 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Beach-cast seaweeds are unexplored feedstock with plentiful biomass in some localities that could be exploited as important sources of natural products. This study evaluated the potential of beach-cast seaweed extracts to inhibit the reverse transcriptase enzyme of the HIV-1. In general, the aqueous extracts showed better biotechnological potential as an antiviral agent than methanolic extracts with lower values of IC50. However, both extracts from Alsidium seaforthii, Osmundaria obtusiloba, Dictyopteris jolyana, and Zonaria tournefortii were highly promising, reaching inhibition above 90%, as well the aqueous extract of Spyridia clavata. Macroalgae are characterized by high levels of sulphated polysaccharides, such as the carrageenan and agar sulphated galactans in red algae, fucans and fucoidans in brown algae, and heterofucans in green algae, which have been related to antiviral biological activity. Furthermore, polyphenols and tannins have been reported as the main metabolites responsible for high antiviral activity in methanol extracts from red and brown algae. One or a combination of these compounds could explain our findings. Nowadays, there is a demand in the industrial and pharmaceutical sectors for natural products with potential bioactivity and beach-cast seaweeds could be a renewable, economically viable, and abundant resource for biotechnological approaches. Therefore, this research could help multiple UN SDGs as 1 (no poverty), 3 (good health and well-being), 5 (gender equality), 6 (clean water and sanitation), 8 (decent work and economic growth), 9 (industry, innovation, and infrastructure), 10 (reduced inequalities), 11 (sustainable cities and communities), 12 (responsible consumption and production), 13 (climate action), 14 (life below water), 17 (partnerships for the goals) through the integration of sustainable use of marine resources benefiting local communities, especially female equality, human and ecosystem health and sustainable consumption and development.
Introduction
The number of humans infected by the human immunodeficiency virus (HIV) in the world is growing exponentially. The World Health Organization estimated that 38 million people were living with HIV in 2019 (WHO, Citation2019), in which acquired immunodeficiency syndrome (AIDS) is one of the greatest contemporary public health diseases. HIV-1 and HIV-2 are two different strains of viral particles consisting of similar kinds of structure and symptoms, but the latter confined to Africa, whereas the former is dispersed to the rest of the world (Artan, Karadeniz, Karagozlu, Kim, & Kim, Citation2010). More than 25 million people infected with HIV live in African countries.
The search for new drugs based on seaweeds with therapeutic properties is driven by an eternal demand for health care and well-being. The indiscriminate use of antibiotics in the past years has led to the resistance of pathogens; therefore, studies have sought new sources of substances with anti-pathogen activity (Zaman et al., Citation2017). Marine macroalgae are an important source of natural products with bioactive properties, including antivirals (Torres, Flórez-Fernández, & Domínguez, Citation2019). In addition, natural products of macroalgae exhibit low toxicity when compared to synthetic drugs (Wang, Ooi, & Ang, Citation2008). Macroalgae have antiviral properties that provide a protective effect against several virus species by obstructing the spread of HIV and other sexually transmitted viruses, such as herpes simplex virus (HSV) and genital warts (Mendis & Kim, Citation2011).
Several bioactive metabolites derived from seaweeds, including terpenes, lipids, proteins, polyphenols, and especially a variety of sulphated polysaccharides, have shown antiviral activity for the infections caused by HIV and other viruses such as HSV, human papillomavirus, dengue virus, as well as coronavirus (SARS-CoV2) (Sangtani, Ghosh, Jha, Parmar, & Bala, Citation2020; Wittine, Saftić, Peršurić, & Kraljević Pavelić, Citation2019). All these compounds have shown a capacity to inhibit different stages of the virus life cycle. Polysaccharides and phenolic compounds from seaweeds have better antiviral activity than other bioactive substances because they prevent viral adsorption (simultaneous-treatment assay) and replication (post-treatment assay) (Kwon et al., Citation2013).
Such anti-viral activity has progressed to clinical trials. For instance, a carrageenan-based vaginal microbicide called Carraguard has been shown to block HIV and other sexually transmitted diseases in vitro (Zeitlin, Whaley, Hegarty, Moench, & Cone, Citation1997). Clinical trials have been carried out on women in South Africa to investigate the effectiveness of the gel as a topical microbicide for the prevention of HIV infections (Spieler, Citation2002). The safety of Carraguard was demonstrated, although protective action against HIV was not confirmed in phase III of clinical trials (Turville et al., Citation2008). Among the bioactive compounds, the polysaccharides are the ones that stand out the most.
The antiviral mechanism of macroalgal polysaccharides focuses on the viral attachment phase by: 1) attaching immediately with virions, 2) connecting to the protein to bind the respective receptors, and/or 3) immunomodulating activities that activate natural killer cells or prompt immune reactions (Shi et al., Citation2017). However, there is still the need for more research to comprehensively understand the antiviral action mechanisms of algae compounds and to benefit from their use as functional ingredients in the pharmaceutical and food industries (Pina-Pérez, Rivas, Martínez, & Rodrigo, Citation2017). The recent COVID-19 pandemic scenario is a perfect example of the need for further efforts to invest in the search for new marine natural products with antiviral activity, and recent research is promising (Gentile et al., Citation2020; Sangtani et al., Citation2020).
In this context, there is an extensive demand for new sources of bioactive products, in which beach-cast macroalgae can be considered emerging and promising sources of novel natural marine bioactivity, where the high availability of biomass is critical to make this activity sustainable. Based on these facts and considering that there are no algal cultures on a commercial scale in Brazil to supply the biomass market for exploitation, the beach-cast algae present in large quantities in the northeast coast and some localities in the southeast and south of Brazil, and also available in other countries, could constitute potential biomass as a source of functional ingredients and new biotechnological applications to meet the demands for new natural products that can be used as a matrix for various ingredients for biotechnological purposes and diverse industries.
