 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Among efforts to explore ways to achieve carbon neutrality globally or regionally, photosynthetic carbon sequestration by algae has been identified as having immense potential. Algae play a crucial role in providing the base of aquatic ecosystems, driving important biogeochemical cycles in oceans and freshwaters and, in so doing, act as a critical component for CO2 drawdown from the atmosphere and ameliorating global change. Furthermore, algae are used extensively in some societies as a source of food and have potential as feedstock for biofuels and as sources of bioactive chemicals. Such activities align strongly with a number of the United Nations Sustainable Development Goals (SDGs). Here we discuss how marine macroalgae might contribute to several of these goals by exploring their potential to enhance aquaculture, contribute to “Blue Carbon” drawdown of CO2 to ameliorate climate change (UN SDGs 13,14) and provide biomass as feedstock for biofuels (UN SDG 7) to reduce reliance on fossil fuel combustion. Though further work is required, we suggest that farming macroalgae in air has great potential for mitigation of CO2 emissions and improvement of aquaculture environments.
Summary: Photosynthetic activity of macroalgae, in addition to driving biosynthesis and biomass accumulation, can cause arise in pH due to CO2 depletion/HCO3–. This can buffer the pH decrease associated with anthropogenic CO2 increases and ameliorate the effects of ocean acidification. Though increasing in magnitude, macroalgal aquaculture still represents only asmall fraction of the Cdrawdown by wild macroalgae populations and currently accounts for drawdown of an even lower fraction of global CO2 emissions. Nonetheless, scaling up of intensive macroalgal aquaculture could be one approach to contribute more to ameliorating anthropogenic CO2 emissions and ocean acidification. Modification of IMTA involving growth of the algae in air rather than in seawater could prove auseful means to help stabilize fluctuations in oxygen and pH in aquaculture operations.
Introduction
The world Ocean is a major sink for anthropogenic CO2 emissions and the photosynthetic activities of algae play a major role in ameliorating the rise in atmospheric CO2 that is driving climate change and ocean acidification (Raven, Citation2017). Algal aquaculture offers a potential route to reducing the levels of CO2 as well as providing sources of food and novel compounds for biotechnology. In this opinion piece, we argue that the assimilation of inorganic carbon by seaweeds (macroalgae) has significant potential to act as a carbon sink or feedstock for bio-renewable energy. We also propose that growing macroalgae in air offers advantages over conventional immersed aquaculture systems.
1. Inorganic carbon uptake and fixation by algae
The inorganic carbon system in seawater involves equilibria between CO2, bicarbonate and carbonate as shown in the following equations with CO2 and bicarbonate being the inorganic carbon species utilized in photosynthesis.
The H+ can then react with carbonate (CO32–), producing more HCO3– at the expense of CO32– when additional CO2 dissolves into seawater.
In seawater, the concentration of CO2 is usually less than 1% of total dissolved inorganic carbon (DIC), while that of bicarbonate (HCO3–) accounts for over 90%. To meet photosynthetic carbon fixation, most algae (including macro- and microalgae) have evolved CO2 concentrating mechanisms (CCMs) that in most cases involve the active uptake and utilization of HCO3− (Beardall & Raven, Citation2020; Beer, Björk, & Beardall, Citation2021), though carboxylation within the cells catalysed by Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) uses only CO2 as substrate. HCO3 – ions are converted to CO2 by carbonic anhydrase before the CO2 can be assimilated to organic matter via Rubisco. This process of inorganic carbon acquisition causes shifts in equilibria between the different inorganic carbon species (CO2, HCO3 – and CO32–) shown in equations 1 and 2 (Hurd et al., Citation2020; Raven, Citation2011), generating OH− (removing protons) and raising the pH of seawater as CO2. Carbon, together with the nutrients nitrogen and phosphorus, is thus assimilated as follows (Gao et al., Citation1991):
Bicarbonate uptake and utilization generates 1 mol OH− inside cells for every mol of bicarbonate taken up, which, to maintain acid base conditions inside the cell, must then be dealt with by OH− extrusion or H+ uptake (Raven, Citation2011, Citation2013). The consequent rise in pH as a result of inorganic carbon utilization can thus play a buffering role against high-CO2-induced acidification (Raven, Citation2017), thereby addressing UN SDG13 (“Take urgent action to combat climate and its impacts”). It is well known that photosynthetic activity of seagrasses (Hendricks et al., 2014) and kelps beds (Pfister, Altabet, & Weigel, Citation2019) can buffer marine systems against ocean acidification, and Zou (Citation2005) has reported a daytime pH rise of up to 1 pH unit in dense cultures of the economically important brown alga Hizikia fusiforme.
