 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Ilex paraguariensis Saint Hilaire (St. Hil) is an important crop in the north of Argentina, mainly cultivated in the Misiones province. Trichoderma genera are well known for their biological control and plant growth-promoting properties. The application of Ilex paraguariensis endophytes to improve crop production is an interesting alternative to the use of agrochemicals. To evaluate the capability of Trichoderma spp. endophytes, it was performed antagonism tests of Trichoderma spp. against phytopathogenic fungi associated with these crops using dual culture assay. It was also evaluated the chitinase production of these strains using a colorimetric Somogyi-Nelson method to determine biocontrol capability of these endophytes. Moreover, the capability of plant growth promotion on tomato seeds was evaluated. We observed that Trichoderma was capable to invade and reduce phytopathogen growth and particularly three strains produced chitinase and induced growth promotion on Lycopersicum esculentum seeds.
Introduction
Ilex paraguariensis Saint Hilaire (St. Hil.), known as yerba mate, is a regional tree of the Atlantic forest in Argentina, Paraguay, and Brazil. Argentina is the first world producer, which takes place mainly in the provinces of Misiones and Corrientes. Actually, there are 165.326 hectares of yerba mate cultivars, of which 90% are in Misiones province (http://www.inym.org.ar, 2016).
It is currently recommended to adopt conservationist practices as zero tillage (using herbicides) on the management of yerba mate crops and the introduction of plant species companions as green roofs or native tree species (Ilany, Ashton, Montagnini, & Martinez, Citation2010; Prat Kricun & Belingheri, Citation2003).
One interesting alternative to replacing synthetic chemical products in the control of diseases is the application of Trichoderma as a biocontroller and promoter agent of plant growth (Harman, Howell, Viterbo, Chet, & Lorito, Citation2004).
The genus Trichoderma is cosmopolitan, and frequently its species are dominant components of the soil microflora in very diverse habitats. This can be attributed to the heterogeneous metabolic capacity of the Trichoderma species and their aggressively competitive nature. Interest in the genus has increased markedly in recent years, as some species have been proposed as biocontrol agents against plant pathogenic fungi (Pitt & Hocking, Citation2009). It is also known that the genus includes plant symbionts (Harman & Kubicek, Citation1998).
Endophytic microorganisms can contribute to the health, growth, and development of plants. Plant growth promotion by endophytic microorganism may result either from indirect effects such as the biocontrol of soil-borne diseases through competition for nutrients, siderophore-mediated competition for iron, antibiosis or the induction of systemic resistance in the plant host, or from direct effects such as the production of phytohormones or by providing the host plant with solubilization of soil phosphorus and iron (Kinkel, Wilson, & Lindow, Citation2000; Kuklinsky-Sobral et al., Citation2004; Sturz, Christie, & Nowak, Citation2000).
The aim of this work was to evaluate the biological control capacity and the plant growth promoting properties in vitro of fourteen Trichoderma strains previously isolated from yerba mate roots. Antagonist dual assays and chitinase production of Trichoderma strains were performed. Plant growth-promoting activity was assessed in vitro by inoculation of Trichoderma on Lycopersicum esculentum seeds under controlled conditions.
Materials and methods
Isolation and maintenance of fungal strains
Trichoderma species used in this research were obtained from surface-sterilized root of yerba mate plants with 2% sodium hypochlorite. For the isolation, small parts of sterilized root were placed in Petri dishes with 2% papa dextrose agar (PDA) with 25 mg l−1 chloramphenicol and were incubated at 28 ± 1° C in the presence of light, until mycelium appeared. Colonies that showed morphologies similar to Trichoderma genera were isolated.
The strains used in this study are maintained in the collection of the Institute of Biotechnology Misiones “Dra. Maria Ebe Reca” named as LBM. Trichoderma strains used were Trichoderma asperellum LBM 193, LBM 194, LBM 195, LBM 197, LBM 198, LBM 204, and LBM206; Trichoderma strigosellum LBM196, LBM201, and LBM 205; Trichoderma asperelloides LBM 199 and LBM 203; Trichoderma hamantum LBM 200 and Trichoderma sp. LBM 202. The phytopathogenic fungi assayed were Fusarium sp. LBM 184 and Phoma sp. LBM 207. Microorganisms were maintained at 4 °C in Potato Dextrose Agar (PDA).