This study aims to evaluate the in vitro potential of methanolic and aqueous extracts from different species of beach-cast seaweeds as antiviral agents by the capacity to inhibit the enzyme reverse transcriptase of the HIV-1 virus (HIV-RT). Beach-cast seaweeds are underexplored feedstock and could be a renewable, economically viable, and abundant resource for biotechnological approaches.
Material and methods
Biological material and extraction procedure
Thirteen selected species and two mixtures (Mix) of beach-cast macroalgae were collected from the northeast (Ceará and Paraíba states) and southeast (Espírito Santo State) of the Brazilian coast. Identity, location, herbarium voucher, and collection data are summarized in . The collected material was cleaned of macro-epiphytes, washed in tap water, centrifuged and then air-dried in the shade. In the laboratory, the material was oven-dried at 40°C until constant dry matter (DM) weight was achieved. This was then ground to a fine powder in a ball mill (MA350, Marconi, Brazil). Each sample was divided into five sub-samples (n = 5) for the extraction procedure (technical replicates). Extraction was performed using solvents of increasing polarity by simple sequential dynamic maceration in hexane, dichloromethane, ethyl acetate, methanol, and water (80°C) in a ratio of 1 g DM: 30 ml of solvent. The maceration extraction sin hexane, dichloromethane, ethyl acetate, and methanol were performed at room temperature and three times for each solvent, changing the respective solvent every 24 h. Water extraction was carried out at 80°C in a water bath, three times for 3 h each. The supernatant from each solvent was filtered and pooled as a single extract sample for each technical replicate, amounting to five crude extracts in hexane, dichloromethane, ethyl acetate, methanol, and water for each sample. Organic extracts were concentrated by oven-drying (at 40°C) and then lyophilizing the sample, while aqueous extracts were directly lyophilized. Extract yield was calculated as a percentage of the initial DM used in the extraction. Both methanolic and aqueous extracts exhibited high yields of compounds of interest, so further quantification analyses were performed with these extracts.
Table 1. List of selected species of beach-cast marine algae harvested from the Brazilian coast at northeast Ceará (CE) and Paraíba (PB) and southeast Espírito Santo (ES).
HIV-reverse transcriptase (HIV-RT) inhibition assay
The antiviral activity of the extracts was analysed according to their ability to inhibit the activity of the HIV-1 virus reverse transcriptase enzyme by the colorimetric method based on the reverse transcriptase test kit (Roche, Germany). The dried extracts were solubilized in 10% DMSO prepared with DEPC (diethylpyrocarbonate) water at a stock concentration of 2 mg ml–1. A preliminary screening of the antiviral activity was performed at a concentration of 400 µg ml–1 for methanolic extracts and 200 µg ml–1 for aqueous extracts to choose promising algal extracts and concentrations to be tested and estimate the IC50 (50% inhibition concentration of the HIV-RT activity). The negative control was done with 10% DMSO with DEPC water and Foscarnet standard solution (sodium phosphonoformate tribasic hexahydrate) in 10% DMSO was used as a positive control. A Foscarnet standard curve at 0–1 μg ml–1 was constructed.
The test kit was used following the manufacturer’s instructions. Sample or standard or negative control (20 μl) was mixed with 20 μl of template/primer containing solution poly(A)+oligo(dT) and nucleotides labelled with biotin and digoxigenin (DIG), 19.5 μl of lysis buffer, and 0.5 μl of HIV-reverse transcriptase (RT) enzyme. The reaction was incubated for 1 h at 37°C under constant shaking. After this step, the reaction mixture was transferred to a 96 well microplate treated with streptavidin, a tetrameric protein that has a strong affinity with biotin. The reaction mixture was incubated once again in a shaker for 1 h at 37°C, binding the RNA/DNA molecules to the wells of the microplate. After the incubation period, the microplate was washed five times with 200 μl of wash buffer. After complete removal of the wash buffer, 200 μl of the solution containing incubation buffer and anti-digoxigenin-peroxidase antibody (anti-DIG-POD) was added to each well, this antibody binds strongly to the digoxigenin present in the labelled nucleotides of RNA/DNA molecule.
In the final step, the microplate was incubated again under a shaker for 1 h at 37°C and then washed five times with 200 μl wash buffer. The buffer was withdrawn completely and, in each well, 200 μl of ABTS solution dissolved in substrate buffer (sodium perborate and citric acid/phosphate buffer) was added. The enzyme peroxidase (bound to the antibody) catalyzes the breakdown of the substrate, releasing hydroxyl radical (OH●), which reacts with ABTS (green colour) forming the ABTS+ radical with intense green colouration. The microplate was incubated for 30 min at room temperature; then the absorbance readings were performed at 405 nm and 490 nm in the UV–Vis microplate spectrophotometer (Epoch Biotek, USA).
The results were expressed as a percentage of inhibition, according to Woradulayapinij, Soonthornchareonnon, & Wiwat (Citation2005):
where: Abs405NC – absorbance of the negative control at 405 nm; Abs490NC – absorbance of the negative control at 490 nm; Abs405S – absorbance of the sample at 405 nm; Abs490S – absorbance of the sample at 490 nm.