Intertidal and most economically important fleshy macroalgae show beneficial responses (enhanced rates of photosynthesis and growth) to increased concentrations of CO2 and a remarkable resilience to the pH drop associated with increased CO2 from anthropogenic emissions (see Gao, Gao, Wang, & Dupont, Citation2020b and references therein). They also exhibit a potential to increase coastal pH, running counter to ocean acidification (Gao & McKinley, Citation1994; Ji & Gao, Citation2021; Noisette & Hurd, Citation2018). Raven (Citation2017) discusses this and other ways in which algal activities can ameliorate global change in the oceans. Farming of macroalgae has been suggested to be capable of CO2 mitigation by 1500 tons CO2 km–2 year–1 (Duarte, Wu, Xiao, Bruhn, & Krause-Jensen, Citation2017). Such a value is unlikely to mean similar macroalgal farming capabilities in different regions and for different species. Consequently, the value cited by Duarte et al. (Citation2017) might significantly underestimate the CO2 sequestration capacity of farmed macroalgae, since sea-farming Laminaria thalli for 7 months has been shown to be able to capture more than 7500 tons of CO2 km–2 (see the review by Gao &and McKinley (Citation1994) and literature therein), assuming the carbon content of the dry biomass to be 25% (Duarte et al., Citation2017). However, it must be recognized that expansion of macroalgal farming to an extent where it can make significant contributions to CO2 mitigation will require extensive areas of cultivation and significant technological and ecological (Chung, Beardall, Mehta, Sahoo, & Stojkovic, Citation2011 and see section 3, below).
2. Potential of macroalgae to act as a carbon sink or feedstock for bio-renewable energy
Sea-farming of economically important macroalgae can play a significant role in increasing the marine carbon sink and the role of blue carbon in the oceans (Chung et al., Citation2011, Citation2013; Duarte et al., Citation2017; Sondak et al., Citation2017), since macroalgal thalli and their debris can be transformed to recalcitrant (refractory) particulate (RPOC) and dissolved organic carbon (RDOC) (Chen et al., Citation2020), which cannot be mineralized by heterotrophs and can be stored for millennia (Jiao et al., Citation2010). During growth of macroalgae, for instance Saccharina kelp, part of their thalli can be torn off by water motion (Zhang et al., Citation2012), and the algal debris can be transported, via ocean currents and sinking, to deep regions of the open ocean, as evidenced by macroalgal eDNA (DNA released into the environment), which has been detected on the seafloor at 4000 m depth (Ortega et al., Citation2019). While the contribution to RPOC and RDOC from macroalgae may differ between different taxa, with about 1.6% of the biomass production by the green tide alga Ulva sp. remaining as RDOC after bacteria-mediated mineralization (Chen et al., Citation2020). Both naturally grown and commercially farmed macroalgae contribute to pools of blue carbon in the oceans, though the proportion of recalcitrant carbon in their biomass is subject to debate (see e.g., Hill et al., Citation2015; Trevathan-Tackett et al., Citation2015). Discrepancies arise as values for recalcitrant carbon in seaweed biomass will depend on cellular composition, particularly cell wall components with different degradation rates, that differs between species (Trevathan-Tackett et al., Citation2015). Further complications about the fate of biomass arise from differences in in situ decomposition capacity and biogeochemical activity in different regions.