Fungal inhibition assay
Dual culture assays were carried out to evaluate the antagonism of Trichoderma strains against phytopathogenic fungi following the methodology described by Desai, Reddy, and Kloepper (Citation2002) with modifications. Mycelial plugs of Trichoderma and phytopathogen were placed on the same dish with PDA medium at 7 cm from each other and were incubated at 28 ± 1 °C. Dishes inoculated only with pathogens served as controls. Three replications of each plate were done. The pathogen growth rate was measured at three, five and seven days after the assay started. The inhibition grade was calculated at day seven. The inhibition of pathogenic fungal growth was evaluated quantitatively using Equationequation (1)(1)
(1)
(1)
(1)
where IR is the percentage of reduction in mycelial growth of the phytopathogen, R1 is the average growth of pathogen in the treated plates, and R2 is the average growth of pathogen in the control plates. The Trichoderma strain that inhibited the pathogen growth 50% or more was considered an effective antagonist (López, Alvarenga, Zapata, Luna, & Villalba, Citation2019).
The Shapiro-Wilk modified test was used to evaluate the normality of the inhibition percentage data using InfoStat program 2018 version (Di Rienzo et al., 2018). The Levene’s test for homogeneity of variances were applied to the antagonism test data using Statgraphics Centurion program version 15.2.06 (StatPoint Inc., 2007). The percentage growth inhibition was tested by one-way analysis of variance (ANOVA) and posteriori Fisher test using InfoStat program 2018 version (Di Rienzo et al., 2018).
At day ten antagonist index was calculated using the Bell scale (Bell, Wells, & Markham, Citation1982). A strain having an index of 3 or 4 is considered an effective antagonist strain. An index of 4 it is considered that Trichoderma completely overgrew the pathogen and covered the entire medium surface and an index of 3 it is considered that Trichoderma occupies 75% surface of the medium surface.
Determination of chitinase activity in vitro
Chitinase production was determined using two different methods: in a solid and liquid medium. The media used for both determinations were: 1.0% chitin (Sigma C-7170), 0.25% K2HPO4, 0.0496% NaH2PO4, 100 µl l−1 minerals solution, 0.15% (NH4)2SO4 and 1.4% agar in solid medium. For chitin determination on solid medium, a plug with mycelial growth of Trichoderma was inoculated on plates and incubated for five days at 28 °C in presence of light. The strains that were capable to growth on this medium could use chitin as carbon source and be considered positive as chitinase producer.
For chitin determination on liquid medium, a plug with mycelial growth of Trichoderma was inoculated in Erlenmeyer containing 10 ml of the media previously described. After nine days of incubation, the technique of the qualitative determination of chitinolytic activity was performed measuring the amount of reducing sugars according to the methodology described by Somogyi- Nelson (Nelson, Citation1944; Somogyi, Citation1952). To this determination, 1 ml of each Trichoderma sample was centrifugate at 13000 rpm during 10 minutes. To 200 µl of the supernatant of the sample was mixed with 200 µl Sogmoyi reagent and placed on a boiled bath for ten minutes. After that, Nelson reagent was added and the colour shift of the samples was observed. Control consists in an uninoculated medium. Samples colour were compared to control yellow colour and, the strains capable of used chitin as carbon source was observed in a colour range between green and blue.
Determination of plant growth-promoting potential of Trichoderma endophytes
In this study, we selected tomato as biological model to evaluate the plant growth promoting properties. Tomato is a good model system for studying plant growth promotion due to its relatively short life cycle, its economic importance as a crop species (Alexander & Grierson, Citation2002) and it is easy to handle.