With the percentage of inhibition at different concentrations, the IC50 of the samples and Foscarnet were calculated with the software GraphPad Prism®6 (Inc., USA) using a sigmoidal fit model. Only methanolic extracts from D. jolyana (ES and PB), Z. tournefortii, A. seaforthii, and O. obtusiloba (ES and PB) and aqueous extracts from D. jolyana (ES and PB), Z. tournefortii, A. seaforthii, O. obtusiloba (ES), and S. clavata were tested in different extract concentrations.
Data analysis
All the analytical analyses were carried out from the extraction of five subsamples (n = 5, technical replicates) and considered for statistical analysis. Statistical analyses were performed using Statistica 10 software, previously tested for normality (Kolmogorov–Smirnov test) and homoscedasticity (Bartlett’s test). The percentage values of antiviral activity were transformed into square root arcsine according to Snedecor (Citation1966). A one-way analysis of variance (ANOVA) was used to observe significant differences (p < 0.05) and when differences were detected, Newman–Keuls post-hoc multiple comparison test was applied.
Results
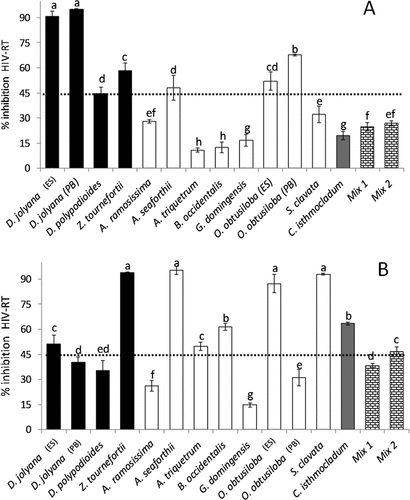
Only the methanolic and aqueous extracts were studied due to the higher yields of compounds of interest (approximately 2.5–29% in methanol and 9.5–37% in aqueous extracts) in comparison to the other solvents (0.18–1% in hexane, 0.2–1.9% in dichloromethane, and 0.2–1% in ethyl acetate extracts) as previously described in Harb, Pereira, Cavalcanti, Fujii, and Chow (Citation2021). A preliminary screening was conducted at a concentration of 400 µg ml–1 for methanolic extracts () and 200 µg ml–1 for aqueous extracts (), in which both extracts of D. jolyana (ES) and (PB), Z. tournefortii, A. seafortii, and O. obtusiloba (ES) inhibited HIV-RT ~45%. Individually, the methanolic extracts of D. polypodioides and O. obtusiloba (PB) and the aqueous extracts of A. triquetrum, B. occidentalis, S. clavata, and C. isthmocladum showed inhibitory percentage over 45%.
Figure 1. Preliminary screening of inhibition of the enzyme HIV-RT (mean ± SD; n = 3) of beach-cast seaweeds for (a) methanolic extracts at the concentration of 400 µg ml−1 and (b) aqueous extracts at the concentration of 200 µg ml−1. letters indicate significant differences (p < 0.05) according to one-way ANOVA and Newman-keuls post-hoc test. black bars: phaeophyceae’s species; white bars: rhodophyta’s species; grey bar: Chlorophyta’s species; drawn bars: mixtures of species. the dotted line corresponds to 45% of antiviral activity.
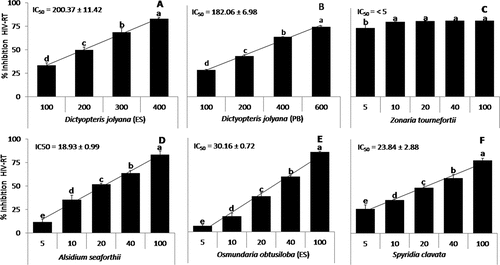
Some of the most promising methanolic and aqueous extracts with higher inhibitory activity (above 45%) were tested at different concentrations to calculate the respective IC50 and are presented in , respectively. Both extracts showed significant differences among concentrations of algal crude extracts with an increasing tendency between concentration and activity, except for the methanolic extract of D. jolyana (PB) (50–400 µg ml–1: 90% of HIV-RT inhibition; ) and aqueous extract of Z. tournefortii (5–100 µg ml–1: 80–85% of HIV-RT inhibition; ). Both exceptions showed the greatest efficiency with inhibitory values close to 100% at the minimum algal crude extract tested and few significant differences were observed among concentrations of these algal extracts; therefore, IC50 was not calculated. The standard curve of Foscarnet (positive control) obtained an IC50 of 0.061 µg ml–1.
Figure 2. (a-f) percentage of inhibition of the enzyme HIV-RT (mean ± SD; n = 3) of beach-cast seaweeds at different concentrations of methanolic extracts (µg ml−1). the calculated values of IC50 (half-maximal inhibitory concentration, µg ml−1) were included for each species. letters indicate significant differences (p < 0.05) according to one-way ANOVA and Newman-keuls post-hoc test.
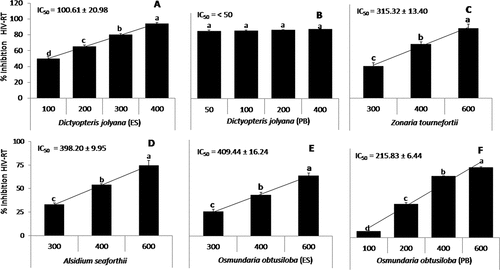
Figure 3. (a-f) percentage of inhibition of the enzyme HIV-RT (mean ± SD; n = 3) of beach-cast seaweeds at different concentrations of aqueous extracts (µg ml−1). the calculated values of IC50 (half-maximal inhibitory concentration, µg ml−1) were included for each species. letters indicate significant differences (p < 0.05) according to one-way ANOVA and Newman-keuls post-hoc test.
shows the comparison of antiviral activity (HIV-RT) from extracts and isolated compounds from macroalgae present in the literature, including the results of the present study. Isolated polysaccharides and diterpenes have been described as having low IC50 values (0.50–1.00 µg ml–1). The IC50 values for crude aqueous and methanolic extracts from attached benthic macroalgae ranged between 12.39 and 300 µg ml–1, while the IC50 from our beach-cast seaweeds ranged from <5 to 409.44 µg ml–1.
