 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Peculiarities of the processes of self-assembly of carbon under pressure up to 8 GPa and temperatures up to 1600°C in pure carbon, hydrocarbon, fluorocarbon, organometallic systems and binary mixtures of all-carbon, hydrocarbon, and fluorocarbon compounds have been revealed in the course of studies of pressure and temperature-induced transformations of different carbon-containing systems. It was shown that the character of the processes of self-assembly of carbon in different systems is controlled in the first place by the mobility of carbon atoms. The low diffusion mobility of carbon atoms in a condensed state at temperatures below 2000° C leads to the fact that in pure carbon systems studied on the examples of fullerite C60 and closed polyhedral carbon nanoparticles, carbon self-organization can occur only due to processes associated with small movements of carbon atoms that ensure the formation of intermolecular bonds in cases of polymerization of C60 or the restructuring of the internal structure of a polyhedral particle, strictly limited to the confines of a single nanoparticle. In the hydrocarbon and fluorocarbon systems, the character of transformation changes drastically due to formation of volatile low-molecular hydrocarbon and fluorocarbon fractions, which ensure a high gas-phase or fluid mobility to carbon atoms. Studies of pressure and temperature-induced transformations of different hydrocarbon, fluorocarbon compounds and their homogeneous binary mixtures revealed a clear synergistic effect of fluorine and hydrogen on processes of carbonization, graphitization, and formation of diamond in these systems in relation to industrially significant reduction of p,T parameters for formation of graphite, diamond and increase in the content of nanosize diamond fractions in the products of transformations of binary mixtures in comparison with pure hydrocarbon and fluorocarbon compounds. Discovery of this synergistic effect opens new opportunities for synthesis of high-purity and doped ultranano-, nano-, submicro-, and micronsized diamonds with the specific properties for different applications in quantum physics and biomedicine. Studies of particularities of self-assembly of carbon in processes of thermal transformations of ferrocene at high pressures demonstrated the possibility of preparation of iron carbide nanoparticles encapsulated into carbon shells, Fe7C3@C and Fe3C@C, considered as perspective magneto-controlled platforms for different biomedical nanocomplexes.
1. Introduction
Remarkable flexibility of sp-sp2-sp3-hybridization states of carbon atom makes carbon a unique chemical element providing a base for design of variety of closed molecular, one-, two-, and 3D polymer materials, drastically different in their properties. By demonstration of the possibility of synthesis of fullerenes, a new nanosize form of carbon, in the course of cooling of carbon plasma, Kroto et al. [Citation1] have drawn the attention of researchers to analysis of the processes of self-assembly of carbon during its condensation from different types of carbon plasma. Progress in this trend of research led to development of effective technologies for preparation of fullerenes [Citation2–4] and discovery of the series of other allothrope modifications of carbon, single- and multi-walled nanotubes [Citation5], spherical onion-like [Citation6], polyhedral [Citation7], and cone-shaped carbon particles [Citation8].
In this work, peculiarities of self-assembly of carbon in the processes of thermal transformations of different types of carbon-containing systems under high pressure are considered. Main objects of studies were the systems based on pure carbon materials and hydrocarbon, fluorocarbon and organometallic compounds, as well as mixtures of all-carbon, hydrocarbon, and fluorocarbon compounds. All these systems are carbon-containing, however the structure, chemical elemental composition of the compounds forming the systems under study, and their relative carbon content differ significantly. The influence of these factors on the processes, p,T parameters and products of various stages induced by high pressures and the temperature of transformations of these systems are considered in this work.
As known [Citation9], thermo-induced transformations of organic compounds in the temperature range up to 1000°С represent a very complex sequence of physico-chemical transformations, which are accompanied by formation of different ordered and disordered states of solid carbon, and designated by a common term – carbonization. Further increase in treatment temperature facilitates the processes of graphitization of the carbonization products [Citation10]. At high-pressure range, in the series of transformations one more stage arises, formation of diamond [Citation11]. From the point of view of theory of physico-chemical evolution of solid state [Citation12], formation of different types of carbon structures in the products of carbonization of carbon-containing systems under high pressures represents different stages of condensation route for formation of solid carbon which turns into different solid forms of carbon as the result of condensation from an oversaturated vapor, solution or vapor-fluid media. The specifics of transformation in this case consist in condensation of carbon that occurs not from pure carbon but either hydrocarbon or other medium of more complex composition. According to current views, time changes of matter proceed by common evolution route consisting in a sequence of stages, such as creation of primary cluster, giving rise to formation of seeds of particular modifications of matter, seeds growth, their aggregation, subsequent structure ordering and maturing of particles. After following this route, the material adopts the form of micro- and macro-size crystals having perfect shape and balanced habitus [Citation12].
Main condition for realization of condensation route to formation of solid material is the occurrence of oversaturation of the medium at the initial moment of time. The extent of oversaturation determines the rate of formation of the solid state of material. Under conditions of significant oversaturation of the media, simultaneous formation of metastable along with stable states of matter is possible. In case of carbon-containing systems the variety of possible metastable states is particularly large. Different nanoforms of carbon, single graphene layers, packings of few graphene layers, fullerenes, spherical and polyhedral onion-like particles, nanoribbons, different states of amorphous carbon, graphite nanoparticles, and diamond, can represent such states. It should be noted that according to size-dependent phase diagram of carbon [Citation13] the ratios of relative stability of different allotropic forms of carbon in the nanosize region are continuously changing along with the size increase.
Classical scheme of condensation route suggests that the processes of formation and growth of new phase from oversaturated media are inevitably terminated by transition of the system from the state of oversaturation to under saturation of the media relatively to the product of reaction. When several allotropic forms of carbon are formed simultaneously, their degrees of under saturation become dissimilar providing a driving force for disappearance of metastable modifications and their conversion into the states possessing the lowest vapor pressure or solubility. The tendency of the dispersed system to transform itself into the lowest surface energy state also facilitates the processes of collective recrystallization under which the disappearance of smaller and disordered particles along with enlargement of larger and ordered forms of matter take place. Namely, in the processes of formation of new phase the existence of material in the nanosize state is limited by initial stages of the evolution route, preceding the stage of particles aggregation. The aim of this work was the evaluation of common and distinct features of the processes of self-assembly of carbon at concluding stages of carbonization in different types of studied systems under high pressures, investigation of the effect of nature of starting compounds on the products of transformations, and studying the possibility of using a high pressure and temperature-induced transformation of different systems for the design of new, particularly nanosized, carbon-containing materials with the desired properties capable of satisfying the requirements for application in perspective quantum-physical and biomedical trends.
The peculiarity of the present work, making it distinguished from other works in this area, is the complex nature of the research conducted. The article for the first time in a generalized form presents the results of a systematic study of induced by high pressures and temperatures transformations of various types of carbon-containing systems, carried out by using a unitary methodological scheme and high-pressure equipment. Thanks to the complex nature of the studies, it was possible for the first time to clearly demonstrate the diversity of nanoscale forms of carbon formed in the processes of these transformations. Within the framework of the studies performed, a comparative study of thermal transformations at high pressures of pure carbon, hydrocarbon, fluorocarbon compounds, and their binary mixtures was carried out for the first time, which made it possible to detect a pronounced synergistic effect of fluorine and hydrogen on the parameters and transformation products of carbon materials, hydrocarbon and fluorocarbon compounds under the conditions of their binary or more complex multicomponent mixtures. The results of this work provided the foundation for development of original methods for synthesis of various nanoscale forms of carbon-containing materials, including the currently most popular nanodiamonds with various impurity-vacancy color centers and super paramagnetic iron carbide nanoparticles encapsulated in carbon shells.