The promotion of plant growth of each of the isolates in Lycopersicum esculentum seeds was evaluated, and the length and fresh biomass of the stem and primary roots were determined of each germinated seed. For this determination, commercial seeds of Lycopersicum esculentum Mill. variety Italian type was disinfected superficially with sodium hypochlorite 2% during three minutes, and three consecutive washes with sterile distilled water for two minutes each one.
For the germination of the seeds, the Murashige and Skoog Basal medium (Sigma-Aldrich, USA) supplemented with 8 g l−1 agar and 10 g l−1 sucrose (Murashige & Skoog, Citation1962), at pH 5.0. Ten seeds per flask were incubated at 24 ± 1 °C for 2 days with a 10 h of photoperiod (Newman, Brown, & Ozbay, Citation2002).
After the germination period, the seeds were inoculated with a spore suspension corresponding to each of Trichoderma strains (1 × 106 spores ml−1). Controls were inoculated with the same volume with distilled sterile water. The test was carried out in triplicate.
The Shapiro-Wilk test was used to analyze the normality of the plant growth promoting data using InfoStat program 2018 version (Di Rienzo et al., 2018). Also, the Levene’s test for homogeneity of variances were applied using Statgraphics Centurion program version 15.2.06 (StatPoint Inc., 2007). The root length, fresh root biomass, stem length, and fresh stem biomass data were statistically tested by one-way analysis of variance (ANOVA) and posteriori Fisher test using InfoStat program 2018 version (Di Rienzo et al., 2018).
Results
Fungal inhibition assay
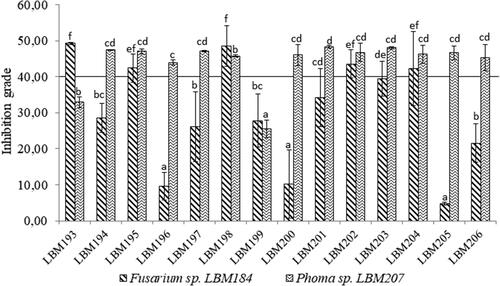
On dual antagonist assay T. asperelloides LBM 193, LBM 195, LBM 198 and LBM 204, Trichoderma sp. LBM 202 and T. asperellum LBM 203 were capable to inhibit in more than 40% the Fusarium sp. LBM 184 growth without significant differences (p > 0.05) between these strains. Therefore, these strains were considered an efficient antagonist against Fusarium.
T. asperelloides LBM 194, LBM 195, LBM 197, LBM 204 and LBM 206, T. strigosellum LBM 196, LBM 201 and LBM 205, T. hamantum LBM 200, Trichoderma sp. LBM 202 and T. asperellum LBM 203 confronted to Phoma sp. LBM 207 were able to inhibit in more than 40% the pathogen growth with no significant differences (p > 0.05) between these strains (). Therefore, these strains were considered an efficient antagonist against Phoma. The statistical analysis has shown in supplementary material 1.
Figure 1. Percentage of pathogen growth inhibition on dual culture assay of Trichoderma spp. confront to Fusarium sp. LBM184 and Phoma sp. LBM 207. Standar error was represented with bars and different letters up to the bars indicate statistically significant differences (p˂0.05). Trichoderma codes: T. asperelloides: LBM 193, LBM 194, LBM 195, LBM197, LBM 198, LBM 204 and LBM 206; T. asperellum: LBM 199 and LBM 203; T. strigosellum: LBM 196, LBM 201 and LBM 205; T. hamatum: LBM 200 and Trichoderma sp.: LBM 202.
The results of the antagonist index calculated after ten days of the assay started showed that T. asperelloides LBM 193, LBM 195, LBM 197 and LBM198, and T. asperellum LBM 203 were capable to invade and reduce in more than 75% the pathogen growth when Trichoderma was confronted to Fusarium sp. LBM 184. On the other hand, T. asperellum LBM 203 was able to invade and reduce Phoma sp. LBM 207 growth in more than 75% ( and ).