Table 2. IC50 values from some studies evaluating antiviral activity (HIV-RT) from isolated and crude extracts from macroalgae.
Discussion
The HIV infection is caused by a virus within the subfamily of retroviruses, also known as lentiviruses or slow viruses. This means that from the moment of infection to the appearance of the first signs of the disease, a long time can pass (Besednova et al., Citation2019). Over the last years, numerous nucleoside analogues have been applied in antiviral therapy and an important role has been described for HIV, herpes viruses, hepatitis B, and hepatitis C infections (Jordheim, Durantel, Zoulim, & Dumontet, Citation2013). The isolation of nucleosides from Brazilian beach-cast seaweeds could be an effective tool for the discovery of new drugs of marine origin, where the high availability of biomass is a fundamental factor for bioprospection and sustainable development. The targets of these nucleoside analogue drugs are the virus-encoded DNA- or RNA-polymerases, such as RT, a central step in viral infection (El Safadi, Vivet-Boudou, & Marquet, Citation2007; Sarafianos et al., Citation2009).
Over the last decade, red and brown algae have attracted much interest in the search of anti-HIV action and fewer reports are found for green algae (Mattos, Romanos, Souza, Sassaki, & Barreto-Bergter, Citation2011; Rodrigues, Torres, de Alencar, Sampaio, & Farias, Citation2009; Vlietinck et al., Citation1998, Yasuhara-Bell & Lu, Citation2010). The highest extract yields obtained for methanolic and aqueous extracts for the species of beach-cast seaweeds suggest that the main matrix of secondary metabolites is composed of polar components. Bioactive polar compounds in macroalgae generally include proteins, lectins, amino acids, phenolic compounds (highly present in methanolic extract), and sulphated polysaccharides (highly present in hot aqueous extract) (Rosales-Mendoza et al., Citation2020; Singh & Walia, Citation2018).
In a previous study by Harb et al. (Citation2021) the chemical composition of the same species and extracts were evaluated. Regarding methanolic extracts, D. jolyana (PB and ES), Z. tournefortii and O. obtusiloba (PB and ES) showed the highest content of phenolic compounds among the 15 extracts analysed. D. jolyana (PB and ES), A. seaforthii, O. obtusiloba (PB) and Z. tournefortii showed better results for carbohydrate content. The sulphation degree of methanolic extracts was higher for the red alga O. obtusiloba (ES and PB) and the brown alga D. jolyana (ES). Regarding aqueous extracts, D. jolyana (PB and ES), Z. tournefortii and O. obtusiloba (PB) showed the highest result for phenolic compounds, but the carbohydrate content was higher for the extracts of D. jolyana (PB and ES), Z. tournefortii and O. obtusiloba (PB). Finally, sulphation degree showed high values for the aqueous extract of red beach-cast seaweed. The species with high content of chemical metabolites from methanolic and aqueous extracts also showed high antiviral activity in the present study.
Sulphated polysaccharides are the most important metabolites contained in large amounts in aqueous extracts of seaweeds (Shi et al., Citation2017). The type of sulphated polysaccharides in macroalgae, such as fucoidan, agar, carrageenan, porphyran, laminarin, galactan, and ulvan, differs depending on the taxonomic group (Klongklaew, Praiboon, Tamtin, & Srisapoome, Citation2020). The results of aqueous extracts showed high activity for the red beach-cast algae A. seaforthii, O. obtusiloba (ES), and S. clavata, these findings might result from the action of sulphated polysaccharides, especially agarans.
Among several kinds of algal polysaccharides, carrageenans from red algae are the most studied and considered safe for human use (Weiner, Citation2016). An isolated polysaccharide from Agardhiella subulata (C) Kraft & M.J. showed activity against HIV, with IC50 values of 0.5 μg ml–1 (Witvrouw et al., Citation1994). In a study conducted on the anti-HIV activity of a mixture of carrageenans from red algae, the antiviral activity of polysaccharides was strong selective inhibitors of HIV-1 replication in human T-cell leukaemia (MT4) cells (Besednova et al., Citation2019). Moreover, the antiviral activity increased with an increase in the molecular weight of the compounds and the degree of their sulphation (Besednova et al., Citation2019).
Few studies assess HIV-RT activity with polar macroalgal extracts. Studies with beach-cast marine algae are even rarer; recently Harb et al. (Citation2021) reported that methanolic and aqueous extracts of Brazilian beach-cast seaweeds are rich in phenolic compounds and sulphated carbohydrates, respectively. Furthermore, these metabolites exhibited a positive correlation with the antioxidant activities found. The antiviral activity described in the present study can be attributed to bioactive polar compounds that are methanolic- and water-soluble. Some polar seaweed’s metabolites such as sulphated polysaccharides, proteins, and phenolic compounds have been described with high antiviral activity for HIV in a recent review (Besednova et al., Citation2019).
Anti-HIV activity is also well reported for brown algae polysaccharides (Ahn et al., Citation2002, Citation2004, Citation2006; Artan et al., Citation2008; Kim & Karadeniz, Citation2011; Queiroz et al., Citation2008). Polysaccharides from Dictyopteris and Zonaria genus contain heterofucans mainly composed of xylose, fucose, galactose, glucose, sulphate, and uronic acid (Khora & Navya, Citation2020).
