibody binding. Following this, the hemocytes were incubated for a further 2 h at room temperature with secondary antibody DyLight 488, Goat Anti-Mouse IgG (Abbkine). Concentrations of secondary antibodies were 2 µg/ml. ActinRed 555 ReadyProbes Reagent (Invitrogen) were used to label the actin fibers. The cell nuclei were stained with DAPI (Enzo Life Sciences). Fluorescence signals were analyzed by fluorescent microscopy using an Axio Vert.A1 fluorescence microscope (Zeiss) with Axio Cam ICc 5 (Zeiss) and ZEN 3.2 blue edition software. This protocol was based on Wronska and Bogus (Citation2020).
Flow cytometry analysis
The hemolymph collected from control group and infected larvae, each consisting of 100 insects, was centrifuged (400 × g, 10 min, 4°C) and washed with PBS. The cells were fixed in 4% paraformaldehyde (Sigma-Aldrich; PFA) in PBS and permeabilized in 0.1% Triton X-100 (Sigma-Aldrich) (also diluted in PBS). Then, the cells were incubated with primary antibodies (the same that were used for immunolocalization) (diluted 1:100) overnight at 4°C. Cells were washed three times in PBS and incubated for 2 h at room temperature with the secondary antibodies: DyLight 488, Goat Anti-Mouse IgG (Abbkine). Readings were acquired on an CyFlow Cube 8 (Sysmex) and analyzed with FCS Express 7 (DeNovo Software). For each experimental condition, 100 μl of each sample was scrutinized. Data were acquired using a 488 nm laser for the detection of each protein on the FL-1 channel. Results were shown as dot plots comparing forward scatter (FSC) with side scatter (SSC). This method was based on Wrońska et al (Wronska et al. Citation2022).
Quantitative determination by ELISA tests
Quantitative histamine, LTC4/D4/E4 and HSF1 analyses were carried out using ELISA tests all from Enzo Life Sciences. The following commercial kits were used: Histamine ELISA kit, Cysteinyl leukotriene ELISA kit and HSF1 ELISA kit. Each test was performed in four independent replicates according to the manufacturer’s instructions. Absorbance was measured using a Synergy HT Microplate Reader (BioTek).
Statistics
All of the aforementioned tests were conducted in three repetitions. The normality of the data was tested using the Kolmogorov–Smirnov test. Brown-Forsythe test was used to determine the homogeneity of variance. One-way ANOVAs followed by orthogonal polynomial contrasts (both linear and quadratic) were used to test differences between treatments. Specifically, Contrast 1 included Control vs (F24+F48) and Contrast 2 included F24 vs F48. OriginPro 9.0.0 (64–bit) SR2 b87 (OriginLab Corporation) software was used to evaluate the normality and homogeneity of the data. IBM SPSS 19.0 software (SPSS, Chicago, IL, USA) was used for orthogonal polynomial contrasts. Significance was set at p = 0.05 in all cases. Raw data and full statistical data are included in Supplementary Table 1–Raw Data.
Results
Immunolocalization of TLR1 and TLR2 in G. mellonella hemocytes
In the control group, immunofluorescence localization based on fluorescence microscopy found the TLR1 receptor to be only faintly present () and TLR2 to be absent (). However, after 24–hour exposure to C. coronatus, major changes were clearly visible in the G. mellonella hemocytes, with both the cell cytoskeleton being more fractured, and the number of cells being greatly lowered, indicating that many of the cells had been destroyed as a result of the infection. Moreover, a fair amount of TLR1 and TLR2 can be noticed in all of the visible hemocytes. After 48–hour exposure, i.e. while those changes were progressing, fewer cells were visible, and their cytoskeleton was even more disrupted; however, the F48 TLR1 was at the same intensity as in F24, while F48 TLR2 was visibly more intense than in F24. In addition, a noticeable amount of TLR2 was observed outside the cells, which indicates a huge number of cells had decomposed.
Figure 1. Immunolocalization of TLR1 in G. mellonella hemocytes: (a) immunodetection of TLR1 (performed as described in materials and methods section), (b) flow cytometry data given as dot plots (SSC versus FSC); green indicates cells containing TLR1. F24 indicates larvae sampled immediately after 24–hour exposure to fungal infection; F48 indicates larvae sampled 24 hours after 24–hour exposure. Scale bars = 25µm.
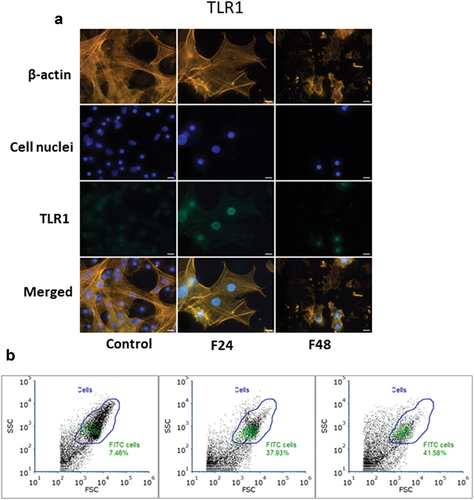
Figure 2. Immunolocalization of TLR2 in G. mellonella hemocytes: (a) immunodetection of TLR2 (performed as described in materials and methods section), (b) flow cytometry data given as dot plots (SSC versus FSC); green indicates cells containing TLR2. F24 indicates larvae sampled immediately after 24–hour exposure to fungal infection; F48 indicates larvae sampled 24 hours after 24–hour exposure. Scale bars = 25µm.