Figure 2. Photographs of PDA plates with Trichoderma and phytopathogenic strains after ten days the assay started. A. Trichoderma confronted to LBM 184 Fusarium sp. B. Trichoderma confronted to LBM 207 Phoma sp. Trichoderma codes: T. asperelloides: LBM 193, LBM 194, LBM 195, LBM197, LBM 198, LBM 204 and LBM 206; T. asperellum: LBM 199 and LBM 203; T. strigosellum: LBM 196, LBM 201 and LBM 205; T. hamatum: LBM 200 and Trichoderma sp.: LBM 202.
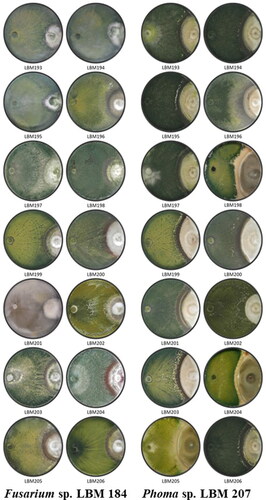
Table 1. Antagonist index after ten days of the assay started. Trichoderma sp. confronted to LBM 184 Fusarium sp. and LBM 207 Phoma sp. Bell scale: 4: Trichoderma overgrows completely to pathogen and covers the whole surface of the medium, 3: Trichoderma overgrows two-thirds of the surface of the medium and 2: Trichoderma and pathogen colonized each half of the surface and nobody seems to dominate the other, 1: the pathogen colonizes the 2/3 parts of the media surface and resists invasion by Trichoderma and 0: the pathogen overgrows completely to Trichoderma covers an area total culture media.
These results showed that T. asperelloides LBM 193, LBM 195, and LBM198, and T. asperellum LBM 203 were the most effective strains against Fusarium sp. LBM 184 because were able to inhibit Fusarium growth by more than 40% and were capable to invade it and reduce its growth in more than 75% after ten days of the assay started. On the other hand, T. asperellum LBM203 was the only strain that inhibited in more than 40% the Phoma growth and, also it was capable to invade and reduce its growth in more than 75% after ten days of the assay started. These results demonstrated that T. asperellum LBM203 was the most effective Trichoderma antagonist against the two pathogens tested.
Determination of chitinase activity in vitro
Quantitative determination of chitinase production has shown that ten Trichoderma strains grown on the selective media and were capable to use chitin as carbon source. The strains that were positive on this determination were T. asperelloides LBM 193, LBM 194, LBM 195, LBM 197, LBM 198, LBM 204 and LBM206; T. asperellum LBM 199 and LBM 203 and, Trichoderma sp. LBM 202 ( and ).
Figure 3. Plates with chitin as carbon source inoculated with Trichoderma strains. A. 1: LBM 206, 2: LBM 198, 3: LBM 205, 4: LBM 201, 5: LBM 203, 6: LBM 202, 7: LBM 193. B. 1: LBM 197, 2: LBM 196, 3: LBM 194, 4: LBM 200, 5: LBM 195, 6: LBM 199, 7: LBM 204. Trichoderma spp. that grown on solid medium were considered positive as chitinase producer. Trichoderma codes: T. asperelloides: LBM 193, LBM 194, LBM 195, LBM197, LBM 198, LBM 204 and LBM 206; T. asperellum: LBM 199 and LBM 203; T. strigosellum: LBM 196, LBM 201 and LBM 205; T. hamatum: LBM 200 and Trichoderma sp.: LBM 202.
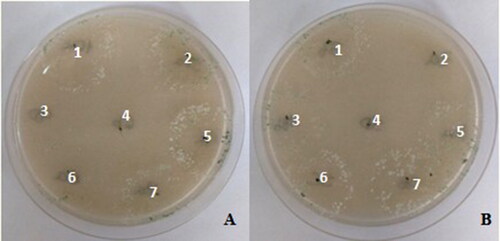
Table 2. Results obtained from chitinase production on solid medium with chitin as carbon source. +: chitinase activity positive and −: chitinase activity negative.
On qualitative determination of chitinase production T. asperelloides LBM 193, LBM 204 and LBM 206 showed highest production of reducing sugars on liquid medium with chitin in comparison with control ( and ).