In terms of achieving goals for carbon neutrality in different countries, progress in green industries, such as expanding algal cultivation and utilization of their biomass for renewable energy can be considered as alternative approaches (Gao & McKinley, Citation1994; Watanabe & Tanabe, Citation2013). Since algae (both macro- and micro-) grow much faster than terrestrial plants (Falkowski & Raven, Citation2013), with their biomass turnover rate being more than 300 times faster than the latter, algal farming in the sea represents a tremendous potential to provide biomass for generation of renewable energy. Various technologies have been developed to transform plant biomass (including algae) to bio-renewable energy (McKendry, Citation2002). While algae have been suggested to have a higher efficiency of oil productivity than terrestrial oil-crops (Chisti, Citation2007; Watanabe & Tanabe, Citation2013), macroalgae are more promising than microalgae in terms of cost-effective sea-farming and other fuel substitutes, such as methane and/or methanol (Gao, Burgess, Wu, Wang, & Gao, Citation2020a). By using bio-renewable energy, there will be zero net addition of CO2 to the atmosphere, since any CO2 released in combustion originated from that captured during recent photosynthesis and can potentially be recaptured in the same way (Moreira & Pires, Citation2016). Furthermore, farming of macroalgae in the sea does not demand land for their growth as do higher plants.
However, to make a significant impact on CO2 emissions would require a massive enhancement of seaweed production globally (Chung et al., Citation2011; Chung, Sondak, & Beardall, Citation2017; Duarte et al., Citation2017). Chung et al. (Citation2011) pointed out that marine macrophytes including macroalgae and seagrasses in the coastal regions account for drawdown of ~1 Pg C year–1, although how much of this assimilated carbon remains sequestered is, as pointed out above, hard to quantify due to different levels of bio-degradation and local variations in biogeochemistry. However, analysis of the data of Chung et al. (Citation2011) shows that the top ten sea-farming countries harvested macroalgal biomass that accounted for <0.01% of those countries’ emissions, a figure that has not appreciably changed to date, using global data from 2019 (FAO, Citation2020, Olivier & Peters, Citation2019). More recently, Krause-Jensen and Duarte (Citation2016) suggested that wild seaweed photosynthesis could contribute about 173 Tg C year–1 (with a range of 61–268 Tg C year–1) globally to C sequestration and Duarte et al. (Citation2017) pointed out that macroalgae aquaculture has a CO2 capture potential of ~0.68 Tg C year–1, but that with current growth rates in aquaculture, the capture potential of macroalgal farming could exceed 6% of the global CO2 sequestration by wild macroalgae by 2050. Clearly then, while macroalgae farming has the potential for some degree of mitigation of CO2 emissions, there is considerable room for improvement. Some increase in production can undoubtedly come from increasing the areas of coastal to open oceans used for their farming (see Ahmed et al., Citation2017; Buschmann et al., Citation2017; Chung et al., Citation2011, Citation2013; Duarte et al., Citation2017; Froehlich, Afflerbach, Frazier, & Halpern, Citation2019). However, increasing the area of ocean set aside for macroalgal aquaculture to a level that would be meaningful in terms of CO2 sequestration is fraught with problems associated with scale up, the necessity to use open ocean areas (which are more prone to physical disturbance from storms) as well as protected coastlines, potential for increases in “dead zone” and the supply of nutrients to support massively increased macroalgal growth (Raven, Citation2017). Indeed, Froehlich et al. (Citation2019) have questioned the feasibility of large-scale global mitigation of climate change through CO2 sequestration by algal farming. Thus, advances in production beyond simply extending the areas used for aquaculture will be necessary if macroalgal farming is to increase to levels above the predictions made in the work cited above.