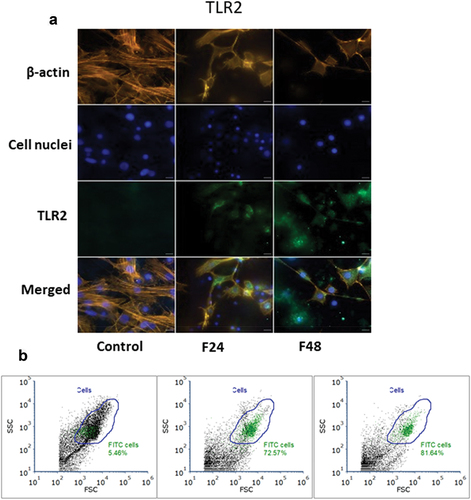
Flow cytometry data were presented as dot plots of side scatter (SSC) versus forward scatter (FSC). All cells present in the sample are marked as “Cells” (blue gate), while cells that contain TLR1 or TLR2 were marked green. The percentage of cells containing the tested receptor was described on the plot.
In the control group, only 7.46% of all cells showed a response to the TLR1 antibody, but this number grew with exposure to C. coronatus, rising from almost 38% after 24 h to about 42% at 48 h after exposure (). In the control group, only 5.46% of all cells demonstrated a response to the TLR2 antibody, but this number grew rapidly from about 73% after 24 h of exposure to almost 82% after 48 h (). These results correspond with those acquired via immunofluorescence microscopy localization. It is also visible that G. mellonella hemocytes are more dispersed after exposure, with more debris present, suggesting that the hemocytes disintegrated due to fungus infection, as visible under the microscope.
Quantitative determination of histamine, HSF1 and cysteinyl leukotriene by ELISA tests
The impact of C. coronatus infection on the concentrations of histamine, HSF1 and Cysteinyl leukotriene in hemocytes of G. mellonella was tested in three groups of larvae. The results of ELISA testing on full hemolymph (lysed cells and plasma) are presented as a graph of means with SD (standard deviation). In all cases, Brown-Forsythe test showed that samples were drawn from distributions with equal variances, and normality test (Kolmogorov–Smirnov test) showed that all of obtained data was drawn from a normally distributed population (p > 0.05).
Histamine () levels increased in G. mellonella larvae hemolymph. It was present at 0.13 ± 0.03 ng/ml in the control group, 0.38 ± 0.05 ng/ml in the F24 group, and 0.22 ± 0.01 ng/ml in F48. The differences between groups were statistically significant. Contrast 1 (Control vs. F24+F48): df = 9, p < 0.001; Contrast 2 (F24 vs. F48): df = 9, p < 0.001.
Figure 3. Changes of concentration of histamine (a), HSF1 (b) and cysteinyl leukotriene (c) in G. mellonella hemolymph after fungal infection, determined by ELISA (Enzo Life Sciences). Data are expressed as mean ± SD; *p < 0.05. All of the raw data are attached in the Supplementary Table 1–Raw data.
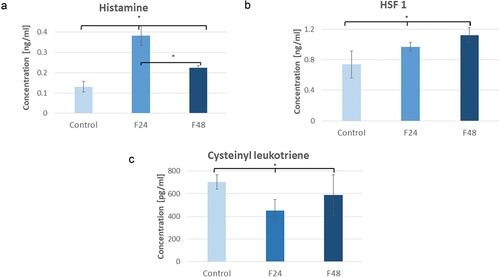
It is important to note that the hemolymph histamine level rose 24 h after exposure to the fungus but then had fallen after another 24 h (F48). HSF1 () increased in all G. mellonella larvae groups. Its concentration was 0.74 ± 0.18 ng/ml in controls, 0.97 ± 0.06 ng/ml in F24, and 1.12 ± 0.10 ng/ml in F48. Statistically significant differences were observed between the control group and each of the treated groups. No significant difference was found between F24 and F48. Contrast 1 (Control vs. F24+F48): df = 9, p = 0.003; Contrast 2 (F24 vs. F48): df = 9, p = 0.105. Due to C. coronatus infection, Cysteinyl leukotriene () lowered its levels in G. mellonella larvae. Its concentration was 701.92 ± 63.08pg/m in controls, 451.88 ± 95.19 pg/ml in F24, and 588.63 ± 177.96 pg/ml in F48. No statistically significant differences were found between F24 and F48. Contrast 1 (Control vs. F24+F48): df = 9, p = 0.038; Contrast 2 (F24 vs. F48): df = 9, p = 0.148. Raw data are presented in Supplementary Table 1.
Discussion
Insects such as G. mellonella, the wax moth, may serve as an alternative to mammals in many toxicological or immunological studies. Since its first description in 1918 in a colony of Apis cerana (Fabricius, 1793) (eastern or Asiatic honeybee), it has increasingly been used in scientific research. It is a known pest of bee colonies, which has allowed it to spread in all continents except Antarctica through its connection to beehives (Kwadha et al. Citation2017).
The life cycle of wax moth is quite short, lasting just over 7 weeks in favorable conditions, but its length depends strongly on the environment, particularly temperature (Smith Citation1965; Fasasi & Malaka Citation2006; Swamy Citation2008; Ellis et al. Citation2013; Kwadha Citation2017; Hosamani et al. Citation2017; Desai et al. Citation2019). Recent papers have shown great interest in G. mellonella as a model organism due to its ease of reproduction and high number of offspring (Binder et al. Citation2016). Most importantly, the genome of the wax moth is also known, which can help in studies based on genetics: the entire transcriptome of immune-challenged larvae was obtained in 2011 and the entire genome was sequenced in 2018 (Vogel et al. Citation2011; Lange et al. Citation2018).