Figure 4. Results of the chitinase determination using the Somogyi-Nelson method. Trichoderma codes: T. asperelloides: LBM 193, LBM 194, LBM 195, LBM197, LBM 198, LBM 204 and LBM 206; T. asperellum: LBM 199 and LBM 203; T. strigosellum: LBM 196, LBM 201 and LBM 205; T. hamatum: LBM 200 and Trichoderma sp.: LBM 202.
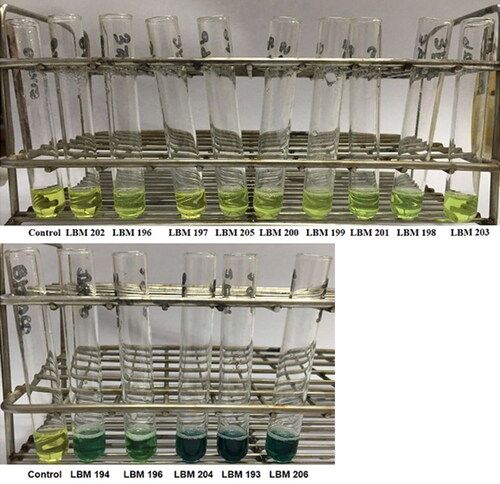
Table 3. Results obtained from chitinase production on liquid medium using Sogmoyi-Nelson determination. +++: highest chitinase activity production, +: chitinase activity production and −: absence of chitinase activity production.
In general, the strains that shown positive results in both determinations were that strains that belongs to T. asperelloides LBM 193, LBM 204 and LBM 206.
Determination of plant growth-promoting potential of Trichoderma endophytes
In the evaluation of plant growth promotion in Lycopersicum esculentum seeds, the one-way analysis of variance indicated that there are statistically significant differences in treatments with: T. asperelloides LBM194, LBM195, LBM198, LBM206, T. asperellum LBM199, LBM203, T. hamatum LBM200, T. strigosellum LBM201, LBM205 and T. sp. LBM202, regarding the control, which demonstrated increase in the length of the primary root (). The treatment with T. strigosellum LBM205 was increased fresh biomass in the primary root of tomato seedlings with statistically significant differences ().
Figure 5. Root length (A), fresh root weigth (B), stem length (C) and fresh stem weigth and of Lycopersicum esculentum plants under the influence of Trichoderma spp. treatments. Different letters up to the bars indicate statistically significant differences (p˂0.05). Trichoderma codes: T. asperelloides: LBM 193, LBM 194, LBM 195, LBM197, LBM 198, LBM 204 and LBM 206; T. asperellum: LBM 199 and LBM 203; T. strigosellum: LBM 196, LBM 201 and LBM 205; T. hamatum: LBM 200 and Trichoderma sp.: LBM 202.
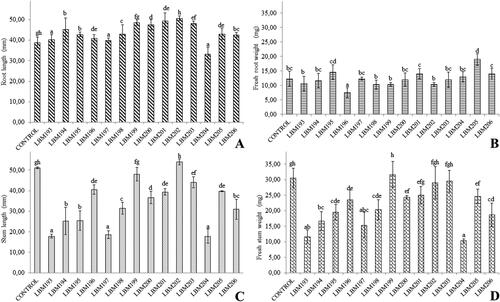
However, the analysis of data showed that Trichoderma treatment does not showed an increase of stem growth statistically significantly with respect to control (). Regarding the determination of fresh biomass increase, no statistically significant increases were observed in the aerial part of the seedlings with respect to the control ().
Under the assay conditions, it could be observed that the species of Trichoderma spp. promoted the growth of the primary root. However, no positive influence on the development of the aerial part of the tomato seedlings was detected.
Discussion
Trichoderma species have been investigated as biological control agents owing to their ability to antagonize plant pathogenic microorganisms. Trichoderma act as biocontrol agents through direct antagonism manifested via enzymatic lysis through secretion of chitinases, β − 1,3-glucanases, proteases and by the secondary metabolites produced (Monte, Citation2001; Geraldine et al., Citation2013). In this research, it was evaluated the ability of Trichoderma spp. isolated from yerba mate roots to reduced phytopathogens growth and its chitinase activity production involved in biological control activity.