2. Materials and methods
Peculiarities of the high pressure and high temperature (HPHT) induced transformations in pure carbon system have been evaluated in this work on the examples of high-purity fullerite C60 supplied by Term USA and polyhedral carbon nanoparticles prepared by annealing of carbon soot at temperature of 2800°С under helium environment [Citation14]. Studies of the transformations in hydrocarbon systems were carried out based on adamantane С10Н16 (Chemapol) and series of polycyclic aromatic compounds, including naphthalene C10H8 (Chemapol), anthracene С14Н10, pentacene С22Н14, perylene С20Н12, and coronene С24Н12, all acquired from Aldrich Chemical Company. Fluorocarbon systems were represented by graphite fluoride CF1.1, octafluoronaphthalene C10F8 (Aldrich Chemical Company, St. Louis, MO), and 1-fluoroadamantane C10H15F (Tokyo Chemical Industry Co., Ltd., Chennai, India). As an example of organometallic compound, ferrocene Fe(C5H5)2 (Aldrich Chemical Company), was selected.
HPHT experiments were carried out using a Toroid – type high-pressure apparatus [Citation15]. Cylinder-shaped pellets of starting materials (4–5 mm diameter and 3 mm height), prepared by cold pressing, were placed into graphite container that simultaneously served as a heater within the high-pressure apparatus. Experimental procedure involved loading of the apparatus to different pressures from 1.5 to 8.0 GPa at room temperature followed by heating the sample to desired temperature in the range from 400 to 1600°C, and then isothermal hold of the sample at this temperature and set loading during variable exposure times from 1 to 300 s.
The pressure was determined by using a calibration curve, i.e. the dependence of pressure in the reaction zone of apparatus on press load at room temperature, created on basis of pressure values for polymorphic transitions in reference metals (bismuth and barium) registered by the detection of corresponding spikes in electric resistance of these metals. The accepted in this work pressure values for known phase transitions were the following: Bi (I–II) = 2.55 ± 0.01 GPa, Bi (II–III) = 2.69 ± 0.01 GPa, and Bi(V–VI) = 7.67 ± 0.18 GPa [Citation16]. This methodology scheme helps in determination of the value of initial pressure (Po) at room temperature used as a nominal pressure in the experimental parameters with an error not exceeding 0.2 GPa. Note, that this error relates to the error for determination of pressure of the initial compressed state of the studied system at room temperature. The actual value of the pressure in the reaction zone during thermobaric transformations, especially accompanied by the release of gaseous products or a significant change in the density of the solid-phase components of the system, may differ significantly from Po. Unfortunately, the implementation of direct control over the evolution of pressure in the reaction zone in the processes of active transformations in the region of the highest pressures and temperatures often turns out to be a very difficult task.
The temperature in the high-pressure chamber was measured directly with chromel-alumel or Pt-Pt/Rh thermocouples. Heating was carried out using a temperature controller module that provides a given heating rate and maintaining the temperature during isothermal holding with a relative accuracy of ±1–5° depending on the temperature range of the experiment. The values of absolute errors in temperature measurement are, respectively ±5–60° and depend on the p,T parameters of the experiment.
The obtained products were quenched to room temperature under pressure and then, after unloading the apparatus, recovered from the reaction zone and analyzed by X-ray diffraction methods, Raman spectroscopy, scanning, and transmission electron microscopy.
3. Results and discussion
3.1. “Pure” carbon system
Up until recently, a textbook p,T phase diagram of carbon [Citation17] considered only three states of solid carbon: graphite, diamond, and carbon phase III, presumably possessing metallic conductivity. However, the discovery of different nanosize forms of carbon indicated that this diagram needs clarification involving the determination of size-dependent phase diagrams. The first theoretical attempts on construction of such phase diagrams showed that within these diagrams besides the areas of thermodynamic stability of graphite and diamond, areas of stability of such nanoforms of carbon as fullerenes and onions become notable [Citation13]. Existence of their own areas of stability for different nanoforms of carbon can lead to disruption of traditional interpretations of phase transformation scheme for bulk macrosize states in the carbon system if it applies to nanosize domain. This means that graphite and diamond will lose the status of most stable phases of carbon at a given p,T parameters in the domain of nanosize structures. This circumstance has to be taken into consideration in the analysis of possible routes of phase transitions in the evolution of the system from an extremely metastable to a stable state of carbon in various size ranges.
Speaking of phase transformations in pure carbon systems, it should be noted that at present time many researchers believe that graphite, as an allotropic form of carbon, is a more or less idealized image model than a real physical object. The thing is that the definition of pure graphite, as well as diamond and carbyne, assumes the presence of free valence on carbon atoms located at end positions in these 1D–3D carbon polymers. Since the presence of free valences is extremely unfavorable from the energy point of view, in a real 1D–3D polymer structures the end carbon atoms are usually bonded to hydrogen and other functional groups with the structure governed by the composition of media, wherein the creation of solid carbon takes place. By taking this note, real graphite should be considered not as a state of pure carbon but as a hydrocarbon wherein the content of hydrogen depends on the method of its preparation [Citation18]. For that reason only an enclosed forms of carbon, such as fullerene-like, nanotubes, polyhedral, and spherical onion-like particles and other carbon structures [Citation19], can be considered as pure states of carbon. Only in these types of systems the thermal transformations at temperatures below 3000°C are truly solid-phase. That’s why the parameters of so-called direct graphite-diamond phase transition, determined by different groups as 13 GPa and 3000°C [Citation17], 11–12 GPa and 3000°C [Citation20], and 15 GPa and 1300°C [Citation21], should be yet considered as a p,T-parameters for diamond formation from hydrocarbons with a variable, although relatively small contents of hydrogen.
As objects of research on the features of carbon self-assembly in pure carbon systems, fullerite C60, a plastic molecular crystal representing a coordination-ordered packing of fullerene C60 molecules, and a disordered mixture of polyhedral nanoscale (30–80 nm) carbon particles were selected.
3.1.1. Fullerite C60
The fullerene C60 molecule is a truncated icosahedron, in 60 vertices of which carbon atoms having triple coordination are located [Citation1,Citation22]. The molecule has 60 single (C–C) and 30 double (C = C) carbon–carbon bonds, the lengths of which are 1.46 and 1.40 Å, respectively. A stable modification of fullerite C60 under normal conditions is the face-centered cubic (fcc) phase C60 with the spatial symmetry group Fmm [Citation23,Citation24]. The distance between the centers of 12 adjacent C60 molecules in the fcc phase is 10.02 Å, the unit cell parameter is 14.17 Å. With a diameter of the nuclear sphere of C60 molecule of 7.1 Å [Citation1], the distance between the nuclear spheres of the nearest C60 molecules is equal to 2.92 Å.
According to the existing classification [Citation25], the atomic states of carbon in the C60 molecule no longer belong to the pure sp2-hybridized states characteristic of graphene, but form their own separate spn (2 < n < 3) class of hybridized states, the so-called intermediate forms of carbon, which include closed nanotubes, toroidal and other types of similar formations built of covalently bonded carbon atoms [Citation19]. Systems of covalently bonded carbon atoms are characterized by high energies of interatomic bonds, resulting in extremely low diffusion mobility of carbon atoms, and record high melting points. As a result, the mechanisms of structural transformations in such systems can be based only on relatively small movements of carbon atoms. High pressures open up opportunities for the implementation of this kind of transformations in molecular systems with unsaturated carbon bonds. These transformations can occur primarily due to the polymerization reactions of fullerene molecules that are in a compressed state, in which the distances between the atoms of two neighboring molecules become comparable to the lengths of interatomic bonds inside C60 molecules. The result of polymerization, depending on the p,T parameters of the fullerite processing, are various types of low-molecular and high-molecular polymer structures with different degrees of structural ordering.
From the point of view of the theory of polymerization, an important characteristic of a monomer is its functionality (f), which means the maximum number of chemical bonds in which a particular monomer is able to participate. For C60, the number of covalent intermolecular bonds may be different in cases of 1D, 2D, or 3D polymerization. According to some model representations, the functionality of C60 in 3D polymers can reach values of 52, 54, and even 60 units per molecule [Citation26]. In this case, polymerization can be both ordered and disordered.
Illustrative examples of orderly polymerization of fullerite, due to the reactions of 2 + 2 cycloaddition of C60 molecules during the treatment of the monomeric C60 phase at pressures up to 9.0 GPa within the thermal stability of C60 at temperatures below ∼1000 K, are the processes of formation of linear and two (tetragonal and rhombohedral) types of 2D C60 polymers, the packing of which in turn form orthorhombic (O), tetragonal (T), and rhombohedral (R) crystalline polymer phases of C60 [Citation27–29]. shows structural models of the dimeric (D) molecule (C60)2 and fragments of various (O, T, R) polymers of C60, indicating the type of symmetry of the isolated monomer units.