Hence, G. mellonella is a very convenient research model organism, which can be used for investigations of conserved patterns of innate mechanisms in both vertebrates and invertebrates. The larvae of the greater wax moth are also broadly used in many aspects of immunological studies (Wojda Citation2017; Kwadha et al. Citation2017).
Results presented in this study are consistent with previous findings reporting that G. mellonella larvae are very sensitive to C. coronatus infection (Wronska et al. Citation2018; Kazek et al. Citation2020). In all cases, it can be observed that exposure to C. coronatus is harmful to G. mellonella larvae. After the infection, insects sustained visible changes in their appearance, black spots and solidify of cuticule which often led to death. Hemolymph collected from infected insects was darker compared to control group. Hemocytes obtained from larvae exposed to sporulating C. coronatus were damaged. Degradation of hemocytes is visible after 24 h from infection and progresses over time. During infection, fewer hemocytes are visible under the microscope and are clearly in worse shape; in addition, large amounts of debris and disintegrated parts of cells can be seen in the hemolymph. All of this can be also noticed in flow cytometry. Changes following C. coronatus infection have been observed in all tests.
In G. mellonella immunity consists of only innate mechanisms: unlike mammals, insects do not possess an adaptive immune system. However, this does not prevent insects from discriminating and recognizing particular infections, and insects are also fully capable of releasing molecules that are efficient in controlling specific classes of pathogens (Ferrandon et al. Citation2007). Their main response to Gram-positive bacteria and fungal infections involves the Toll pathway (Lindsay & Wasserman Citation2014). It is important to note that there are major similarities between insect and mammalian innate immune signaling pathways: like insects, mammals also use Toll-like receptors (TLRs) to activate antibacterial and fungal immune responses (Kumar et al. Citation2009; Chtarbanova & Imler Citation2011; Szatmary Citation2012). In Drosophila, Toll does not directly interact with pathogens but instead receives signals from recognition proteins in the hemolymph. A Toll ligand – Spaetzle – concentrate a signal of microbial presence. Spaetzle is produced as the result of pathogen recognition by the PGRPs (pathogen recognition peptidoglycan recognition proteins): PGRP-SA, PGRP-SD, GNBP1, and GNBP3 (Weber et al. Citation2003; Stokes et al. Citation2015).
Works on D. melanogaster TLRs had a great impact on understanding their role in the human immune system. The Toll pathway was discovered to be involved in the cellular immune response, namely, the phagocytosis of microbes, and the encapsulation of parasites. For example, the cellular immune response is activated in D. melanogaster following infection with parasitic Leptopilina boulardi, and highly elevated levels of circulating plasmatocytes can be observed in larvae hemolymph. It has also been found that the Toll pathway is able to adjust hemocyte density by controlling hemocyte proliferation. Also, Toll signaling is necessary for melanization in Drosophila larvae (Valanne et al. Citation2011; Myllymaki & Ramet Citation2014; Kleino & Silverman Citation2014).
Our present findings indicate that antibodies directed against human TLR1 and TLR2 are able to recognize similar receptors in G. mellonella larvae hemolymph. Immunofluorescence localization showed both TLR1 and TLR2 to be located in plasmatocytes, particularly in the cytoplasm and around the nuclei. In healthy larvae, neither receptor was observed outside the cells. Based on the immunolocalization and flow cytometry, it was concluded that TLR1 was present in higher levels than TLR2 in the control group. After infection by C. coronatus, the levels of both tested compounds increased dramatically, although these did not increase over time. In both cases, infection resulted in the disintegration of hemocytes, and the TLRs could be observed outside the cells. All of this may indicate that both TLR1 and TLR2 were engaged in the immune response of G. mellonella larvae to the C. coronatus infection. However, due to limited resources, preventing to apply isotype-matching irrelevant antibodies control, the possibility that the antibodies cross-react with non-specific epitopes cannot be presently excluded.
A study from 2004 found that the expression of TLR2 and TLR4 mRNA was enhanced by histamine in HUVEC (human umbilical vein endothelial cells), occurring as a response to Gram-negative and Gram-positive bacterial components (Talreja et al. Citation2004; Branco et al. Citation2018). It was also found that the Toll-like receptor (TLR) agonists Pam2CSK4 and LPS activate basophils through TLR2 and TLR4, respectively, resulting in IL-4, IL-6 and histamine production (Alkan et al. Citation2018).
A study from 2019 showed that the infection of the Drosophila melanogaster larvae with bacteria Enterobacter ludwigii had a major impact on histamine levels in insects. Once the bacteria entered the body of D. melanogaster, it started to release ROS (reactive oxygen species) into the hemolymph; this had a critical impact on the life cycle, appearance and behavior of the insect, all of which were connected with neurodegeneration. Interestingly, histamine was found to be absent in the infected flies. Moreover, the authors noticed an uptake of histamine into PNS neurons (peripheral nervous system neurons) (Priyadarsini et al. Citation2019).
In the present study, however, histamine levels rose significantly in the hemolymph after 24 h of exposure to C. coronatus, but then fell after another 24 h. This may indicate not only that histamine play a role in the immune system of the insects but also that C. coronatus infection could be associated with neurodegenerative aspects.
Unfortunately, no information currently exists in the literature regarding whether CysLTs play a role in insect immunology. Our present findings confirm the presence of CysLT in G. mellonella larvae. Its levels lowered significantly during the infection, but there is no data on exact mechanism, and it stands against the role of the CysLTs in human immunology (Bisgaard Citation2001; Singh et al. Citation2010). That may suggest that CysLTs have a different role in insects than CysLTs in mammals. As such, more studies in this area are needed to understand the role of CysLTs in insects.