On dual culture assay, approximately 45% and 80% of Trichoderma strains showed more than 40% growth inhibition of Fusarium sp. LBM 184 and Phoma sp. LBM 207, respectively. On the other hand, the antagonist index showed that T. asperelloides LBM 193, LBM 195, LBM 197 and LBM198, and T. asperellum LBM 203 were efficient antagonists against Fusarium sp. LBM 184 and T. asperellum LBM 203 was most effective against Phoma sp. LBM 207. The inhibitory activity of the antagonist strains against phytopathogenic fungi in this work was similar to the findings reported by other authors (Carrero-Carrón et al., Citation2016; Taghdi et al., Citation2015). Our results showed that the same phytopathogen it could be inhibited by different Trichoderma strains and different strains of the same antagonist species have not the similar effectiveness when confronting to the same phytopathogen. It was observed that the antagonist Trichoderma effect depends on what pathogen were exposed to. Others research indicates that the level of antagonism by Trichoderma varies when this is confronted with different pathogens making necessary a specific selection of Trichoderma isolates for each plant pathogen (Castillo et al., Citation2011).
In this research, 71% of the strains were positive on solid medium with chitin and 21% of Trichoderma strains were positive on liquid medium. It has been reported that the use of chitin as carbon source in liquid medium induced T. asperellum to produce N-acetylglucosaminidase, β − 1,3-glucanase, chitinase, and protease (Silva, Ulhoa, Batista, Yamashita, & Fernandes, Citation2011) and chitinolytic enzymes have been implicated as a factor to contribute to the ability of Trichoderma spp. to act as biological control agents (Lorito et al., Citation1993). It can be evidenced in this research since the strains that produced chitinase activity demonstrated to be efficient antagonists of the pathogens tested.
There are many studies of Trichoderma spp. showing their capacity to promote plant growth on different crops (Bae et al., Citation2009; Macías-Rodríguez, Guzmán-Gómez, García-Juárez, & Contreras-Cornejo, Citation2018; Yedidia, Benhamou, & Chet, Citation1999) but there is limited knowledge about the effect of Trichoderma endophytes isolated from yerba mate crops. T. asperelloides LBM 194, LBM 195, LBM 198, LBM206; T. asperellum LBM 199, LBM 203; T. hamatum LBM 200; T. strigosellum LBM 201, LBM 205 and Trichoderma sp. LBM202 have shown that were able to promote plant growth in vivo on tomato seeds. Mastouri, Bjorkman, and Harman (Citation2010) have described that the treatment of tomato seeds (Lycopersicum esculentum) with T. harzianum accelerates the germination of these, increases the vigor of the seedlings and induces physiological protection in plants against oxidative damage.
To our understanding, in vivo assay on greenhouse conditions gives us an idea from what happened on natural conditions. Bergottini et al. (Citation2015) reported that three native strains produced a highly significant increase in biomass yield in soil of yerba mate seedlings, in comparison to the non-native PGPR strain Azospirillum brasilense 245. This points out to the importance of using native strains as effective bio-inoculants (Fages & Arsac, Citation1991). There are few studies about the benefit of Trichoderma spp. on yerba mate crops. Therefore, the Trichoderma strains that demonstrated plant growth promoting potential in this study will used for furthers assay on yerba mate crop.
Conclusions
The antagonism and enzymes assay have shown that Trichoderma isolates reduced the growth of phytopathogens and, therefore, it can be involved in integrated disease management of yerba mate pathogens. The degree of antagonism varied between and within species of Trichoderma against plant pathogens. This capacity makes them a suitable mean of control for the integrated management of the disease.