A proposed novel approach to improve aquaculture environments and increase macroalgal biomass production
In aquaculture for marine animals, the chemical environment is usually altered rapidly due to feeding and subsequent heterotrophic degradation of residual organic matter, including their faeces, especially in closed waters, such as ponds. The resulting accumulation of ammonia and enhanced anoxia are harmful for many farmed marine animals as well as generally degrading the environment. It is well established that an Integrated Multi-Trophic Aquaculture (IMTA) approach can improve such chemical environments (Buschmann, Varela, Hernández-González, & Huovinen, Citation2008; Mahmood, Fang, Jiang, & Zhang, Citation2016; Yang et al., Citation2006), and at the same time enhance shrimp and fish production (Ahmed et al., Citation2017; Barrington, Chopin, & Robinson, Citation2009; Marinho-Soriano, Nunes, Carneiro, & Pereira, Citation2009; Viera et al., Citation2011; Xu, Fang, & Wei, Citation2008). Growing macroalgae jointly with animals can also increase the level of probiotics and suppress pathogens (Mangott et al., Citation2020), reflecting extra benefits that can flow from using an IMTA system and thereby falling under the aegis of UN-SDG 14 (“Conserve and sustainably use the oceans, seas and marine resources for sustainable development”).
Although growing macroalgae in the IMTA system can raise dissolved O2 concentration and improve water quality during the daytime, the diel changes of pH can be expected to be rather different in IMTA vs aquaculture with animals alone. Inorganic carbon use through photosynthesis and the consequent production of OH−during the day would buffer the pH drop caused by animal respiratory CO2 production. However, O2 consumption and the pH drop during the night would be greater due to the respiratory activity of both animals and algae (and other aerobic organisms, such as bacteria and protozoa), reducing the respiration index (RI = log10 (pO2/pCO2)) (Brewer & Peltzer, Citation2009) and lowering pH, which is harmful for animals. The pH drop or acidification of seawater is known to negatively affect many invertebrates (Kurihara, Citation2008), reducing growth, and also makes shrimps taste bitter (Dupont, Hall, Calosi, & Lundve, Citation2014), which could be attributed to a food chain impact induced by the lowered pH (Jin, Hutchins, & Gao, Citation2020). Phytoplankton and zooplankton tend to have higher contents of phenolics (toxic) under the influence of lowered pH (Jin et al., Citation2015), which might be responsible for the lowered growth and bitter taste of shrimps. In addition, changes in fatty acid composition under the influence of lowered pH affect copepod growth and development (Rossoll et al., Citation2012).
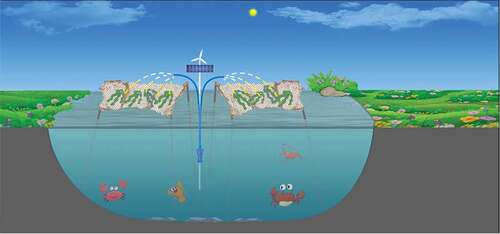
Therefore, we propose here an approach for spatially separating (air and water) the growth of macroalgae and animals, with the macroalgae growing in air under a spray (). Basically, macroalgae can be placed or fixed on a net, which can be mounted above the aquaculture ponds (), so that they do not compete for O2 with the farmed animals during night. While exposing the macroalgae, such as Gracilaria, Porphyra and Ulva to air, seawater can be continuously pumped and sprayed over them, so that they are not dehydrated. They will also photosynthesize faster due to increased CO2 availability to the macroalgae exposed in air, since CO2 diffuses about 10 thousand times faster in air than in water (Denny, Citation1993). In addition, the diffusion boundary layers surrounding the algal thalli can be reduced due to the cascading seawater; in parallel, the algal thalli take up nutrients quickly due to reduced diffusive barrier and photosynthesize faster due to water-current-induced thinned diffusion boundary layer for CO2 and O2 (Gao, Xu, Zheng, & Ke, Citation2012). Enhanced or sustained photosynthesis in emersed thalli (without or with minor dehydration) have been reported for a number of macroalgae including Fucus spiralis (Madsen & Maberly, Citation1990), Pyropia yezoensis (Gao & Aruga, Citation1987; Zhou et al., Citation2014) Mastocarpus papillatus (Bell, Citation1993), Colpomenia peregrina Oates, Citation1985), Ulva linza, Ishige okamurae and Gloiopeltis furcata (Gao, Ji, & Aruga, Citation1999). The extent of such photosynthetic enhancement due to direct air exposure differs due to species and environmental conditions (such as temperature and light). For instance, at temperatures of 15–20°C, the photosynthetic CO2 fixation rate in air increased by 50% in P. yezoensis (Gao & Aruga, Citation1987) and up to 100% in F. spiralis (Madsen & Maberly, Citation1990) when the thalli were not dehydrated.