Understanding the mechanisms of the interaction between the innate immune system and HSPs will make it possible to rationally modulate immune responses, either towards immunity or towards tolerance (Vabulas et al. Citation2002; Pockley & Henderson Citation2018).
A recent study in our group also confirmed the presence of HSPs in G. mellonella larvae hemolymph (Wronska & Bogus Citation2020). HSPs were found to be present in both the control groups and larvae infected with C. coronatus. Although HSP60, HSP27 and HSP90 levels were seen to increase after fungal infection, HSP70 did not change (Wronska & Bogus Citation2020). Following on from these findings, the present study examined whether HSF1 was also present in the G. mellonella larvae hemolymph and whether its concentration changes following C. coronatus infection. Under optimal conditions, HSFs regulate the heat shock response. There are at least five groups of HSFs in vertebrates: HSF1, HSF2, HSF3, HSF4, and HSFY (Garbuz Citation2017). HSF1 demonstrates activation in stress conditions, and so was chosen for the present study. HSF2 is associated with embryo development, HSF4 is a clone from human, mouse, and rat genomes, and HSFY is encoded by a Y-chromosomal gene. Although HSF3 is similar to HSF1, it is found in birds and activates at higher temperatures (Garbuz Citation2017).
In general, the heat shock response system is highly evolutionarily conserved, e.g., D. melanogaster HSF is capable of inducing the transcription of heat shock genes in mammalian cells (Clos et al. Citation1993; Garbuz Citation2017). In normal conditions, D. melanogaster HSF and vertebrate HSF1 occur as monomers in complex with Hsp90 in the cell, like many other transcription factors whose activity is regulated with similar chaperones (Abravaya et al. Citation1992; Xu & Lindquist Citation1993; Whitesell et al. Citation1998; Kosano et al. Citation1998). In this study, the presence of HSF1 was detected in both control and infected G. mellonella larvae. Although a rise in HSF1 level was noted after infection, time generally had no effect. Although little data exists on the impact of infection on HSF1 in insects, it can be assumed that a similar process may take place in other invertebrate species. In Liriomyza trifolii, HSF1 was involved in heat stress, where its levels generally decreased with rising temperatures (Chang et al. Citation2021), and in D. melanogaster, HSF1 did not exhibit any pronounced differences based on sex or age of the insect (Shilova et al. Citation2020). It was also described that HSF1 activated and up regulated Hsp70, during diapause in H. armigera (Chen et al. Citation2019).
Conclusions
A fully grown, sporulating colony of C. coronatus causes major damage in G. mellonella larvae. Although no significant changes in CysLT were found between control and infected larvae, the other substances tested during the research presented in this paper visibly reacted to the pathogen. Both histamine and HSF1 levels increased after exposure to C. coronatus. Immunolocalization of TLR1 and TLR2 based on fluorescence microscopy and flow cytometry found that both receptors increased their levels following fungus infection. The results indicate that G. mellonella demonstrates a similar cellular response to humans in some cases. As such, G. mellonella larvae represents a promising candidate for a human-like immunological model in further studies on the diseases connected with immunodeficiency or drug development.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2023.2276353
Additional information
Funding
References
- Abravaya K, Myers MP, Murphy SP, Morimoto RI. 1992. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes & Development 6(7):1153–1164. DOI: 10.1101/gad.6.7.1153.
- Alkan M, Sayes F, Ramadan A, Machavoine F, Dy M, Schneider E, Thieblemont N. 2018. Basophil activation through TLR2 and TLR4 signaling pathways. AIMS Allergy and Immunology 2(3):126–140. DOI: 10.3934/Allergy.2018.3.126.
- Ashida M, Brey PT. 1995. Role of the integument in insect defense: Pro-phenol oxidase cascade in the cuticular matrix. Proceedings of the National Academy of Sciences of the United States of America 92(23):10698–10702. DOI: 10.1073/pnas.92.23.10698.
- Bai Y, Suzuki T. 2022. Activity-dependent circuitry plasticity via the regulation of the histamine receptor level in the Drosophila visual system. Molecular & Cellular Neuroscience 119:103703. DOI: 10.1016/j.mcn.2022.103703.
- Bania J, Samborski J, Bogus M, Polanowski A. 2006. Specificity of an extracellular proteinase from Conidiobolus coronatus and its inhibition by an inhibitor from insect hemolymph. Archives of Insect Biochemistry and Physiology 62(4):186–196. DOI: 10.1002/arch.20134.
- Binder U, Maurer E, Lass-Florl C. 2016. Galleria mellonella: An invertebrate model to study pathogenicity in correctly defined fungal species. Fungal Biology 120(2):288–295. DOI: 10.1016/j.funbio.2015.06.002.
- Bisgaard H. 2001. Pathophysiology of the cysteinyl leukotrienes and effects of leukotriene receptor antagonists in asthma. Allergy 56(Suppl 66):7–11. DOI: 10.1034/j.1398-9995.56.s66.2.x.
- Boguś M, Szczepanik M. 2000. Histopathology of Conidiobolus coronatus (Entomophthorales) infection in Galleria mellonella (Lepidoptera) larvae. Acta Parasitologica 45(1):48–54.
- Bogus MI, Kędra E, Bania J, Szczepanik M, Czygier M, Jabłoński P, Pasztaleniec A, Samborski J, Mazgajska J, Polanowski A. 2007. Different defense strategies of Dendrolimus pini, Galleria mellonella, and Calliphora vicina against fungal infection. Journal of Insect Physiology 53(9):909–922. DOI: 10.1016/j.jinsphys.2007.02.016.