To our understanding, future trials of these strains with promising qualities are necessary to find a sustainable eco-friendly alternative for regional crops of yerba mate.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alexander, L., & Grierson, D. (2002). Ethylene biosynthesis and action in tomato: A model for climacteric fruit ripening. Journal of Experimental Botany, 53(377), 2039–2055. doi:10.1093/jxb/erf072
- Bae, H., Sicher, R. C., Kim, M. S., Kim, S. H., Strem, M. D., Melnick, R. L., & Bailey, B. A. (2009). The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. Journal of Experimental Botany, 60(11), 3279–3295. doi:10.1093/jxb/erp165
- Bell, D. K., Wells, H. D., & Markham, C. R. (1982). In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathology, 72(4), 379–382. doi:10.1094/Phyto-72-379
- Bergottini, V. M., Otegui, M. B., Sosa, D. A., Zapata, P. D., Mulot, M., Rebord, M., … Junier, P. (2015). Bio-inoculation of yerba mate seedlings (Ilex paraguariensis St. Hil.) with native plant growth-promoting rhizobacteria: A sustainable alternative to improve crop yield. Biology and Fertility of Soils, 53, 749–755. doi:10.1007/s00374-015-1012-5
- Carrero-Carrón, I., Trapero-Casas, J. L., Olivares-García, C., Monte, E., Hermosa, R., & Jiménez-Díaz, R. M. (2016). Trichoderma asperellum is effective for biocontrol of Verticillium wilt in olive caused by the defoliating pathotype of Verticillium dahliae. Crop Protection., 88, 45–52.
- Castillo, F. D. H., Berlanga Padilla, A. M., Gabriel Gallegos Morales, G., Cepeda Siller, M., Rodriguez Herrera, R., Aguilar Gonzales, C. N., & Castillo Reyes, F. (2011). In vitro Antagonist Action of Trichoderma Strains Against Sclerotinia sclerotiorum and Sclerotium cepivorum. American Journal of Agricultural and Biological Sciences, 6, 410–417. doi:10.3844/ajabssp.2011.410.417
- Desai, S., Reddy, M. S., & Kloepper, J. W. (2002). Comprehensive testing of biocontrol agents. In Gnanamanickam SS. Biological control of crop diseases (pp. 387–420). Boca Raton: CRC Press.
- Di Rienzo, J. A., Casanoves, F., Balzarini, M. G., Gonzalez, L., Tablada, M., & Robledo, C. W. InfoStat versión 2018. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. Retrieved from http://www.infostat.com.ar.
- Fages, J., & Arsac, J. F. (1991). Sunflower inoculation with Azospirillum and other plant growth promoting rhizobacteria. Plant and Soil, 137(1), 87–90. doi:10.1007/BF02187437
- Geraldine, A. M., Cardoso Lopes, F. A., Costa Carvalho, D. D., Barbosa, E. T., Rodrigues, A. R., Brandão, R. S., … Lobo Junior, M. (2013). Cell wall-degrading enzymes and parasitism of sclerotia are key factors on field biocontrol of white mold by Trichoderma spp. Biological Control., 67(3), 308–316. doi:10.1016/j.biocontrol.2013.09.013
- Harman, G. E., & Kubicek, C. P. (Eds.) (1998). Trichoderma and Gliocladium: Enzymes, biological control and commercial application (1st ed., Vol. 2, pp. 393.). London: Taylor & Francis Ltd.