Figure 1. Structural models of the dimeric (D) molecule (C60)2 and fragments of various (O, T, R) polymers of C60 indicated by the type of symmetry of the isolated monomer units (a). MD simulated amorphous carbon structures based on polymerized states of fullerite C60 with different content of sp3 bonds [Citation30] (b).
![Figure 1. Structural models of the dimeric (D) molecule (C60)2 and fragments of various (O, T, R) polymers of C60 indicated by the type of symmetry of the isolated monomer units (a). MD simulated amorphous carbon structures based on polymerized states of fullerite C60 with different content of sp3 bonds [Citation30] (b).](/cms/asset/28e77d44-7a8d-4651-9ecb-ea9071407236/tfdi_a_2193212_f0001_c.jpg)
At pressures above 9.0 GPa, 3D polymerization of C60 [31–34] is observed. It should be noted that in contrast to the 1D and 2D polymerized states, attempts to obtain the 3D crystalline phase of C60 still remain unsuccessful, despite the existence of several theoretical models of this kind of phases [Citation31,Citation35]. The reason for this is the disordered nature of 3D polymerization of C60, the product of which are also disordered 3D polymer structures of carbon.
The elementary act of the 2 + 2 cycloaddition reaction is associated with the partial breaking of double (C = C) bonds in neighboring C60 molecules and the formation of single (C–C) intermolecular bonds. Thus, the carbon atoms involved in the formation of intermolecular bonds move from states with triple coordination to states with quadruple coordination, i.e. to sp3 hybridized states. This transition is accompanied by certain distortions of the structure of the original C60 molecular cluster. As the share of sp3 carbon states in the system increases, starting from a certain point, the separation of an individual C cluster as a single structure-forming unit by any physical methods becomes virtually impossible. The system transitions from the state of molecular carbon polymer to the state of amorphous atomic polymer of carbon.
The unifying feature of the 1D, 2D, and low molecular weight fractions of 3D C60 polymers formed at high pressures and temperatures is that all of them can be classified as weak gels [Citation36]. A distinctive feature of weak gels is that the monomers in them are bound into macromolecules (chemically bonded clusters) of finite size, the entirety of which is called the sol fraction. However, a feature of weak gels based on 3D polymers is their tendency to sol-gel transitions, as a result of which the bulk of monomers become included in an infinite 3D network of chemical bonds, denoted as a gel fraction. In a pure carbon system, which is fullerite C60, the classical patch on the sol-gel transition, i.e. the problem of forming a continuous covalent-bound carbon network, acquires great practical significance, since 3D networks of this kind with different contents of carbon atoms in the sp3 hybridized state form a special class of amorphous carbon materials with a wide range of variations in physical properties. Some structural models of such materials, simulated by methods of molecular dynamics [Citation30], are illustrated in .
From a thermodynamic point of view, fullerite C60 and polymer structures based on C60 molecules are highly metastable states of the carbon system relative to graphite and diamond [Citation37], so the processing of C60 fullerite at temperatures above the limit of thermal stability of the C60 cluster in the pressure range up to 9.0 GPa is accompanied by the formation of graphite-like and weakly ordered hard-to-graphitize carbon materials, and at pressures above 9.0 GPa and temperatures above 2000 K the formation of diamonds in the system is noted [Citation31–34].
In general, the results of studies show that the behavior of fullerite C60 at high pressures in many respects resembles the behavior of a system possessing a geometric frustration. With the random nature of the formation of covalent carbon-carbon bonds with any of the 12 neighboring C60 molecules, it is possible to obtain numerous “degenerate” amorphous polymerized states of the system that do not coincide, strictly speaking, in their structure, but have the same energy.
3.1.2. Polyhedral carbon nanoparticles
For studies of the peculiarities of self-assembly of carbon in a “pure” carbon system, polyhedral nanosize (30–80 nm) particles have also been selected. The defining aspects of the transformations of polyhedral carbon nanoparticles in the temperature range up to 1600°C are a truly solid-phase character of such transformations and low diffusion mobility of carbon atoms. As the result, the processes of self-assembly of carbon in these conditions are usually limited by the framework of individual nanoparticles and consist in the change of internal structure of these particles. Herewith the processes of aggregation of nanoparticles and their collective recrystallization do not explicitly occur. The processes of collective recrystallization of studied nanoparticles at temperature of 1600°C do not evolve even under pressure of 8 GPa which one would expect to be beneficial for densification of the system and substantial shortening of distances between carbon atoms of neighboring nanoparticles. Main changes of polyhedral nanoparticles occurring at 8 GPa and 1600°C [Citation38] are related to reconstruction of internal structure and shape of initial nanoparticles associated with lacking an internal cavity. This means that the effect of pressure is expressed in the transformation of initial nanoparticles into a more dense form of carbon. Depending on initial ratio of the total volume of polyhedral nanoparticle to its internal cavity volume, the transformation may proceed both with preservation of polyhedral form and a loss of polyhedrality and its transition into a spherical onion-shaped particle.
The character of changes can be seen in showing the TEM images of assembly of initial polyhedral nanoparticles () and typical HRTEM images of these nanoparticles () as well as similar images of the assembly () and individual nanoparticles () formed during treatment of starting material at 8.0 GPa and 1600°C for 20 s. Thus, in the studied range of pressures and temperatures, most transformations of carbon system consisting of polyhedral carbon nanoparticles are mainly limited to individual nanoparticles. For this reason, the whole system continues to be nanosized. At the same time the formation of nanodiamond, noted in onion-like nanoparticles under electron beam irradiation [Citation39], has not been clearly observed in this case.
Figure 2. TEM (a, d) and HRTEM (b, c, e, f) images of initial polyhedral carbon nanoparticles (a, b, c) and products of their treatment at 8.0 GPa and 1600°C (d, e, f). Reproduced with permission from ref [Citation38], Copyright (2011) Elsevier.
![Figure 2. TEM (a, d) and HRTEM (b, c, e, f) images of initial polyhedral carbon nanoparticles (a, b, c) and products of their treatment at 8.0 GPa and 1600°C (d, e, f). Reproduced with permission from ref [Citation38], Copyright (2011) Elsevier.](/cms/asset/54460595-3e01-4534-903e-81361526d123/tfdi_a_2193212_f0002_b.jpg)
3.2. Hydrocarbon systems
Studies of thermal transformations of hydrocarbon compounds and related natural materials have been the subject of vast number of works, which enabled the detailed learning of major steps of carbonization of different types of organic compounds and subsequent structural ordering of carbonization products at ambient and autogenic pressure [Citation9,Citation10,Citation40]. In particular, in the course of studies of carbonization of polycyclic aromatic hydrocarbons [Citation9,Citation40], it has been established that the initial stage of these transformations is the disproportionation reaction proceeding by atomic hydrogen being detached from one molecule, e.g. naphthalene, and subsequently hydrogenating the other molecule of naphthalene. As the result of reaction from one hand, free radical molecules, which undergo condensation into large flat aromatic clusters, arise. From the other hand, hydrogenated aromatic molecules, easily involved into processes of further hydrogenation accompanied by destruction of initial aromatic molecule and formation of different low-molecular volatile hydrocarbon fractions, also emerge. Thus, the processes of carbonization of hydrocarbons are likely to proceed in two evolutionary directions. One of them leads to formation of low-volatile high-molecular flat aromatic compounds that become precursors for graphite formation. Another direction is related to emergence of more and more volatile low-molecular fractions of hydrocarbons up to methane and free radicals. These most mobile fractions of hydrocarbons become the main suppliers of carbon for construction of different types of metastable nanosize carbon structures. The same hydrocarbon fractions provide effective transport of carbon in the processes of collective recrystallization of these metastable forms of carbon. Formation of flat polycyclic clusters becomes characteristic not only for pyrolysis of polycyclic aromatic compounds but also for transformations of the other types of organic compounds.