- Boguś MI, Ligęza‐Żuber M, Polańska MA, Mosiewicz M, Włóka E, Sobocińska M. 2018. Fungal infection causes changes in the number, morphology and spreading ability of Galleria mellonella haemocytes. Physiological Entomology 43(3):214–226. DOI: 10.1111/phen.12246.
- Bogus MI, Wieloch W, Ligeza-Zuber M. 2017. Coronatin-2 from the entomopathogenic fungus Conidiobolus coronatus kills Galleria mellonella larvae and incapacitates hemocytes. Bulletin of Entomological Research 107(1):66–76. DOI: 10.1017/S0007485316000638.
- Bogus MI, Wrońska AK, Kaczmarek A, Boguś-Sobocińska M. 2021. In vitro screening of 65 mycotoxins for insecticidal potential. PLoS One 16(3):e0248772. DOI: 10.1371/journal.pone.0248772.
- Branco A, Yoshikawa FS, Pietrobon AJ, Sato MN. 2018. Role of histamine in modulating the immune response and inflammation. Mediators of Inflammation 2018:9524075. DOI: 10.1155/2018/9524075.
- Browne N, Heelan M, Kavanagh K. 2013. An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence 4(7):597–603. DOI: 10.4161/viru.25906.
- Carswell H, Young JM. 1985. The characteristics of histamine H1-agonist-stimulated breakdown of inositol phospholipids differ between regions of Guinea-pig brain. Biochemical Society Transactions 13(6):1188–1189. DOI: 10.1042/bst0131188.
- Chang YW, Wang Y-C, Zhang X-X, Iqbal J, Lu M-X, Du Y-Z. 2021. Transcriptional regulation of small heat shock protein genes by heat shock factor 1 (HSF1) in Liriomyza trifolii under heat stress. Cell Stress and Chaperones 26(5):835–843. DOI: 10.1007/s12192-021-01224-2.
- Chen W, Geng S-L, Song Z, Li Y-J, Wang H, Cao J-Y. 2019. Alternative splicing and expression analysis of HSF1 in diapause pupal brains in the cotton bollworm, Helicoverpa armigera. Pest Management Science 75(5):1258–1269. DOI: 10.1002/ps.5238.
- Chtarbanova S, Imler JL. 2011. Microbial sensing by Toll receptors: A historical perspective. Arteriosclerosis, Thrombosis, and Vascular Biology 31(8):1734–1738. DOI: 10.1161/ATVBAHA.108.179523.
- Clos J, Rabindran S, Wisniewski J, Wu C. 1993. Induction temperature of human heat shock factor is reprogrammed in a Drosophila cell environment. Nature 364(6434):252–255. DOI: 10.1038/364252a0.
- Coates CJ, Lim J, Harman K, Rowley AF, Griffiths DJ, Emery H, Layton W. 2019. The insect, Galleria mellonella, is a compatible model for evaluating the toxicology of okadaic acid. Cell Biology and Toxicology 35(3):219–232. DOI: 10.1007/s10565-018-09448-2.
- Cook SM, McArthur JD. 2013. Developing Galleria mellonella as a model host for human pathogens. Virulence 4(5):350–353. DOI: 10.4161/viru.25240.
- Dayalan Naidu S, Dinkova-Kostova AT. 2017. Regulation of the mammalian heat shock factor 1. The FEBS Journal 284(11):1606–1627. DOI: 10.1111/febs.13999.
- Denno ME, Privman E, Borman RP, Wolin DC, Venton BJ. 2016. Quantification of histamine and Carcinine in Drosophila melanogaster tissues. ACS Chemical Neuroscience 7(3):407–414. DOI: 10.1021/acschemneuro.5b00326.
- Desai A, Siddhapara MR, Patel PK, Prajapati AP. 2019. Biology of greater wax moth, Galleria mellonella. On artificial diet. Journal of Experimental Zoology, India 22(2):1267–1272.
- Desalermos A, Fuchs BB, Mylonakis E. 2012. Selecting an invertebrate model host for the study of fungal pathogenesis. PLoS Pathogens 8(2):e1002451. DOI: 10.1371/journal.ppat.1002451.
- Elias MS, Evans PD. 1983a. Histamine in the insect nervous system: Distribution, synthesis and metabolism. Journal of Neurochemistry 41(2):562–568. DOI: 10.1111/j.1471-4159.1983.tb04776.x.
- Elias MS, Evans PD. 1983b. Histamine in the insect nervous system: Distribution, synthesis and metabolism. Journal of Neurochemistry 41(2):562–568. DOI: 10.1111/j.1471-4159.1983.tb04776.x.
- Ellis JD, Graham JR, Mortensen A. 2013. Standard methods for wax moth research. Journal of Apicultural Research 52(1):1–17. DOI: 10.3896/IBRA.1.52.1.10.
- Fasasi K, Malaka SLO. 2006. Life cycle and impact of greater waxmoth, Galleria mellonella L. (Lepidoptera: Pyralidae) feeding on stored beeswax. Nigerian Journal of Entomology 23:13–17. DOI: 10.36108/NJE/6002/32.0130.
- Ferrandon D, Imler J-L, Hetru C, Hoffmann JA. 2007. The Drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nature Reviews: Immunology 7(11):862–874. DOI: 10.1038/nri2194.
- Fischer N, Ruef C, Ebnöther C, Bächli EB. 2008. Rhinofacial Conidiobolus coronatus infection presenting with nasal enlargement. Infection 36(6):594–596. DOI: 10.1007/s15010-008-8056-5.
- Garbuz DG. 2017. Regulation of heat shock gene expression in response to stress. Molekuliarnaia Biologiia 51(3):400–417. DOI: 10.1134/S0026893317020108.