- Harman, G. E., Howell, C. R., Viterbo, A., Chet, I., & Lorito, M. (2004). Trichoderma species-opportunistic, avirulent plant symbionts. Nature Reviews Microbiology, 2(1), 43–56. doi:10.1038/nrmicro797
- Ilany, T., Ashton, M., Montagnini, F., & Martinez, C. (2010). Using agroforestry to improve soil fertility: Effects of intercropping on Ilex paraguariensis (yerba mate) plantations with Araucaria angustifolia. Agroforestry Systems, 80(3), 399–409. doi:10.1007/s10457-010-9317-8
- Kinkel, L., Wilson, M., & Lindow, S. (2000). Plant species and plant incubation conditions influence variability in epiphytic bacterial population size. Microbial Ecology, 39(1), 1–11. doi:10.1007/s002489900182
- Kuklinsky-Sobral, J., Araújo, W. L., Mendes, R., Geraldi, I. O., Pizzirani-Kleiner, A. A., & Azevedo, J. L. (2004). Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environmental Microbiology, 12, 1244–1251. doi:10.1111/j.1462-2920.2004.00658.x
- López, A. C., Alvarenga, A. E., Zapata, P. D., Luna, M. F., & Villalba, L. L. (2019). Trichoderma spp. from Misiones, Argentina: Effective fungi to promote plant growth of the regional crop Ilex paraguariensis St. Hil. Mycology, 10(4), 210–221. doi:10.1080/21501203.2019.1606860
- Lorito, M., Harman, G. E., Hayes, C. K., Broadway, R. M., Tronsmo, A., Woo, S. L., & Di Pietro, A. (1993). Chitinolytic enzymes produced by Trichoderma harzianum: Antifungal activity of purified endochitinase and chitobiosidase. Phytopathology, 83(3), 302–307. doi:10.1094/Phyto-83-302
- Macías-Rodríguez, L., Guzmán-Gómez, A., García-Juárez, P., & Contreras-Cornejo, H. A. (2018). Trichoderma atroviride promotes tomato development and alters the root exudation of carbohydrates, which stimulates fungal growth and the biocontrol of the phytopathogen Phytophthora cinnamomi in a tripartite interaction system. FEMS Microbiology Ecology, 94(9)
- Mastouri, F., Bjorkman, T., & Harman, G. E. (2010). Seed treatment with Trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedlings. Phytopathology, 100(11), 1213–1221. doi:10.1094/PHYTO-03-10-0091
- Monte, E. (2001). Understanding Trichoderma: Between biotechnology and microbial ecology. International Microbiology, 4, 1–4.
- Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum, 15(3), 473–493. doi:10.1111/j.1399-3054.1962.tb08052.x
- Nelson, N. J. (1944). A photometric adaptation of the Somogyi method for the determination of glucose. Journal of Biological Chemistry, 153, 375–380.
- Newman, S. E., Brown, W. M., & Ozbay, N. (2002). The effect of the Trichoderma harzianum strains on the growth of tomato seedlings. In XXVI International Horticultural Congress: Managing Soil-Borne Pathogens: A Sound Rhizosphere to Improve Productivity, 635, 131–135.
- Pitt, J. I., & Hocking, A. D. (2009). Fresh and Perishable Foods. In: Fungi and Food Spoilage (pp. 383–400). Boston, MA: Springer .
- Prat Kricun, S. D., & Belingheri, L. D. (2003). Cosecha tradicional de la yerba mate. Cerro Azul: INTA, EEA. p. 12.
- Silva, B. D. S., Ulhoa, C. J., Batista, K. A., Yamashita, F., & Fernandes, K. F. (2011). Potential fungal inhibition by immobilized hydrolytic enzymes from Trichoderma asperellum. Journal of Agricultural and Food Chemistry, 59(15), 8148–8154. doi:10.1021/jf2009815
- Somogyi, M. (1952). Notes on sugar determination. Journal of Biological Chemistry, 195, 19– 23.
- StatPoint Inc. (2007). STATGRAPHICS Centurion XV, version 15.2.06. Warrenton, USA.
- Sturz, A. V., Christie, B. R., & Nowak, J. (2000). Bacterial endophytes: Potential role in developing sustainable systems of crop production. Critical Reviews in Plant Sciences, 19(1), 1–30. doi:10.1080/07352680091139169
- Taghdi, Y., Hermosa, R., Domínguez, S., Rubio, M. B., Essalmani, H., Nicolas, C., & Monte, E. (2015). Effectiveness of composts and Trichoderma strains for control of Fusarium wilt of tomato. Phytopathologia Mediterranea, 54, 232–240.
- Yedidia, I., Benhamou, N., & Chet, I. (1999). Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Applied and Environmental Microbiology, 65(3), 1061–1070. 1999doi:10.1128/AEM.65.3.1061-1070.1999