In the study of Thuy et al. (Citation2015), fucoidan isolated from three species of brown algae possessed anti-HIV activity in the cell lines. Trinchero et al. (Citation2009) showed that galactofucan fractions from brown algae were active against HIV-1 in vitro. It was proved that the inhibitory effect is not due to the inactivation of the virus, but by blocking the early stages of virus replication and, therefore, the authors recommend these substances as good candidates as preventive and therapeutic drugs against HIV infection. Studies reported that laminaran, a water‐soluble polysaccharide found in some species of Dictyopteris (Karaki et al., Citation2013), potently prevents the replication and proliferation of HIV via suppressing the viral binding with lymphocytes (Ahmadi, Zorofchian Moghadamtousi, Abubakar, & Zandi, Citation2015).
Among the beach-cast seaweeds from the area of collection, green algae species are the least frequent. This influenced our study because we did not have other Chlorophyta species in abundance. In addition, the C. isthmocladum collected was trapped in rhodoliths, which is also an indication of the abundance of this species in rhodolith banks in the region.
Few studies have evaluated anti-HIV potential from Codium species. Ahn et al. (Citation2002) studied the anti-HIV potential of two species of this genus and the methanolic extracts did not show activity to inhibit HIV-RT, in our findings C. isthmocladum showed low antiviral activity for the methanolic extract. In contrast, the results obtained for the aqueous extracts of the beach-cast C. isthmocladum exhibited promising HIV-RT inhibition. Species from the genus Codium biosynthesize water-soluble sulphated arabinans and galactans with bioactivity already reported for these metabolites (Fernández, Arata, & Ciancia, Citation2014). These substances may have been responsible for the high activity found for the species in the aqueous extract. These results agree with Amorim (Citation2018) for the aqueous extract of non-beach-cast C. isthmocladum that also showed high antiviral activity HIV-RT. This scenario demonstrates that further studies are needed on the antiretroviral potential of C. isthmocladum to clarify the substances responsible for its anti-HIV action.
Other polar metabolites with high antiviral HIV-RT are proteins, mainly lectins that can be found in methanolic extracts. Lectins of red, brown, and green algae (Singh & Walia, Citation2018) are considered as potential candidates for preventing sexual transmission of HIV as a microbicide (Alexandre et al., Citation2012). They not only inhibit the infection of cells with HIV but can effectively prevent the transmission of the pathogen from infected cells to uninfected CD4 + T lymphocytes (Huskens & Schols, Citation2012). In a study carried out by Sato et al. (Citation2011), a binding lectin was isolated from green macroalgae. The lectin showed antiviral activity against HIV-1 infections and influenza viruses (Queiroz et al., Citation2008). The mechanism of action anti-HIV-1 occurs when lectin or glycoproteins, bind to the carbohydrate moiety of the virus to inhibit its attachment to the target cells and also to hinder the replication of viral RNA (Sangtani et al., Citation2020).
Especially in methanolic extracts, red and green seaweeds can contain different amounts of phenolic compounds such as flavonoids, glycosylated flavonoids, phenolics acids, catechins, and bromophenols responsible for antiviral activity in polar extracts, while phlorotannins are the major polyphenolic secondary compounds synthesized mainly by marine brown seaweed (Deyab, Elkatony, & Ward, Citation2016; Gómez-Guzmán, Rodríguez-Nogales, Algieri, & Gálvez, Citation2018; Yoshie-Stark, Hsieh, & Suzuki, Citation2003).
Brown algae showed the highest antiviral activity among the studied beach-cast seaweeds, even in a very low extract concentration. Despite the high percentage of antiviral inhibition, the IC50 values of the species were superior to the standard IC50 of Foscarnet (0.061 µg ml–1). However, it is still evident the antiviral potential of the beach-cast seaweeds, especially for D. jolyana (ES – methanolic extract) and Z. tournefortii (aqueous extract), which the IC50 could not be calculated due to the high activity reached in all concentrations tested. In the literature, these species showed promising antiviral activity for other viruses such as HSV (Bianco et al., Citation2013) and Hepatitis Β for the Zonaria genus (Premnathan, Chandra, Bajpai, & Kathiresan, Citation1992).
Moreover, phlorotannins are polar metabolites rich in the chemical composition of brown macroalgae, commonly found in Z. tournefortii and species from Dictyopteris genus (Murray, Dordevic, Ryan, & Bonham, Citation2018; Nunes et al., Citation2017). According to the mechanism of action, these substances can act at different stages of viral infection and can inhibit adsorption, reverse transcriptase, and transcription (Ahn et al., Citation2004). Therefore, it is suggested that phlorotannins contributed to the high HIV-RT response of brown beach-cast algae.
In the literature, most studies with macroalgae evaluate the antiviral activity of an isolated substance, such as Queiroz et al. (Citation2008), Trinchero et al. (Citation2009), and Cavalcanti et al. (Citation2011), this explains the low IC50 values reported by these authors. Santos, Torres, dos Santos, Motta, & Chow (Citation2019) analysing the antiviral activity in the hot water extract from Sargassum vulgare C.Agardh observed seasonal differences in the antiviral activity of the species, indicating that the environmental conditions in which the macroalgae are exposed may exert influence on the production of bioactive substances. Studies have been suggested to evaluate the seasonal variation of antiviral activity for the selected beach-cast species.