- Gottar M, Gobert V, Matskevich AA, Reichhart JM, Wang C, Butt TM, Belvin M, Hoffmann JA, Ferrandon D. 2006. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell 127(7):1425–1437. DOI: 10.1016/j.cell.2006.10.046.
- Gusach A, Luginina A, Marin E, Brouillette RL, Besserer-Offroy É, Longpré J-M, Ishchenko A, Popov P, Patel N, Fujimoto T, Maruyama T, Stauch B, Ergasheva M, Romanovskaia D, Stepko A, Kovalev K, Shevtsov M, Gordeliy V, Han GW, Katritch V, Borshchevskiy V, Sarret P, Mishin A, Cherezov V. 2019. Structural basis of ligand selectivity and disease mutations in cysteinyl leukotriene receptors. Nature Communications 10(1):5573. DOI: 10.1038/s41467-019-13348-2.
- Hosamani V, Hanumantha Swamy BC, Kattimani KN, Kalibavi CM. 2017. Studies on Biology of greater wax moth (Galleria mellonella L.). International Journal of Current Microbiology and Applied Sciences (IJCMAS) 6(11):3811–3815. DOI: 10.20546/ijcmas.2017.611.447.
- Huang X, Liu Y, Xi L, Zeng K, Mylonakis E. 2018. Galleria mellonella as a model invertebrate host for the study of muriform cells of dematiaceous fungi. Future Microbiology 13(9):1021–1028. DOI: 10.2217/fmb-2018-0036.
- Jacobsen ID. 2014. Galleria mellonella as a model host to study virulence of Candida. Virulence 5(2):237–239. DOI: 10.4161/viru.27434.
- Jiang H, Vilcinskas A, Kanost MR. 2010. Immunity in lepidopteran insects. Advances in Experimental Medicine and Biology 708:181–204.
- Kazek M, Kaczmarek A, Wrońska AK, Boguś MI. 2020. Conidiobolus coronatus induces oxidative stress and autophagy response in Galleria mellonella larvae. PLoS One 15(2):e0228407. DOI: 10.1371/journal.pone.0228407.
- Kazek M, Kaczmarek A, Wrońska AK, Boguś MI. 2021. Dodecanol, metabolite of entomopathogenic fungus Conidiobolus coronatus, affects fatty acid composition and cellular immunity of Galleria mellonella and calliphora vicina. Scientific Reports 11(1):15963. DOI: 10.1038/s41598-021-95440-6.
- Kedra E, Bogus MI. 2006. The influence of Conidiobolus coronatus on phagocytic activity of insect hemocytes. Journal of Invertebrate Pathology 91(1):50–52. DOI: 10.1016/j.jip.2005.06.013.
- Kleino A, Silverman N. 2014. The Drosophila IMD pathway in the activation of the humoral immune response. Developmental and Comparative Immunology 42(1):25–35. DOI: 10.1016/j.dci.2013.05.014.
- Kosano H, Stensgard B, Charlesworth MC, McMahon N, Toft D. 1998. The assembly of progesterone receptor-Hsp90 complexes using purified proteins. The Journal of Biological Chemistry 273(49):32973–32979. DOI: 10.1074/jbc.273.49.32973.
- Kumar H, Kawai T, Akira S. 2009. Toll-like receptors and innate immunity. Biochemical and Biophysical Research Communications 388(4):621–625. DOI: 10.1016/j.bbrc.2009.08.062.
- Kwadha CA, Ong’amo GO, Ndegwa PN, Raina SK, Fombong AT. 2017. The biology and control of the Greater Wax Moth, Galleria mellonella. Insects 8:8(2. DOI: 10.3390/insects8020061.
- Lange A, Beier S, Huson DH, Parusel R, Iglauer F, Frick JS. 2018. Genome Sequence of Galleria mellonella (Greater Wax Moth). Genome Announcements 6(2). DOI: 10.1128/genomeA.01220-17.
- Lemaitre B, Hoffmann J. 2007. The host defense of Drosophila melanogaster. Annual Review of Immunology 25(1):697–743. DOI: 10.1146/annurev.immunol.25.022106.141615.
- Lindsay SA, Wasserman SA. 2014. Conventional and non-conventional Drosophila Toll signaling. Developmental and Comparative Immunology 42(1):16–24. DOI: 10.1016/j.dci.2013.04.011.
- Lionakis MS. 2011. Drosophila and Galleria insect model hosts: New tools for the study of fungal virulence, pharmacology and immunology. Virulence 2(6):521–527. DOI: 10.4161/viru.2.6.18520.
- McCurdy JD, Olynych TJ, Maher LH, Marshall JS. 2003. Cutting edge: Distinct toll-like receptor 2 activators selectively induce different classes of mediator production from human mast cells. Journal of Immunology (Baltimore, Md: 1950) 170(4):1625–1629. DOI: 10.4049/jimmunol.170.4.1625.
- Michel T, Reichhart J-M, Hoffmann JA, Royet J. 2001. Drosophila toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature 414(6865):756–759. DOI: 10.1038/414756a.
- Moncada DC, Montes M, Molina V, Velásquez JB, Gómez CI. 2016. Orofacial infection by Conidiobolus coronatus. Biomedica 36:15–22. DOI: 10.7705/biomedica.v36i2.2806.
- Myllymaki H, Ramet M. 2014. JAK/STAT pathway in Drosophila immunity. Scandinavian Journal of Immunology 79(6):377–385. DOI: 10.1111/sji.12170.
- Ozato K, Tsujimura H, Tamura T. 2002. Toll-like receptor signaling and regulation of cytokine gene expression in the immune system. Biotechniques 33:66–8, 70, 72 passim. DOI: 10.2144/Oct0208.
- Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. 2004. Cutting edge: Direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. Journal of Immunology (Baltimore, Md: 1950) 173(6):3589–3593. DOI: 10.4049/jimmunol.173.6.3589.
- Peters-Golden M, Gleason MM, Togias A. 2006. Cysteinyl leukotrienes: multi-functional mediators in allergic rhinitis. Clinical and Experimental Allergy: Journal of the British Society for Allergy and Clinical Immunology 36(6):689–703. DOI: 10.1111/j.1365-2222.2006.02498.x.
- Piacenza N, Kaltner F, Maul R, Gareis M, Schwaiger K, Gottschalk C. 2021. Distribution of T-2 toxin and HT-2 toxin during experimental feeding of yellow mealworm (Tenebrio molitor). Mycotoxin Research 37(1):11–21. DOI: 10.1007/s12550-020-00411-x.
- Pockley AG, Henderson B. 2018. Extracellular cell stress (heat shock) proteins—immune responses and disease: An overview. Philosophical Transactions of the Royal Society of London: Series B, Biological Sciences 373(1738):20160522. DOI: 10.1098/rstb.2016.0522.
- Priyadarsini S, Sahoo M, Sahu S, Jayabalan R, Mishra M. 2019. An infection of Enterobacter ludwigii affects development and causes age-dependent neurodegeneration in Drosophila melanogaster. Invertebrate Neuroscience: In 19(4):13. DOI: 10.1007/s10158-019-0233-y.
- Rabindran SK, Giorgi G, Clos J, Wu C. 1991. Molecular cloning and expression of a human heat shock factor, HSF1. Proceedings of the National Academy of Sciences of the United States of America 88(16):6906–6910. DOI: 10.1073/pnas.88.16.6906.
- Ramarao N, Nielsen-Leroux C, Lereclus D. 2012. The insect Galleria mellonella as a powerful infection model to investigate bacterial pathogenesis. Journal of Visualized Experiments: JoVe 2012(70):e4392. DOI: 10.3791/4392.
- Renwick J, Daly P, Reeves EP, Kavanagh K. 2006. Susceptibility of larvae of Galleria mellonella to infection by Aspergillus fumigatus is dependent upon stage of conidial germination. Mycopathologia 161(6):377–384. DOI: 10.1007/s11046-006-0021-1.
- Rillich J, Stevenson PA. 2018. Serotonin mediates depression of aggression after acute and chronic social defeat stress in a model insect. Frontiers in Behavioral Neuroscience 12:233. DOI: 10.3389/fnbeh.2018.00233.
- Sehnal F. 1966. Kritisches studium der Bionomie und Biometrik der in Verschiedenen Lebensbedingungen Gezuchteten Wachsmotte Galleria Mellonella L (Lepidopera). Zeitschrift Fur Wissenschaftliche Zoologie 174(1–2):53–.
- Shamovsky I, Nudler E. 2008. New insights into the mechanism of heat shock response activation. Cellular and Molecular Life Sciences: CMLS 65(6):855–861. DOI: 10.1007/s00018-008-7458-y.
- Shilova V, Zatsepina O, Zakluta A, Karpov D, Chuvakova L, Garbuz D, Evgen’ev M. 2020. Age-dependent expression profiles of two adaptogenic systems and thermotolerance in Drosophila melanogaster. Cell Stress and Chaperones 25(2):305–315. DOI: 10.1007/s12192-020-01074-4.
- Singh RK, Gupta S, Dastidar S, Ray A. 2010. Cysteinyl leukotrienes and their receptors: Molecular and functional characteristics. Pharmacology 85(6):336–349. DOI: 10.1159/000312669.
- Smith TL. 1965. External morphology of the Larva, Pupa, and adult of the Wax Moth, Galleria mellonella L. Journal of the Kansas Entomological Society 38(3):287–310.
- Stokes BA, Yadav S, Shokal U, Smith LC, Eleftherianos I. 2015. Bacterial and fungal pattern recognition receptors in homologous innate signaling pathways of insects and mammals. Frontiers in Microbiology 6:19. DOI: 10.3389/fmicb.2015.00019.
- Swamy B. 2008. Bionomics and biometrics of Greater Wax Moth Galleria mellonella Linnaeus. Asian Journal of Biological Sciences 3(1):49–51.
- Szatmary Z. 2012. Molecular biology of toll-like receptors. General Physiology and Biophysics 31(4):357–366. DOI: 10.4149/gpb_2012_048.
- Talreja J, Kabir MH, B Filla M, Stechschulte DJ, Dileepan KN. 2004. Histamine induces Toll-like receptor 2 and 4 expression in endothelial cells and enhances sensitivity to Gram-positive and Gram-negative bacterial cell wall components. Immunology 113(2):224–233. DOI: 10.1111/j.1365-2567.2004.01946.x.
- Thivierge M, Stankova J, Rola-Pleszczynski M. 2006. Toll-like receptor agonists differentially regulate cysteinyl-leukotriene receptor 1 expression and function in human dendritic cells. The Journal of Allergy and Clinical Immunology 117(5):1155–1162. DOI: 10.1016/j.jaci.2005.12.1342.
- Uvnas B. 1964. Release processes in mast cells + their activation by injury. Annals of the New York Academy of Sciences 116(A3):880–. DOI: 10.1111/j.1749-6632.1964.tb52554.x.
- Uvnas B, Aborg CH, Bergendorff A. 1970. Storage of histamine in mast cells. Evidence for an ionic binding of histamine to protein carboxyls in the granule heparin-protein complex. Acta Physiologica Scandinavica: Supplementum 336:1–26.