Spray culture has been previously tested for Gracilaria (Hanisak, Citation1987; Moeller, Griffen, & Lee, Citation1982; Pickering, Gordon, & Tong, Citation1995) and a range of other red, brown and green macroalgae (Haglund & Pedersen, Citation1988), though not to date applied to IMTA systems. Reported growth rates from spray culture of macroalgae were comparable to those of immersed alga, though emersed cultures tended to be less affected by epiphytes (Pickering et al., Citation1995). Haglund and Pedersen (Citation1988) reported growth rates of 3.4% day–1 for Gelidium and up to 1.1% day–1 for Fucus vesiculosus, while values of 1.4% day–1 are cited by Pickering et al. (Citation1995) for Gracilaria. Empirically, spray cultures of macroalgae are practically feasible and could be extended to a wider range of species. Further work is required to investigate which macroalgal species respond well to growth in spray culture. The energy required for powering the water-spray can be obtained from solar or wind energy generators and, therefore, such an approach could be environmentally sustainable, though a thorough cost/benefit analysis is required, with emphasis on the feasibility of co-location of wind or solar energy systems in areas suitable for seaweed aquaculture. In addition, the harvested biomass of macroalgae can be used as food, as stock for generation of renewable energy (Gao & McKinley, Citation1994) and/or for grow-out of abalone in the sea (Viera, Courtois de Viçose, Fernández-Palacios, & Izquierdo, Citation2016; Viera et al., Citation2011).
Figure 1. Illustration of the proposed approach for a water-air separated aquaculture system. Macroalgae are placed about the animal farming ponds and sprayed with seawater pumped from the pond, so that the algae remove nutrients from the farming ponds without competing for O2 with animals during the night, improving water quality.
Acknowledgments
The study was supported by National Natural Science Foundation of China (41720104005, 41890803, 41721005). The authors are grateful to the laboratory engineers Xianglan Zeng, Wenyan Zhao for their logistic and technical supports.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahmed, N., Stuart, W., Bunting, S. W., Glaser, M., Flaherty, M. S., & Diana, J. S. (2017). Can greening of aquaculture sequester blue carbon? AMBIO, 46, 468–477.
- Barrington, K., Chopin, T., & Robinson, S. (2009). Integrated multi-trophic aquaculture (IMTA) in marine temperate waters. In D. Soto (Ed.), Integrated mariculture: A global review (Vol. 529, pp. 7–46). Rome, FAO: FAO Fisheries and Aquaculture Technical Paper.
- Beardall, J., & Raven, J. A. (2020). Structural and biochemical features of carbon acquisition in algae. In A. Larkum, A. Grossman, and J. Raven (Eds.), Photosynthesis in algae: Biochemical and physiological mechanisms. Advances in photosynthesis and respiration (including bioenergy and related processes) (Vol. 45, pp.141–160). Cham: Springer, doi:10.1007/978-3-030-33397-3_7
- Beer, S., Björk, M., & Beardall, J. (2021). Carbon dioxide vs. bicarbonate utilisation. In K. S. Gao, H. D A., & J. Beardall (Eds.), Research methods of environmental physiology in aquatic sciences (pp. 153–164). Singapore: Springer.
- Bell, E. C. (1993). Photosynthetic response to temperature and desiccation of the intertidal alga Mastocarpus papillatus. Marine Biology, 117, 337–346.
- Brewer, P. G., & Peltzer, E. T. (2009). Limits to marine life. Science, 324, 347–348.
- Buschmann, A. H., Camus, C., Infante, J., Neori, A., Israel, I., Hernández-González, M. C., … Critchley, A. T. (2017). Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. European Journal of Phycology, 52, 391–406.
- Buschmann, A. H., Varela, D. A., Hernández-González, M. C., & Huovinen, P. (2008). Opportunities and challenges for the development of an integrated seaweed-based aquaculture activity in Chile: Determining the physiological capabilities of Macrocystis and Gracilaria as biofilters. Journal of Applied Phycology, 20, 571–577.