Structural self-assembly of carbon in the pyrolysis process of different organic compounds involves an important stage of formation through van der Waals interactions of flat or slightly bent aromatic clusters, being in fact fragments of graphene layers built from 10 to 20 hexagonal carbon rings, and small 2–5 layered packings of these clusters called basic structural units [Citation41]. Such packings of flat clusters can in their turn form a larger size associates having a spherical or cylindrical symmetry. An example of formation of associates with spherical symmetry are the particles of carbon soot which in cases of enlargement of the fragments of graphene layers to the size of closed carbon spheroids can transform into an onion-like nanoparticle. Another example of closed carbon structures, which can be formed on base of the associates of packings of carbon layers, are polyhedral nanoparticles, occasionally called polyhedral carbon black or graphitized carbon black [Citation41]. Lamellae, a linear chain of basic structural units serving as the basis for formation of graphite, provide an example of cylindrical formation of basic structural units with the infinite radius of curvature. At treatment temperatures of ∼1000–1500°С, basic structural units arrange into a separate columns. The extended corrugated carbon layers are formed at temperatures of ∼2000°C through the formation of a network of carbon bonds between fragments of graphene layers of adjacent columns. Effective annealing of defects accompanied by formation of extended flat graphene layers, development of the processes of 3D ordering and formation of crystalline graphite start at temperatures of ∼2500°C and above [Citation41].
Performed research showed that the most important distinction of the character of thermal transformations of hydrocarbon systems from the transformations of “pure” carbon system consists in that these transformations, as a rule, are not already a solid-phase but present a complex combination of a solid-, liquid-, and gas-phase transformations. The appearance at particular stages of the evolution of system of free hydrogen atoms and different gas- and fluid-like components, capable of serving as transport agents for carbon atoms, substantially alters the processes and parameters of formation of different solid states of carbon. Pressure is an additional factor modifying the processes of thermal transformations of hydrocarbon compounds.
Applying the high-pressure results in reduction of temperature thresholds for major stages of processes of carbonization and graphitization of hydrocarbon materials and emergence of the step related to formation of diamond [Citation42–44]. The first attempt of systematic study of behavior of various organic compounds at 12–13 GPa, according to the current pressure scale and 1300–3000°C in the absence of a metal-catalyst, was made by Wentorf [Citation21]. The work was carried out in a “Belt” high-pressure apparatus using titanium as sample container material. In the course of these studies, Wentorf showed that the final product of the transformations of naphthalene, anthracene, and chrysene at 12 GPa and 2000°C is graphite, whereas the treatment of adamantane, camphene, pyrene, fluorene, and polyethylene under the same conditions leads to diamond formation with a 60% yield. The increase of the synthesis temperature up to 2300°C allowed diamond to form from anthracene, but with a rather low yield not exceeding 30%. Moreover, Wentorf pointed out that the diamonds thus obtained were quite unusual: they were snow-white “soft” diamonds, with the appearance and mechanical properties of paraffin wax or chalk. Nevertheless, the X-ray diffraction patterns of these products were those of diamond, but with broadened diffraction lines. Therefore, the transformation has produced very small nano-sized diamond crystallites. As a result, it was assumed that the very nature of the starting carbonaceous material affects decisively the nature of the products of pyrolysis under pressure, even at temperature of 2000°C or higher.
The more detailed studies of the behavior of hydrocarbon compounds under high pressures, performed by Yakovlev et al. [Citation44], showed that synthesis of common “hard” diamond from naphthalene and other hydrocarbons still can be successfully realized without the use of metal catalysts. Furthermore, the authors [Citation44] did not find a well-defined influence of the pristine molecular structures on the final stages of the thermal conversions, although diamond synthesis was performed at p, T values lower than those used in [Citation21]. Later, “direct” diamond synthesis from various precursors including terpene family of hydrocarbons, furfuryl alcohol resin, phenolic resin, and mesophase pitch has been realized by Onodera and Suito [Citation45]. They showed that, when using Ta for the sample container and heater, diamond formation is observed for all hydrocarbons (camphene, adamantane, and fluorene) studied in the 6–12 GPa pressure range at temperatures up to 1600°C.
While analyzing the given data, one would like to focus on remark made in the work [Citation21]. During the studies of temperature dependence of rates of formation of diamond from different carbon materials, Wentorf noted the lack of permanence of the activation energy value for this process and suggested that this is perhaps related to diversity of the types of structure acting as direct carbon precursors for formation of diamond in different systems. To a certain extent, this can also be the reason for the difference in p,T parameters of formation of diamond from different hydrocarbon compounds.
Microscopy studies of the products of thermal transformations of hydrocarbon compounds under high pressures, carried out in works [Citation11,Citation46], indeed revealed a variety of forms of carbon formed during these transformations. Thus, in the samples, obtained by treatment of naphthalene at 8.0 GPa and temperature of 1300°C and isothermal exposure time of 60 s, micron size particles of highly ordered graphite and small globular formations of carbon were present along with the micron size diamond crystals comprising about 90% of the transformation product ().
Figure 3. SEM micrographs of the sample obtained through naphthalene treatment at 8 GPa and 1300°C: D—diamond, G—graphite, CG—carbon globule. Reproduced with permission from ref [Citation46], Copyright (2006) Elsevier.
![Figure 3. SEM micrographs of the sample obtained through naphthalene treatment at 8 GPa and 1300°C: D—diamond, G—graphite, CG—carbon globule. Reproduced with permission from ref [Citation46], Copyright (2006) Elsevier.](/cms/asset/90d9fe4a-e7e2-48bb-8240-bd0282bb23d1/tfdi_a_2193212_f0003_b.jpg)
High-resolution TEM analysis of carbon globules showed that a variety of nanosize forms of carbon, including carbon nano-onions, polyhedral nanoparticles, graphitic ribbons and nanosize diamonds, are present inside their composition ().
Figure 4. HRTEM images of nanosized carbon structures observed in the sample obtained through naphthalene treatment at 8 GPa and 1280°C: spherical onion-like (a), faceted polyhedral particles (b), graphite ribbons (c), and nanocrystalline diamonds (d). Reproduced with permission from ref [Citation46], Copyright (2006) Elsevier.
![Figure 4. HRTEM images of nanosized carbon structures observed in the sample obtained through naphthalene treatment at 8 GPa and 1280°C: spherical onion-like (a), faceted polyhedral particles (b), graphite ribbons (c), and nanocrystalline diamonds (d). Reproduced with permission from ref [Citation46], Copyright (2006) Elsevier.](/cms/asset/d1157853-bb94-4d4f-83f9-56825da71dab/tfdi_a_2193212_f0004_c.jpg)
The presence of nanoforms of carbon at the level of several wt.% along with the micronsize particles of graphite and diamond in the samples suggests that they belong to remnants of different metastable states of carbon formed at the stage of oversaturation of the system and surviving through the processes of collective recrystallization and growth of the most rigid states of carbon. Thus, given the variety of states of carbon which are actually formed in the processes of thermal transformations of hydrocarbon compounds, it is hard to deny the possibility of different mechanisms of diamond formation in these systems, noted by Wentorf [Citation21]. From thermodynamics point of view, formation of diamond from different metastable nanosize forms of carbon means the possibility of a direct transformation of any metastable carbon modification into a more stable under given p,T conditions state of carbon system bypassing the entire sequence of gradual transformations through other intermediate energy states.
Although taking into account that micro-sized particles of highly ordered graphite are the dominant component in the transformation products of naphthalene and other polycyclic aromatic hydrocarbons near the temperature threshold for the start of mass formation of diamond at 8.0 GPa, being ∼1300°C [Citation11], it should be proposed that transformation of graphite-like material into diamond, occuring in a certain amount of hydrogen environment, is the main mechanism of diamond formation during carbonization of hydrocarbons at high pressures and temperatures. The content of the nanoscale fraction in the transformation products of naphthalene and other studied hydrocarbons at 8.0 GPa and 1300°C, as a rule, does not exceed 1 wt.%.