According to Cos, Vlietinck, Berghe, and Maes (Citation2006), extracts with promising biological activities should have an IC50 value below 100 μg ml–1. From this perspective, the most promising species in the present study were the aqueous extracts of the brown alga Z. tournefortii and the red algae A. seaforthii, S. clavata, and O. obtusiloba, with IC50 values that ranged from <5 to 30.16 µg ml–1 and the methanolic extracts of D. jolyana from ES (100.61 µg ml–1) and PB (<50 µg ml–1).
Comparing the IC50 results of beach-cast algae from ES (present study) with the attached ones from the same place in the study of Amorim (Citation2018), it is possible to note that the aqueous extracts of beach-cast algae, especially the red algae (A. seaforthii – 18.93 µg ml–1, S. clavata – 23.84 µg ml–1 and O. obtusiloba – 30.16 µg ml–1), showed higher antiviral activity than non-beach-cast macroalgae (S. cymosum – 50 µg ml–1, P. gymnospora – 70 µg ml–1, C. minima – 100 µg ml–1 and D. plagiogramma – 100 µg ml–1). These results can imply that beach-cast algae have a huge potential, similar to attached macroalgae. However, it is important to take into account the type of drying, extraction, solvents, biotic and abiotic factors that can influence these algal responses. Thus, further studies are needed to assess the fluctuation of the antiviral response of beach-cast seaweeds.
The present study is the first report that evaluated the biotechnological potential of beach-cast algae as an antiviral agent. Such applied feature brings environmental benefits by mitigating coastal pollution, generating valuable usable resources, job opportunities and financial incomes, in addition to ensuring sustainable socio-economic development. Thus, this research contributes to multiple UN SDGs by using, life below water to bring about reduction of poverty and inequalities and promote good health and well-being, gender equality, decent work opportunities and economic growth, clean water and sanitation, sustainable cities and communities, and responsible consumption and production, aggregating innovation development and partnerships for the goals.
The biotechnological potential of the studied beach-cast seaweeds is highly promising, reaching percentages of inhibition above 90%. Methanolic and aqueous extracts showed an efficient inhibitory activity for brown and red algae; therefore, we can suggest that beach-cast seaweeds could be a potential renewable source of natural antiviral activity, including economically viable and abundant resources for prospective approaches. The in vitro results of the antiviral potential of beach-cast seaweeds suggest the utility of a greater investment into research to elucidate which substances are present in the methanolic and aqueous extracts that are responsible for the activity, in addition to understanding the mechanisms of action for inhibiting HIV-RT.
Author statement
This manuscript has not been published in whole or in part nor is it being considered for publication elsewhere. The authors have no conflicts of interest to declare.
Acknowledgments
We thank Cia das Algas for the support with the expeditions for collections of the raw material.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Funding
References
- Ahmadi, A., Zorofchian Moghadamtousi, S., Abubakar, S., & Zandi, K. (2015). Antiviral potential of algae polysaccharides isolated from marine sources: A review. BioMed Research International, 2015, 1–10.
- Ahn, M. J., Yoon, K. D., Kim, C. Y., Kim, J. H., Shin, C. G., & Kim, J. (2006). Inhibitory activity on HIV‐1 reverse transcriptase and integrase of a carmalol derivative from a brown alga. Ishige Okamurae. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives, 20, 711–713.
- Ahn, M. J., Yoon, K. D., Kim, C. Y., Min, S. Y., Kim, Y. U., Kim, H. J., … Kim, S. H. (2002). Inhibition of HIV-1 reverse transcriptase and HIV-1 integrase and antiviral activity of Korean seaweed extracts. Journal of Applied Phycology, 14, 325–329.
- Ahn, M. J., Yoon, K. D., Min, S. Y., Lee, J. S., Kim, J. H., Kim, T. G., & Kim, J. (2004). Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga Ecklonia cava. Biological & Pharmaceutical Bulletin, 27, 544–547.
- Alexandre, K. B., Gray, E. S., Mufhandu, H., McMahon, J. B., Chakauya, E., O’Keefe, B. R., & Morris, L. (2012). The lectins griffithsin, cyanovirin-N and scytovirin inhibit HIV-1 binding to the DC-SIGN receptor and transfer to CD4+ cells. Virology, 423, 175–186.
- Amorim, A. M. P. B. (2018). Study of antioxidants, bioactive potential and chemical composition of Chnoospora minima, Dictyopteris plagiogramma, Padina gymnospora, Sargassum cymosum (Ochrophyta) and Codium isthmocladum (Chlorophyta). master dissertation. Institute of Bioscience. University of São Paulo. 122. doi.10.11606/D.41.2019.tde-18022019-091636
- Artan, M., Karadeniz, F., Karagozlu, M. Z., Kim, M. M., & Kim, S. K. (2010). Anti-HIV-1 activity of low molecular weight sulfated chitooligosaccharides. Carbohydrate Research, 345, 656–662.
- Artan, M., Li, Y., Karadeniz, F., Lee, S. H., Kim, M. M., & Kim, S. K. (2008). Anti-HIV-1 activity of phloroglucinol derivative, 6, 6′-bieckol, from Ecklonia cava. Bioorganic & Medicinal Chemistry, 16, 7921–7926.
- Besednova, N. N., Zvyagintseva, T. N., Kuznetsova, T. A., Makarenkova, I. D., Smolina, T. P., Fedyanina, L. N., & Zaporozhets, T. S. (2019). Marine algae metabolites as promising therapeutics for the prevention and treatment of HIV/AIDS. Metabolites, 9, 87.
- Bianco, É. M., De Oliveira, S. Q., Rigotto, C., Tonini, M. L., Da rosa guimarães, T., Bittencourt, F., & Martins, C. D. L. (2013). Anti-infective potential of marine invertebrates and seaweeds from the Brazilian coast. Molecules, 18, 5761–5778.