- Chen, J., Li, H. M., Zhang, Z. H., He, C., Shi, Q., Jiao, N. Z., & Zhang, Y. Y. (2020). DOC dynamics and bacterial community succession during long-term degradation of Ulva prolifera and their implications for the legacy effect of green tides on refractory DOC pool in seawater. Water Research, 185, 116268.
- Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25, 294–306.
- Chung, I. K., Beardall, J., Mehta, S., Sahoo, D., & Stojkovic, S. (2011). Using marine macroalgae for carbon sequestration: A critical appraisal. Journal of Applied Phycology, 23, 877–886.
- Chung, I. K., Oak, J. H., Lee, J. A., Shim, J. A., Kim, J. G., & Park, K.-S. (2013). Installing kelp forests/seaweed beds for mitigation and adaptation against global warming: Korean Project Overview. ICES Journal of Marine Science, 70, 1038–1044.
- Chung, I. K., Sondak, C. F. A., & Beardall, J. (2017). The future of seaweed aquaculture in a rapidly changing world. European Journal of Phycology, 52, 495–505.
- Denny, M. W. (1993). Air and water. The biology and physics of life’s media. Princeton, NJ: Princeton University Press.
- Duarte, C. M., Wu, J. P., Xiao, X., Bruhn, A., & Krause-Jensen, D. (2017). Can seaweed farming play a role in climate change mitigation and adaptation? Frontiers in Marine Science, 4, 100.
- Dupont, S., Hall, E., Calosi, P., & Lundve, B. (2014). First evidence of altered sensory quality in a shellfish exposed to decreased pH relevant to ocean acidification. Journal of Shellfish Research, 33, 857–861.
- Falkowski, P. G., & Raven, J. A. (2013). Aquatic photosynthesis. New Jersey: Princeton University Press.
- FAO. (2020). The State of World Fisheries and Aquaculture 2020. Sustainability in action. Rome, DOI:10.4060/ca9229en.
- Froehlich, H. E., Afflerbach, J. C., Frazier, M., & Halpern, B. S. (2019). Blue growth potential to mitigate climate change through seaweed offsetting. Current Biology, 29, 3087.
- Gao, K. S., & Aruga, Y. (1987). Preliminary studies of the photosynthesis and respiration of Porphyra yezoensis under emersed conditions. Journal of the Tokyo University of Fisheries, 47, 51–65.
- Gao, K. S., Aruga, Y., Asada, K., Ishihara, T., Akano, T., & Kiyohara, M. (1991). Enhanced growth of the red alga Porphyra yezoensis Ueda in high CO2 concentrations. Journal of Applied Phycology, 3, 355–362.
- Gao, G., Burgess, J. G., Wu, M., Wang, S. J., & Gao, K. S. (2020a). Using macroalgae as biofuel: Current opportunities and challenges. Botanica Marina, 63, 355–370.
- Gao, K. S., Gao, G., Wang, Y. J., & Dupont, S. (2020b). Impacts of ocean acidification under multiple stressors on typical organisms and ecological processes. Marine Life Science & Technology, 2, 279–291.
- Gao, K. S., Ji, Y., & Aruga, Y. (1999). Relationship of CO2 concentrations to photosynthesis of intertidal macroalgae during emersion. Hydrobiologia, 398/399, 355–359.
- Gao, K. S., & McKinley, K. R. (1994). Use of macroalgae for marine biomass production and CO2 remediation: A review. Journal of Applied Phycology, 6, 45–60.
- Gao, K. S., Xu, J. T., Zheng, Y. Q., & Ke, C. H. (2012). Measurement of benthic photosynthesis and calcification in flowing-through seawater with stable carbonate chemistry. Limnology and Oceanography-Methods, 10, 555–559.
- Haglund, K., & Pedersen, M. (1988). Spray cultivation of seaweeds in recirculating brackish water. Aquaculture, 72, 181–189.
- Hanisak, M. D. (1987). Cultivation of Gracilaria and other macroalgae in Florida for energy production. In K. T. Bird & P. H. Benson (Eds.), Seaweed cultivation for renewable resources (pp. 191–218). Amsterdam: Elsevier.