Possible mechanism of such “hydrogen catalyzed” transformation has been proposed in work [Citation47]. According to the proposed mechanism, formation of diamond from graphite-like material occurs due to the transition of edge carbon atoms in the graphite structure as the result of hydrogenation of sp2 to sp3 state and formation of transitional hydrogenated graphite-diamond layer enabling the transformation of graphite structure into a diamond structure.
Note that introduction into the “pure” carbon system of even minor quantities of hydrogen, in the form of some hydrocarbon, leads to significant change of the character of transformations of this system. For instance, presents the SEM images of initial polyhedral carbon nanoparticles (), products of their treatment at 8.0 GPa and 1600°C (), and products of treatment of homogeneous binary mixture of polyhedral nanoparticles with naphthalene containing 1 wt.% hydrogen at 8.0 GPa, 1000°C and 1300°C ().
Figure 5. SEM images of initial polyhedral carbon nanoparticles (a), products of their treatment at 8.0 GPa and 1600°C (b), and products of treatment of binary mixture of polyhedral carbon nanoparticles with naphthalene at 8.0 GPa, 1000°C (c) and 1300°C (d). D—diamond, G—graphite. Reproduced with permission from ref [Citation38], Copyright (2011) Elsevier.
![Figure 5. SEM images of initial polyhedral carbon nanoparticles (a), products of their treatment at 8.0 GPa and 1600°C (b), and products of treatment of binary mixture of polyhedral carbon nanoparticles with naphthalene at 8.0 GPa, 1000°C (c) and 1300°C (d). D—diamond, G—graphite. Reproduced with permission from ref [Citation38], Copyright (2011) Elsevier.](/cms/asset/2398cf06-9340-4d2b-b230-b4fdb48b84c1/tfdi_a_2193212_f0005_b.jpg)
According to these data, treatment of polyhedral nanoparticles at specified p,T parameters does not lead to any notable external changes of the starting material. The system remains mainly nanosized. However, under conditions of binary mixture with naphthalene the character of transformations of polyhedral nanoparticles at the same p,T parameters of treatment changes entirely. As follows from , the product of transformation of binary mixture at 1000°C is micronsize particles of layered graphite-like material. The micron size particles of highly ordered graphite and monocrystals of diamond became main products of transformations of binary mixture at 1300°C. Abrupt change in the dimensionality type of the system, converted from nano- to micron size range, shows a quality change in the mechanism of transformations due to significant increase in mobility of carbon atoms which provided the opportunity for effective crystallization of graphite, diamond formation, and collective recrystallization in carbon system.
This kind of “catalytic” effect of hydrogen on transformations of polyhedral nanoparticles can be explained by two factors: (i) chemical activity of hydrogen capable of hydrogenating the unsaturated carbon compounds, and (ii) its ability of playing the role of transportation agent for carbon atoms.
3.3. Fluorocarbon systems
Studies of thermal transformations of fluorocarbon compounds under high pressures by examples of highly fluorinated graphite (C1F1.1) and octafluoronaphthalene (C10F8) demonstrated the qualitative similarity of the processes of carbonization of these compounds with the processes of carbonization of hydrocarbons [Citation48]. However, in the case of fluorocarbon systems a noticeable reduction (by ∼150–200°C) of temperature thresholds of the main stages of thermal transformations associated with thermal destruction of initial compounds, the beginning of the formation of basic structural units, their structural ordering and formation of monocrystals of high perfection graphite are observed. In addition, two size fractions of carbon materials are clearly standing out in the products of the final stages of carbonization of fluorocarbon compounds.
By way of illustration, the SEM and HRTEM images of carbonization products of octafluoronaphthalene (C10F8) at 8.0 GPa and 800°С are shown in . SEM survey image of C10F8 transformation products () clearly demonstrates the presence of two size fractions of carbon materials – a nanoscale (N) and nominally a microsize (M) one. A detailed SEM image of the M fraction () shows that it is formed by a set of flat polygonal particles of graphite-like material of submicron sizes. At the same time, the detailed SEM image of the N fraction () indicates that it is an agglomeration of nanoscale particles resembling ultradisperse nanodiamonds in appearance. However, HRTEM images of these nanoparticles () show that these are not nanodiamonds, but closed 2–5-layer onion-like or polyhedral nanoparticles measuring ∼5–20 nm with an admixture of some other forms of weakly ordered carbon. With an increase in the processing temperature in the system, the formation of single crystals of high-crystalline graphite with lateral dimensions of up to ten microns and submicron particles of less perfect graphite-like material is observed. They are formed in the processes of collective recrystallization of microsize and, accordingly, nanoscale fractions of carbon observed in carbonization products of fluorocarbon materials at 8.0 GPa and moderate temperatures of ∼800°C.
Figure 6. SEM images (a, b, c) of carbonization products of octafluoronaphthalene at 8.0 GPa, and 800°С (N – nanosized fraction, M – microsized fraction) and the TEM image (d) of nanosized onion-like carbon particles representing a metastable fraction of carbon formed in the process of thermal transformations of fluorocarbon compounds under moderate treatment temperatures. Reproduced with permission from ref [50], Copyright (2016) The American Chemical Society.
![Figure 6. SEM images (a, b, c) of carbonization products of octafluoronaphthalene at 8.0 GPa, and 800°С (N – nanosized fraction, M – microsized fraction) and the TEM image (d) of nanosized onion-like carbon particles representing a metastable fraction of carbon formed in the process of thermal transformations of fluorocarbon compounds under moderate treatment temperatures. Reproduced with permission from ref [50], Copyright (2016) The American Chemical Society.](/cms/asset/a4903445-0e0c-41f6-bd85-b59e6ded7f32/tfdi_a_2193212_f0006_c.jpg)
The most important difference in the nature of transformations of hydrocarbon and fluorocarbon compounds turned out to be that the formation of diamond is not observed in fluorocarbon systems at 8 GPa in all studied range of temperatures up to 1600°C [Citation48]. This fact suggests that fluorine does not have the same “catalytic” effect on the conversion of graphite-like material into diamond as hydrogen [Citation47], despite the fact that fluorine, like hydrogen, is able to transform carbon from sp2 to sp3 states in the processes of fluorination of unsaturated carbon bonds of a graphite-like material. Obviously, this is due to a difference in atomic radii of hydrogen and fluorine and energies of the С-Н и С-F chemical bonds. As a result, the replacement of hydrogen atoms by fluorine during the process of reconstruction of 2-layered packings of graphene layers of sp2 carbons into a 3-layered diamond structure of sp3 carbon due to formation of hydrogenated transition layer, in case of fluorination leads to such a significant deformation of the structure of transition layer that the formation of diamond by this mechanism becomes thermodynamically unprofitable.
3.4. Fluorocarbon-hydrocarbon systems
Studies of the behavior of homogeneous mixtures of hydrocarbon and fluorocarbon compounds at high pressures and temperatures, performed by examples of binary mixtures of naphthalene (C10H8) with highly fluorinated graphite (CF1.1) and octafluoronaphthalene (C10F8), have identified a pronounced synergistic impact of fluorine and hydrogen on processes of transformations of hydrocarbon and fluorocarbon compounds under conditions of their binary mixtures [Citation48–50]. Synergistic effect is manifested primarily by significant temperature drop for the main stages of thermal transformations of binary mixtures in comparison with the pure hydrocarbon and fluorocarbon compounds. So, in particular, the active processes of destruction, observed at 8.0 GPa for pure naphthalene and fluorinated graphite under short times of isothermal exposure at temperatures above 600°C and 500°C, respectively, occur under conditions of their binary mixture already at temperatures ∼400°C. Formation of highly ordered graphite, observed under the same pressure and temperatures of 1200°C, 1000°C, and 1000°С, from naphthalene, fluorinated graphite, and octafluoronaphthalene, in case of binary mixtures of naphthalene with fluorocarbon compounds occurs at a temperature of 900°C. And finally, at 8.0 GPa the temperature threshold for the onset of diamond formation, which for naphthalene and other polycyclic aromatic compounds is ∼1300°С, in case of binary mixtures of naphthalene with octafluoronaphthalene and fluorinated graphite decreases accordingly to ∼1000°C and ∼900°С. It should be noted that despite the fact that in cases of pure fluorocarbon compounds the formation of diamond at a pressure of 8.0 GPa has not been observed in all studied range of temperatures up to 1600°C, under the conditions of binary mixtures of naphthalene with octafluoronaphthalene and fluorinated graphite formation of diamond occurs with almost 100% yield. This means that the transformation into diamond is undergone by the carbon materials being products of carbonization of both the hydrocarbon and fluorocarbon components of mixtures [Citation48,Citation50]. The reason for the noticeable differences in the parameters of the same stages of transformations of pure hydrocarbon and fluorocarbon compounds under conditions of their binary mixtures is the difference of mechanisms of these transformations occurring in pure hydrocarbon (C-H), fluorocarbon (C-F), and combined carbon-hydrogen-fluorine (C-H-F) systems. The results obtained allow us to conclude that the processes of dehydrogenation and defluorination in cases of carbonization of individual hydrocarbon and fluorocarbon compounds are thermally activated.