- Vabulas RM, Wagner H, Schild H. 2002. Heat shock proteins as ligands of toll-like receptors. Current Topics in Microbiology and Immunology 270:169–184.
- Valanne S, Wang JH, Ramet M. 2011. The Drosophila Toll signaling pathway. Journal of Immunology (Baltimore, Md: 1950) 186(2):649–656. DOI: 10.4049/jimmunol.1002302.
- Vihervaara A, Sistonen L. 2014. HSF1 at a glance. Journal of Cell Science 127(Pt 2):261–266. DOI: 10.1242/jcs.132605.
- Vogel H, Altincicek B, Glöckner G, Vilcinskas A. 2011. A comprehensive transcriptome and immune-gene repertoire of the lepidopteran model host Galleria mellonella. BMC Genomics 12(1):308. DOI: 10.1186/1471-2164-12-308.
- Weber AN, Tauszig-Delamasure S, Hoffmann JA, Lelièvre E, Gascan H, Ray KP, Morse MA, Imler J-L, Gay NJ. 2003. Binding of the Drosophila cytokine Spätzle to Toll is direct and establishes signaling. Nature Immunology 4(8):794–800. DOI: 10.1038/ni955.
- White M. 1999. Mediators of inflammation and the inflammatory process. The Journal of Allergy and Clinical Immunology 103(3 Pt 2):S378–81. DOI: 10.1016/S0091-6749(99)70215-0.
- Whitesell L, Sutphin PD, Pulcini EJ, Martinez JD, Cook PH. 1998. The physical association of multiple molecular chaperone proteins with mutant p53 is altered by geldanamycin, an Hsp90-binding agent. Molecular Cell Biology 18(3):1517–1524. DOI: 10.1128/MCB.18.3.1517.
- Wieloch W, Boguś MI, Ligęza M, Koszela-Piotrowska I, Szewczyk A. 2011. Coronatin-1 isolated from entomopathogenic fungus Conidiobolus coronatus kills Galleria mellonella hemocytes in vitro and forms potassium channels in planar lipid membrane. Toxicon 58(4):369–379. DOI: 10.1016/j.toxicon.2011.07.007.
- Włóka E, Boguś MI, Wrońska AK, Drozdowski M, Kaczmarek A, Sobich J, Gołębiowski M. 2022. Insect cuticular compounds affect Conidiobolus coronatus (Entomopthorales) sporulation and the activity of enzymes involved in fungal infection. Scientific Reports 12(1):13641. DOI: 10.1038/s41598-022-17960-z.
- Wojda I. 2017. Immunity of the greater wax moth Galleria mellonella. Insect Science 24(3):342–357. DOI: 10.1111/1744-7917.12325.
- Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249(4975):1431–1433. DOI: 10.1126/science.1698311.
- Wronska AK, Kaczmarek A, Kazek M, Boguś MI. 2021. Infection of Galleria mellonella (Lepidoptera) larvae with the Entomopathogenic fungus Conidiobolus coronatus (Entomophthorales) induces apoptosis of hemocytes and affects the concentration of eicosanoids in the Hemolymph. Frontiers in Physiology 12:774086. DOI: 10.3389/fphys.2021.774086.
- Wronska AK, Bogus MI. 2019. Harman and norharman, metabolites of the entomopathogenic fungus Conidiobolus coronatus (Entomophthorales), affect the serotonin levels and phagocytic activity of hemocytes, insect immunocompetent cells, in Galleria mellonella (Lepidoptera). Cell Bioscience 9:29.
- Wronska AK, Bogus MI. 2020. Heat shock proteins (HSP 90, 70, 60, and 27) in Galleria mellonella (Lepidoptera) hemolymph are affected by infection with Conidiobolus coronatus (Entomophthorales). PloS One 15(2):e0228556. DOI: 10.1371/journal.pone.0228556.
- Wronska AK, Boguś MI, Kaczmarek A, Kazek M. 2018. Harman and norharman, metabolites of entomopathogenic fungus Conidiobolus coronatus (Entomopthorales), disorganize development of Galleria mellonella (Lepidoptera) and affect serotonin-regulating enzymes. PLoS One 13(10):e0204828. DOI: 10.1371/journal.pone.0204828.
- Wronska AK, Boguś MI, Włóka E, Kazek M, Kaczmarek A, Zalewska K. 2018. Cuticular fatty acids of Galleria mellonella (Lepidoptera) inhibit fungal enzymatic activities of pathogenic Conidiobolus coronatus. PLoS One 13(3):e0192715. DOI: 10.1371/journal.pone.0192715.
- Wronska AK, Kaczmarek A, Sobich J, Grzelak S, Boguś MI. 2022. Intracellular cytokine detection based on flow cytometry in hemocytes from Galleria mellonella larvae: A new protocol. PLoS One 17(9):e0274120. DOI: 10.1371/journal.pone.0274120.
- Wrońska AK, Loor JJ. 2018. Cuticular fatty acids of Galleria mellonella (Lepidoptera) inhibit fungal enzymatic activities of pathogenic Conidiobolus coronatus. PloS One 13(3):e0192715. DOI: 10.1371/journal.pone.0192715.
- Wu G, Liu Y, Ding Y, Yi Y. 2016. Ultrastructural and functional characterization of circulating hemocytes from Galleria mellonella larva: Cell types and their role in the innate immunity. Tissue and Cell 48(4):297–304. DOI: 10.1016/j.tice.2016.06.007.
- Xu Y, Lindquist S. 1993. Heat-shock protein Hsp90 governs the activity of pp60v-src kinase. Proceedings of the National Academy of Sciences of the United States of America 90(15):7074–7078. DOI: 10.1073/pnas.90.15.7074.