- Hill, R., Bellgrove, A., Macreadie, P. I., Petrou, K., Beardall, J., Steven, A., & Ralph, P. J. (2015). Can macroalgae contribute to blue carbon? An Australian perspective. Limnology and Oceanography, 60, 1689–1706.
- Hurd, C. L., Beardall, J., Comeau, S., Cornwall, C. E., Havenhand, J. N., Munday, P. L., … McGraw, C. M. (2020). Ocean acidification as a multiple driver: How interactions between changing seawater carbonate parameters affect marine life. Marine and Freshwater Research, 71, 263–274.
- Ji, Y., & Gao, K. S. (2021). Effects of climate change factors on marine macroalgae: A review. Advances in Marine Biology, 88, 91–136.
- Jiao, N. Z., Herndl, G. J., Hansell, D. A., Benner, R., Kattner, G., Wilhelm, S. W., … Azam, F. (2010). Microbial production of recalcitrant dissolved organic matter: Long-term carbon storage in the global ocean. Nature Reviews Microbiology, 8, 593–599.
- Jin, P., Hutchins, D. A., & Gao, K. S. (2020). The impacts of ocean acidification on marine food quality and its potential food chain consequences. Frontiers in Marine Science, 7, 780.
- Jin, P., Wang, T. F., Liu, N. N., Dupont, S., Beardall, J., Boyd, P. W., … Gao, K. S. (2015). Ocean acidification increases the accumulation of toxic phenolic compounds across trophic levels. Nature Communications, 6, 8714.
- Krause-Jensen, D., & Duarte, C. M. (2016). Substantial role of macroalgae in marine carbon sequestration. Nature Geoscience, 9, 737–742.
- Kurihara, H. (2008). Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Marine Ecology Progress Series, 373, 275–284.
- Madsen, T. V., & Maberly, S. C. (1990). A comparison of air and water as environments for photosynthesis by the intertidal alga Fucus spiralis (Phaeophyta). Journal of Phycology, 26, 24–30.
- Mahmood, T., Fang, J. G., Jiang, Z. J., & Zhang, J. (2016). Seasonal nutrient chemistry in an integrated multi-trophic aquaculture region: Case study of Sanggou Bay from North China. Chemistry and Ecology, 32, 149–168.
- Mangott, A., Nappi, J., Delli Paoli Carini, A., Goncalves, P., Hua, K., Domingos, J. A., … Thomas, T. (2020). Ulva lactuca as a functional ingredient and water bioremediator positively influences the hepatopancreas and water microbiota in the rearing of Litopenaeus vannamei. Algal Research, 51, 102040.
- Marinho-Soriano, E., Nunes, S. O., Carneiro, M. A. A., & Pereira, D. C. (2009). Nutrients‘ removal from aquaculture wastewater using the macroalgae Gracilaria birdiae. Biomass and Bioenergy, 33, 327–331.
- McKendry, P. (2002). Energy production from biomass (part 2): Conversion technologies. Bioresource Technology, 83, 47–54.
- Moeller, H. W., Griffen, G., & Lee, V. (1982). Aquatic biomass production on land using seawater spray. Int. Gas Technol. Meetings January, 25–28, 237–248.
- Moreira, D., & Pires, J. C. M. (2016). Atmospheric CO2 capture by algae: Negative carbon dioxide emission path. Bioresource Technology, 215, 371–379.
- Noisette, F., & Hurd, C. (2018). Abiotic and biotic interactions in the diffusive boundary layer of kelp blades create a potential refuge from ocean acidification. Functional Ecology, 32, 1329–1342.
- Oates, B. R. (1985). Photosynthesis and amelioration of desiccation in the intertidal saccate alga Colpomenia peregrina. Marine Biology, 89, 109–119.
- Olivier, J. G. J., & Peters, J. A. H. W. (2019). Trends in global CO2 and total greenhouse gas emissions: 2019 report. PBL Netherlands Environmental Assessment Agency, The Hague.