Under the conditions of binary mixtures, the additional thermochemical channels arise for the processes of carbonization of hydrocarbon and fluorocarbon components of the mixture, which are associated with active mutual fluorine-hydrogen chemical interaction and formation of hydrogen fluoride (HF) molecules capable of significantly modifying the mechanisms, activation energies, and parameters of transformations of both hydrocarbon and fluorocarbon compounds.
Comparative analysis of diamond materials, formed by the high pressure-high temperature induced transformations of pure hydrocarbon compounds and their binary mixtures with fluorocarbons, revealed another important feature of the transformations in conditions of binary mixtures having great practical importance. It is related to the fact that in the products of transformations of binary mixtures, massive formation of nanosized crystals of diamond is observed along with the micronsized diamond crystals which are a typical product of transformation of pure hydrocarbon compounds [Citation11]. SEM images () of diamond materials, obtained by the transformations of naphthalene and its binary mixture with fluorinated graphite, clearly demonstrate this feature. HRTEM image of single-crystal nanodiamond () shows a high degree of crystalline perfection of diamond materials obtained in growth systems based on binary mixtures of hydrocarbon and fluorocarbon compounds.
Figure 7. SEM (a,b), TEM (c) and HRTEM (d) images of diamond materials produced by treatment of mixtures of fluorinated graphite with naphthalene at 8.0 GPa and temperature of 1000°C. Reproduced with permission from ref [Citation48], Copyright (2011) The American Chemical Society.
![Figure 7. SEM (a,b), TEM (c) and HRTEM (d) images of diamond materials produced by treatment of mixtures of fluorinated graphite with naphthalene at 8.0 GPa and temperature of 1000°C. Reproduced with permission from ref [Citation48], Copyright (2011) The American Chemical Society.](/cms/asset/8455891d-71fc-4be4-9472-e3756a6e9589/tfdi_a_2193212_f0007_b.jpg)
Simultaneous presence of two size fractions of diamond in the products of transformations of mixtures of hydrocarbon and fluorocarbon compounds, unlike pure hydrocarbon compounds, can be explained by difference in the compositions of carbon fractions produced before the onset of diamond formation at 8.0 GPa in pure hydrocarbon systems at temperatures of ∼1300°C and binary mixtures of hydrocarbon and fluorocarbon compounds at temperatures of ∼900–1000°С. The active course of the processes of collective recrystallization at 1300°C leads to the production under these conditions of “large” microscale particles of high-perfection graphite which become the main component of hydrocarbon systems, serving as direct precursor for diamond formation. In case of binary mixtures, decrease of the temperatures of the main stages of transformation leads to the observation in the products of transformations, along with the particles of submicron and micron-sized graphite-like materials, of the significant share of less ordered nanosized forms of carbon. Based on these two qualitatively different forms of carbon, the formation of micron-sized and nano-sized fractions of diamond ultimately occurs. Wherein the relative content of different size fractions in the products of transformation of binary systems can vary depending on the composition of starting growth mixture, p-T parameters and treatment time. This makes it possible to successfully obtain ultranano-(up to 10 nm), nano-(10–100 nm), submicro-(100–1000 nm), and micro-(over 1000 nm) size fractions of diamond.
It is interesting to note that the use of pure fluorinated hydrocarbon compounds, such as 1-fluoroadamantane (C10H15F), as a growth system, makes it possible to obtain nanoscale fractions of diamond being very uniform in size with virtually 100% yield. presents an SEM image of such a homogeneous nanoscale fraction of diamond with an average particle size of 20 ± 5 nm.
Figure 8. SEM image of nanoscale diamond fraction with an average particle size of 20 ± 5 nm, obtained as a result of HPHT treatment of C10H15F at 8.0 GPa and 1300°С.
It should be emphasized that the nature of the transformation of 1-fluoroadamantane at high pressures and temperatures is largely similar to the nature of the transformations of another halogen derivative of adamantane, namely 1-chloroadamantane (C10H15Cl), in the products of thermal transformations of which at 8–9 GPa, the mass formation of nanoscale fractions of diamond was also noted [Citation51]. At the same time, the size range of the resulting diamond fractions was quite clearly determined by the treatment temperature of 1-chloroadamantane.
3.5. Synthesis of diamond materials with specified properties
Implementation of synthesis of diamond under high pressures and temperatures based on hydrocarbon growth systems, which do not contain a traditional metal catalysts, allows the production of the most pure diamond materials. Besides that, the introduction of doping additives in the form of specific heterorganic compounds into a growth mixture enables the synthesis of ultranano-, nano-, submicro-, and micron-sized fractions of diamond materials with different types of electroactive and optically active impurity centers which opens the opportunity for synthesis of diamond materials with the desired properties, so called functional diamonds. It should be noted that due to the recent appearance of several perspective quantum-physical and biomedical application trends, the nano-sized diamond materials with the impurity-vacancy MV (NV, SiV, GeV, SnV, and PbV) optical centers [Citation52–57] became of high demand.
In this work, it is shown that the introduction into the composition of the initial hydrocarbon or fluorocarbon-hydrocarbon growth mixtures of nitrogen, silicon or germanium- containing compounds, such as acridine (C13H9N), aminotetrazole (CH3N5), tetrakis (trimethylsilyl)silane (C12H36Si5), tetraphenyl germanium (C24H20Ge) and others, allows to successfully synthesize various size fractions of diamond with a specified content of NV, SiV, GeV color centers, which can be used as single-photon emitters for quantum physical applications [Citation58–61], and highly efficient multiphoton intracellular emitters for two-color intracellular imaging systems, intravital intracellular thermometry, and intracellular markers for directed drug delivery in biomedicine [Citation62].
The introduction of hydrocarbon compounds with a high content of the carbon isotope 13C into the growth system makes it possible to obtain diamonds with a variable specified content of isotopes 12C and 13C, as well as to synthesize diamond “core-shell” nanoparticles with different isotopic composition of the core and shell and different content of impurity-vacancy centers [Citation63]. Particles such as “core–shell” diamond nanoparticles with NV centers and a highly isotopically enriched 13C shell are considered as a promising hyperpolarization agent.
It was found that the nanodiamonds with impurity-vacancy centers, synthesized by high pressure and temperature induced metal catalyst free transformations of hydrocarbon growth systems, possess a number of advantages relatively to nanodiamonds obtained by using the other synthesis methods, such as explosive technologies [Citation64–66], methods of chemical deposition of carbon from the gas phase (CVD) [Citation67–72], ion implantation [Citation73,Citation74], mechanical and ultrasound milling of macro- and micron-sized fractions of diamond materials [Citation75,Citation76], high pressure synthesis by using a metal catalyst growth systems [Citation77–79].