- Cavalcanti, D. N., de Oliveira, M. A. R., De-Paula, J. C., Barbosa, L. S., Fogel, T., Pinto, M. A., … Teixeira, V. L. (2011). Variability of a diterpene with potential anti-HIV activity isolated from the Brazilian brown alga Dictyota menstrualis. Journal of Applied Phycology, 23, 873–876.
- Cos, P., Vlietinck, A. J., Berghe, D. V., & Maes, L. (2006). Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. Journal of Ethnopharmacology, 106, 290–302.
- Deyab, M., Elkatony, T., & Ward, F. (2016). Qualitative and quantitative analysis of phytochemical studies on brown seaweed. Dictyota dichotoma. International Journal of Engineering Development and Research, 4, 2321–2330.
- El Safadi, Y., Vivet-Boudou, V., & Marquet, R. (2007). HIV-1 reverse transcriptase inhibitors. Applied Microbiology and Biotechnology, 75, 723–737.
- Fernández, P. V., Arata, P. X., & Ciancia, M. (2014). Polysaccharides from Codium species: Chemical structure and biological activity their role as components of the cell wall. Advances in Botanical Research, 71, 253–278.
- Gentile, D., Patamia, V., Scala, A., Sciortino, M. T., Piperno, A., & Rescifina, A. (2020). Putative inhibitors of SARS-CoV-2 main protease from a library of marine natural products: A virtual screening and molecular modeling study. Marine Drugs, 18, 225.
- Gómez-Guzmán, M., Rodríguez-Nogales, A., Algieri, F., & Gálvez, J. (2018). Potential role of seaweed polyphenols in cardiovascular-associated disorders. Marine Drugs, 16, 250.
- Harb, T. B., Pereira, M. S., Cavalcanti, M. I. L. G., Fujii, M. T., & Chow, F. (2021). Antioxidant activity and related chemical composition of extracts from Brazilian beach-cast marine algae: Opportunities of turning a waste into a resource. Journal of Applied Phycology, 33, 3383–3395.
- Huskens, D., & Schols, D. (2012). Algal lectins as potential HIV microbicide candidates. Marine Drugs, 10, 1476–1497.
- Jordheim, L. P., Durantel, D., Zoulim, F., & Dumontet, C. (2013). Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nature Reviews. Drug Discovery, 12, 447–464.
- Karaki, N., Sebaaly, C., Chahine, N., Faour, T., Zinchenko, A., Rachid, S., & Kanaan, H. (2013). The antioxidant and anticoagulant activities of polysaccharides isolated from the brown algae Dictyopteris polypodioides growing on the Lebanese coast. Journal of Applied Pharmaceutical Science, 3, 43–51.
- Khora, S. S., & Navya, P. (2020). Bioactive polysaccharides from marine macroalgae. Encyclopedia of Marine Biotechnology, 121–145. doi:10.1002/9781119143802.ch6
- Kim, S. K., & Karadeniz, F. (2011). Anti-HIV activity of extracts and compounds from marine algae. Advances in Food and Nutrition Research, 64. doi:10.1016/B978-0-12-387669-0.00020-X
- Klongklaew, N., Praiboon, J., Tamtin, M., & Srisapoome, P. (2020). Antibacterial and antiviral activities of local Thai green macroalgae crude extracts in pacific white shrimp (Litopenaeus vannamei). Marine Drugs, 18, 140.
- Kwon, H. J., Ryu, Y. B., Kim, Y. M., Song, N., Kim, C. Y., Rho, M. C., & Park, S. J. (2013). In vitro antiviral activity of phlorotannins isolated from Ecklonia cava against porcine epidemic diarrhea coronavirus infection and hemagglutination. Bioorganic & Medicinal Chemistry, 21, 4706–4713.
- Mattos, B. B., Romanos, M. T. V., Souza, L. M. D., Sassaki, G., & Barreto-Bergter, E. (2011). Glycolipids from macroalgae: Potential biomolecules for marine biotechnology? Revista Brasileira De Farmacognosia, 21, 244–247.
- Mendis, E., & Kim, S. K. (2011). Present and future prospects of seaweeds in developing functional foods. Advances in Food and Nutrition Research, 64, 1–15.
- Murray, M., Dordevic, A. L., Ryan, L., & Bonham, M. P. (2018). An emerging trend in functional foods for the prevention of cardiovascular disease and diabetes: Marine algal polyphenols. Critical Reviews in Food Science and Nutrition, 58, 1342–1358.
- Nunes, N., Ferraz, S., Valente, S., Barreto, M. C., & De Carvalho, M. P. (2017). Biochemical composition, nutritional value, and antioxidant properties of seven seaweed species from the Madeira Archipelago. Journal of Applied Phycology, 29, 2427–2437.
- Pina-Pérez, M. C., Rivas, A., Martínez, A., & Rodrigo, D. (2017). Antimicrobial potential of macro and microalgae against pathogenic and spoilage microorganisms in food. Food Chemistry, 235, 34–44.
- Premnathan, M., Chandra, K., Bajpai, S. K., & Kathiresan, K. (1992). A survey of some Indian marine plants for antiviral activity. Botanica Marina, 35, 321–324.
- Queiroz, K. C. S., Medeiros, V. P., Queiroz, L. S., Abreu, L. R. D., Rocha, H. A. O., Ferreira, C. V., & Leite, E. L. (2008). Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomedicine & Pharmacotherapy, 62, 303–307.
- Rodrigues, J. A., Torres, V. M., de Alencar, D. B., Sampaio, A. H., & Farias, W. R. (2009). Extraction and anticoagulant activity of sulfated polysaccharides from the red marine alga. Halymenia Pseudofloresia. Revista Ciência Agronômica, 40, 224.