- Ortega, A., Geraldi, N. R., Alam, I., Kamau, A. A., Acinas, S. G., Logares, R., … Duarte, C. M. (2019). Important contribution of macroalgae to oceanic carbon sequestration. Nature Geoscience, 12, 748–754.
- Pfister, C. A., Altabet, M. A., & Weigel, B. L. (2019). Kelp beds and their local effects on seawater chemistry, productivity, and microbial communities. Ecology, 100, e02798.
- Pickering, T. D., Gordon, M. E., & Tong, L. J. (1995). A preliminary trial of a spray culture technique for growing the agarophyte Gracilaria chilensis (Gracilariales, Rhodophyta). Aquaculture, 130, 43–49.
- Raven, J. A. (2011). Praeger Review: Effects on marine algae of changed seawater chemistry with increasing atmospheric CO2. Biology and Environment, 111, 1–17.
- Raven, JA. (2013). Half a century of pursuing the pervasive proton. Progress in Botany, 74, 3–34.
- Raven, J A. (2017). The possible roles of algae in restricting the increase in atmospheric CO2 and global temperature. European Journal of Phycology, 52, 506–522.
- Rossoll, D., Bermu´dez, R., Hauss, H., Schulz, K. G., Riebesell, U., Sommer, U., & Winder, M. (2012). Ocean acidification-induced food quality deterioration constrains trophic transfer. PloS One, 7, e34737.
- Sondak, C. F. A., Ang, P. O., Jr., Beardall, J., Bellgrove, A., Boo, S. M., Gerung, G. S., … Chung, I. K. (2017). Carbon dioxide mitigation potential of seaweed aquaculture beds (SABS). Journal of Applied Phycology, 29, 2363–2373.
- Trevathan-Tackett, S. M., Kelleway, J., Macreadie, P. I., Beardall, J., Ralph, P., & Bellgrove, A. (2015). Comparison of marine macrophytes for their contributions to blue carbon sequestration. Ecology, 96, 3043–3057.
- Viera, M. D. P., Courtois de Viçose, G., Fernández-Palacios, H., & Izquierdo, M. (2016). Grow-out culture of abalone Haliotis tuberculata coccinea Reeve, fed land-based IMTA produced macroalgae, in a combined fish/abalone offshore mariculture system: Effect of stocking density. Aquaculture Research, 47, 71–81.
- Viera, M. P., de Vicose, G. C., Gómez-Pinchetti, J. L., Bilbao, A., Fernandez-Palacios, H., & Izquierdo, M. S. (2011). Comparative performances of juvenile abalone (Haliotis tuberculata coccinea Reeve) fed enriched vs non-enriched macroalgae: Effect on growth and body composition. Aquaculture, 319, 423–429.
- Watanabe, M. M., & Tanabe, Y. (2013). Biology and industrial potential of Botryococcus braunii. In A. Richmond & Q. Hu (Eds.), Handbook of microalgal culture (pp. 369–387). Oxford: Wiley-Blackwell.
- Xu, Y. J., Fang, J. G., & Wei, W. (2008). Application of Gracilaria lichenoides (Rhodophyta) for alleviating excess nutrients in aquaculture. Journal of Applied Phycology, 20, 199.
- Yang, Y. F., Fei, X. G., Song, J. M., Hu, H. Y., Wang, G. C., & Chung, I. K. (2006). Growth of Gracilaria lemaneiformis under different cultivation conditions and its effects on nutrient removal in Chinese coastal waters. Aquaculture, 254, 248–255.
- Zhang, J. H., Fang, J. G., Wang, W., Du, M. R., Gao, Y. P., & Zhang, M. L. (2012). Growth and loss of mariculture kelp Saccharina japonica in Sungo Bay, China. Journal of Applied Phycology, 24, 1209–1216.
- Zhou, W., He, L., Yang, F., Lin, A. P., Zhang, B. Y., Niu, J. F., & Wang, G. C. (2014). Pyropia yezoensis can utilize CO2 in the air during moderate dehydration. Chinese Journal of Oceanology and Limnology, 32, 358–364.
- Zou, D. (2005). Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture, 250, 726–735.