Abandonment of using the metal catalysts excludes the presence of any metal inclusions into the transformation products and enables synthesis of the most pure to date diamond materials characterized by a high degree of crystal perfection and minimal degrees of internal strains comparable with the best samples of low-strained bulk diamonds [Citation80]. The obtained high degree of crystal perfection of the synthesized diamond is due to the fact that unlike the CVD technologies formation of diamond in this case occurs in the region of its thermodynamic stability at pressures near 8 GPa and higher temperatures of ∼1100–1500°С which provide a sufficiently effective annealing of different structural defects. As a result, the opportunity arises for synthesis of nanodiamonds exhibiting a record narrow photoluminescence band close to the bandwidth determined by the lifetime of the excited state of impurity-vacancy center [Citation81] which is particularly important for quantum optical technologies. The proposed methodology also allows successful variation of the composition and relative content of different doping impurities in diamonds due to the appropriate choice of composition of starting growth mixtures.
3.6. Organometallic compounds
Research interest in thermo-induced transformations of organometallic compounds is mainly motivated by the opportunities they open for preparation of different types of nano-sized materials including a quasi-spherical nanoparticles with metal containing core encapsulated into сarbon or more complex layered shells. Given the qualitative likeness of the structure of these particles to the makeup of our planet, they are sometimes assigned to the category of so-called Earthicle [Citation82]. Note, that the processes of thermal destruction of organometallic compounds, and above all ferrocene Fe(C5H5)2, are studied in sufficient details both experimentally and theoretically [Citation83–86]. As a result, it is shown that the destruction of ferrocene at high temperatures proceeds in several stages. In the first stage, a rupture occurs of the C-H bonds of pentagonal fragments of ferrocene molecules, then of the C-C, and finally of the Fe-C bonds [Citation83,Citation85]. Therefore, the size and phase composition of materials, formed in condensation processes of the products of destruction of ferrocene, significantly depends on temperature. Condensation of gaseous mixtures of iron atoms, low-molecular carbon and hydrocarbon clusters, resulting from laser photolysis of ferrocene at temperatures of 2500–2800°С, is accompanied by formation of nano-sized droplets of pure α- and γ-iron, enclosed into carbon shells. These shells have a double-layered structure. Inner layers of the shell are usually built from a two-dimensionally ordered turbostratic states of carbon, while the outer layers by the amorphous ones [Citation86].
The use of high pressures allows to modify the mechanism of transformations and accordingly the fractional composition of the products of transformations [Citation87,Citation88]. By realizing the processes of thermal destruction of ferrocene at pressures and temperatures somewhat lower than the temperature range corresponding to a 100% atomization of Fe-C clusters, one can create the conditions under which the processes of condensation will involve not only the iron atoms but also iron-carbon clusters kept in the system. As a result, not the nanoparticles of pure iron become the main product of condensation of metal-containing components of the system, say at pressure of 8.0 GPa, but a different iron carbides, including an oversaturated by carbon amorphous carbides FeхСу of variable composition dominant at treatment temperatures of 800–1200°С, and a crystalline carbides Fe7С3 and Fe3С, prevailing in samples at treatment temperatures of 1200–1400 and 1400–1600°С, respectively [Citation88].
Due to high content of carbon in the starting compound Fe(С5Н5)2, where the Fe:C atomic ratio is 1:10, all carbon, released during the decomposition of ferrocene, cannot be entirely absorbed by the carbides formed. Therefore, it has to condense also in the forms of pure carbon. Self-assembly of carbon under these conditions occurs within the framework of two subsystems, in fact, carbide and purely carbon.
Primary states of carbide subsystem, formed during the initial stages of material’s condensation at pressures and temperatures, slightly exceeding the limits of thermal stability of ferrocene at appropriate pressures, represent sphere-shaped 2–5 nm size nanoparticles of amorphous iron carbide FexCy, which do not have a well-defined carbon shell (). In this case, the bulk mass of carbon appears in a weekly ordered amorphous state comprising a common carbon matrix where the nanoparticles of iron carbide are dispersed ().
Figure 9. TEM and HRTEM images of the products of ferrocene treatment at 8 GPa and 800 (a), 1100 (b, c), and (d) 1500°C (d). Reproduced with permission from ref [Citation88], Copyright (2018) The American Chemical Society.
![Figure 9. TEM and HRTEM images of the products of ferrocene treatment at 8 GPa and 800 (a), 1100 (b, c), and (d) 1500°C (d). Reproduced with permission from ref [Citation88], Copyright (2018) The American Chemical Society.](/cms/asset/97b6e2f2-62e9-4dde-9d8f-0bd71696a4a0/tfdi_a_2193212_f0009_b.jpg)
Sequential increase of temperature or duration of isothermal exposure, besides the increase of average size of produced particles capable of reaching at elevated treatment temperatures a submicron and micron sizes, lead to development of the processes of structural ordering within framework of both carbide and carbon subsystems.
In case of carbide subsystem, the processes of structural ordering at 8.0 GPa are related to gradual decrease of carbon content in the composition of amorphous nanoparticles of carbide FexCy and their crystallization first in the form of crystalline phase of iron carbide Fe7C3, and then, as the carbon content declines further, in the form of iron carbide Fe3C. The released carbon in this case creates a carbon shell around the carbide nanoparticles resulting in Fe7С3@C и Fe3С@C nanostructures (с,d)) [Citation88]. A definite contribution to the formation of carbon shell can be also made by other carbon and hydrocarbon components appearing at the decomposition of ferrocene. The structure of the shells is rather heterogeneous. It shows the fragments formed by both amorphous carbon and 2–5 layered turbostratic packings of graphene planes (). It is interesting to note that in cases of low density carbon shell, which does not provide complete isolation of the carbide core from the surrounding atmosphere, the extraction of samples from the high pressure apparatus may be accompanied by spontaneous oxidation of the carbide cores with oxygen and the formation of an additional layer of iron oxide in the shell structure [Citation89].
At the same time, it is clear that the self-assembly of the overwhelming bulk of carbon takes place within a purely carbon, or more precisely a hydrocarbon subsystem, considering the possibility of participation of emerging hydrogen in the transformation processes. For this reason, the main stages of self-assembly of carbon remind the pressure and temperature induced transformations of hydrocarbons.
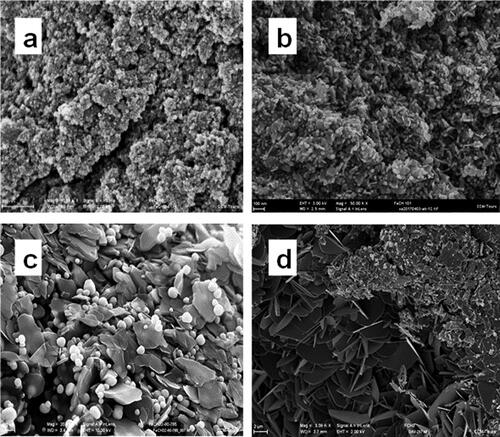
As in the case of pure hydrocarbon systems, the amorphous states of carbon, formed at the initial stages of condensation of carbonization products of organometallic systems, are becoming structurally ordered as the processing temperature rises. SEM images of materials obtained as a result of ferrocene processing at 8.0 GPa and various temperatures () make it possible to trace the structural evolution of the carbon subsystem from states of amorphous carbon, dominating at reduced processing temperatures (), to single crystals of graphite with a high degree of crystalline perfection (). The spherical light particles that stand out on the SEM images represent encapsulated iron carbide particles.
Figure 10. SEM images of the products of ferrocene treatment at 8 GPa and 800 (a), 1100 (b), 1300 (c), and 1500°C (d). Light spherical particles are iron carbides coated with a carbon shell; dark flake-like platelets represent graphite.
However, analysis of the transformation products of ferrocene reveals some distinctive features of transformations of organometallic systems at high pressures and temperatures in comparison with a pure hydrocarbon system. An important distinguishing feature of the self-organization of carbon in an organometallic system, besides the formation of iron carbide particles encapsulated into carbon shells, is the possibility of the formation of various types of carbon nanotubes (). The formation of these carbon structures is observed mainly in samples obtained in the region of reduced pressures (2–6 GPa) and temperatures (900–1200°C) of ferrocene treatment.
Figure 11. SEM images of the products of ferrocene treatment at 2.5 GPa, 900 (a,b,c), and 1100°C(b). Light spherical particles are iron carbides coated with a carbon shell.