- Rosales-Mendoza, S., García-Silva, I., González-Ortega, O., Sandoval-Vargas, J. M., Malla, A., & Vimolmangkang, S. (2020). The potential of algal biotechnology to produce antiviral compounds and biopharmaceuticals. Molecules, 25, 4049.
- Sangtani, R., Ghosh, A., Jha, H. C., Parmar, H. S., & Bala, K. (2020). Potential of algal metabolites for the development of broad-spectrum antiviral therapeutics: Possible implications in COVID-19 therapy. Phytotherapy Research, 35, 2296–2316.
- Santos, J. P., Torres, P. B., dos Santos, D. Y., Motta, L. B., & Chow, F. (2019). Seasonal Effects on Antioxidant and anti-HIV Activities of Brazilian Seaweeds. Journal of Applied Phycology, 31, 1333–1341.
- Sarafianos, S. G., Marchand, B., Das, K., Himmel, D. M., Parniak, M. A., Hughes, S. H., & Arnold, E. (2009). Structure and function of HIV-1 reverse transcriptase: Molecular mechanisms of polymerization and inhibition. Journal of Molecular Biology, 385, 693–713.
- Sato, Y., Hirayama, M., Morimoto, K., Yamamoto, N., Okuyama, S., & Hori, K. (2011). High mannose-binding lectin with preference for the cluster of α1–2-mannose from the green alga Boodlea coacta is a potent entry inhibitor of HIV-1 and influenza viruses. Journal of Biological Chemistry, 286, 19446–19458.
- Shi, Q., Wang, A., Lu, Z., Qin, C., Hu, J., & Yin, J. (2017). Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydrate Research, 453-454, 1–9.
- Singh, R. S., & Walia, A. K. (2018). Lectins from Red Algae and Their Biomedical Potential. Journal of Applied Phycology, 30, 1833–1858.
- Snedecor, G. W. (1966). Metodos estadisticos: Aplicacion a la investigacion agricola y biologica (No. C015. 027). Compania Editorial Continental SA.
- Spieler, R. (2002). Seaweed compound’s anti-HIV efficacy will be tested in Southern Africa. The Lancet, 359, 1675.
- Thuy, T. T. T., Ly, B. M., Van, T. T. T., Van Quang, N., Tu, H. C., Zheng, Y., & Ai, U. (2015). Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydrate Polymers, 115, 122–128.
- Torres, M. D., Flórez-Fernández, N., & Domínguez, H. (2019). Integral utilization of red seaweed for bioactive production. Marine Drugs, 17, 314.
- Trinchero, J., Ponce, N. M., Córdoba, O. L., Flores, M. L., Pampuro, S., Stortz, C. A., … Turk, G. (2009). Antiretroviral activity of fucoidans extracted from the brown seaweed Adenocystis utricularis. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives, 23, 707–712.
- Turville, S. G., Aravantinou, M., Miller, T., Kenney, J., Teitelbaum, A., Hu, L., & Lifson, J. D. (2008). Efficacy of Carraguard®-based microbicides in vivo despite variable in vitro activity. PloS One, 3, e3162.
- Vlietinck, A. J., De Bruyne, T., Apers, S., & Pieters, L. A. (1998). Plant-derived leading compounds for chemotherapy of human immunodeficiency virus (HIV) infection. Planta Medica, 64, 97–109.
- Wang, H., Ooi, E. V., & Ang, P. O. (2008). Antiviral activities of extracts from Hong Kong seaweeds. Journal of Zhejiang University. Science. B, 9, 969–976.
- Weiner, M. L. (2016). Parameters and pitfalls to consider in the conduct of food additive research, Carrageenan as a case study. Food and Chemical Toxicology, 87, 31–44.
- WHO, World Health Organization. (2019). Retrieved from https://www.who.int/hiv/data/en/accessedat15/17/2020
- Wittine, K., Saftić, L., Peršurić, Ž., & Kraljević Pavelić, S. (2019). Novel antiretroviral structures from marine organisms. Molecules, 24, 3486.
- Witvrouw, M., Este, J. A., Mateu, M. Q., Reymen, D., Andrei, G., Snoeck, R., & De Clercq, E. (1994). Activity of a sulfated polysaccharide extracted from the red seaweed Aghardhiella tenera against human immunodeficiency virus and other enveloped viruses. Antiviral Chemistry & Chemotherapy, 5, 297–303.
- Woradulayapinij, W., Soonthornchareonnon, N., & Wiwat, C. (2005). In vitro HIV type 1 reverse transcriptase inhibitory activities of Thai medicinal plants and Canna indica L. rhizomes. Journal of Ethnopharmacology, 101, 84–89.
- Yasuhara-Bell, J., & Lu, Y. (2010). Marine compounds and their antiviral activities. Antiviral Research, 86, 231–240.
- Yoshie-Stark, Y., Hsieh, Y. P., & Suzuki, T. (2003). Distribution of flavonoids and related compounds from seaweeds in Japan. Journal-Tokyo University of Fisheries, 89, 1–6.
- Zaman, S. B., Hussain, M. A., Nye, R., Mehta, V., Mamun, K. T., & Hossain, N. (2017). A review on antibiotic resistance: Alarm bells are ringing. Cureus, 9, 6.
- Zeitlin, L., Whaley, K. J., Hegarty, T. A., Moench, T. R., & Cone, R. A. (1997). Tests of vaginal microbicides in the mouse genital herpes model. Contraception, 56, 329–335.