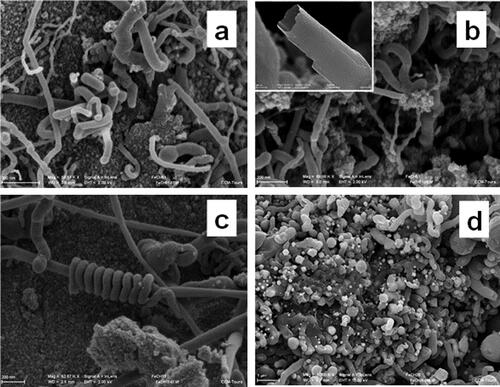
According to , the formation of carbon nanotubes partially filled with iron carbide particles may occur simultaneously with the formation of iron carbide nanoparticles encapsulated in carbon shells. The emergence of tubular carbon structures in the products of transformation of ferrocene may result from either coagulation of a number of carbide particles having a carbon shells or due to the classic version of carbon nanotube growth on metallic substrate [Citation90], whose role in this case can be played by iron carbide particles present in the system. At the same time, the fact that the formation of carbon tubes is mainly observed in the region of moderate processing temperatures, that is, in conditions of still a fairly high hydrogen content in the system, suggests that hydrogen can also play a certain role in the formation of these tubes, providing gas transport mobility of carbon atoms necessary for the effective growth of extended carbon structures.
4. Conclusion
Comparative study of the evolution of states of solid carbon in the high pressure and temperature induced processes of transformations of genuinely carbon and different types of carbon-containing systems has shown that the process efficiency of the physico-chemical evolution of the solid states of carbon is primarily determined by mobility of carbon atoms in the appropriate system. Low values of diffusion mobility of carbon atoms in pure carbon system at temperatures up to 1600°C leads to transformations occurring only through small atomic movements, such as polymerization in the case of fullerite C60 or restructuring of the internal structure, and being strictly limited to the framework of a single particle, as in the case of polyhedral nanoparticles. The processes of collective recrystallization are practically frozen in this case. Therefore, the system based on polyhedral carbon nanoparticles retains a metastable nanoscale character at temperature of 1600° C and maximum pressures of the order of 8.0 GPa.
System introduction of hydrogen or fluorine, chemical elements capable of providing high gas–fluid transport mobility of carbon atoms in the studied range of temperatures due to formation of volatile fractions of low-molecular hydrocarbon and fluorocarbon compounds in the course of thermal transformations, lead to significant decrease of temperature thresholds for the beginning of active stages of mutual transformations of different metastable states of solid carbon and formation of graphite and diamond as the most stable forms of carbon. Studying processes of “secondary” structural ordering of the products of carbonization of studied systems revealed the characteristics of condensation routes of physico-chemical evolution of the solid states of matter associated with the origin, growth, aggregation and structural ordering of particles, materials formed, in different types of carbon-containing systems. The results of the work indicate that the induced by high pressures and temperatures transformations of growth systems based on hydrocarbon compounds and their mixtures with fluorocarbon and heteroorganic compounds with different doping additives not containing a traditional metal catalysts, open new opportunities for production of ultranano-, nano-, submicro- and micro-sized fractions of diamond materials with tailored properties. Implementation of condensation routes of formation of matter at high pressures and temperatures based on organometallic compounds of ferrocene type also enables synthesis of variable size fractions of particles of amorphous FexCy and crystalline (Fe7C3, Fe3C) carbides encapsulated into a carbon shells.
| Abbreviations | ||
| CVD | = | Chemical Vapor Deposition |
| HPHT | = | High Pressure, High Temperature |
| p,T | = | pressure, temperature |
| GPa | = | Giga pascal |
| SEM | = | Scanning Electrons Microscopy |
| TEM | = | Transmission Electrons Microscopy |
| HRTEM | = | High Resolution Electrons Microscopy |
Acknowledgments
VD acknowledges support of Russian Foundation for Basic Research, TP of Human Frontier Science Program, and VKof Nanoplazz Technologies Inc.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Additional information
Funding
Notes on contributors
V. A. Davydov
Valeri Davydov is a Chief Researcher in the laboratory of perspective materials and technologies in the Vereshchagin Institute of High Pressure Physics of RAS. The trends of his research activity are related to physico-chemical transformations of different types of carbon-containing systems under high pessures and temperatures (HPHT) and development of methods for synthesis of new carbon materials with desired properties. In the recent years his works were devoted to HPHT synthesis of different nanosize states of carbon materials and their property studies. Under framework of these studies it has been developed a unique methodology for preparation of nanosize diamonds with variable NV, SiV, and GeV color centers, considered as the most promising candidates for applications as single-photon emitters in quantum physics, biology and medicine, from metal catalyst-free halogenated carbon systems. He holds a professoriate Doctor of Sciences degree (2015) and a doctorate PhD degree (1975) in Chemistry from the Lomonosov Moscow State University.
V. N. Agafonov
Viatcheslav Agafonov Professor Emeritus of University of Tours, France. His research is focused on crystallogenesis and structural characterization of carbon compounds (fullerenes, nanotubes, nanodiamonds) by X ray diffraction methods (powder and single crystals); Raman spectroscopy; Electron Microscopy (Scanning and Transmission), Electron Paramagnetic Resonance. Doctorate: Ph.D. in Chemistry (Chemistry of Materials), Moscow State University, 1975. Ph.D. in Crystal Chemistry, University of Paris VI, Jussieu, France, 1985. Professor Agafonov has co-authored 175 publications (H-index 30; 4374 citations).
T. Plakhotnik
Taras Plakhotnik, PhD, Associate Professor in the School of Mathematics and Physics at the University of Queensland, QLD 4072, Australia; His main research interest is in using of nanoparticles for sensing at nanoscale. The range of sensing application of HTHP nanodiamonds with imbedded luminescent defects such as silicon-vacancy, nitrogen-vacancy and others is quickly expending. Biological compatibility and small size of nanodiamond enables nanothermometry of biological cells, a highly controversial but interesting topic, and a subject of intense discussion in the literature. One of the drawbacks of luminescent HTHP diamonds, their inhomogeneity (by size and optical properties) within a batch is one of the unsolved problems of the crystal growth which hinders applications. Therefore, Dr. Plakhotnik is actively involved in the search for a solution of this problem.
V. N. Khabashesku
Valery Khabashesku holds an Adjunct Professor and Lecturer appointments in the Department of Materials Science and Nanoengineering at Rice University, Houston, TX; previously working at Baker Hughes energy company as Head of the R&D Nanotechnology Center of Excellence and Sr Technology Advisor, during 20 yr period a faculty member in Chemistry at Rice University and Chemical Engineering departments at University of Houston; holds a professoriate Doctor of Sciences degree (1998) and a doctorate PhD degree (1980) in Organic Chemistry from the Russian Academy of Sciences and M.Sc. degree in chemistry from Lomonosov Moscow State University, awarded a 2001 State Prize of Russian Federation in science and technology. Research and expertise are in materials chemistry, functional nanomaterials and oilfield and biomedical applications of nanotechnology; authored over 400 technical papers and holds 93 US patents (H-index 40, 10334 citations).
References
- Kroto HW, Heath JR, O’Brien SC, et al. C60: buckminsterfullerene. Nature. 1985;318(6042):162–163.
- Krätschmer W, Lamb LD, Fostiropoulos K, et al. Solid C60: a new form of carbon. Nature. 1990;347(6291):354–358.
- Howard JB, McKinnon JT, Makarovsky Y, et al. Fullerenes C60 and C70 in flames. Nature. 1991;352(6331):139–141.
- Lieber CM, Chen CC. Preparation of fullerenes and fullerene-based materials. In: Ehrenreich H, Spaepen F, editors. Solid state phys. Cambridge (MA): Academic Press; 1994. p. 109–148.
- Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354(6348):56–58.
- Ugarte D. Curling and closure of graphitic networks under electron-beam irradiation. Nature. 1992;359(6397):707–709.
- Gogotsi Y, Libera JA, Kalashnikov N, et al. Graphite polyhedral crystals. Science. 2000;290(5490):317–320.
- Ge M, Sattler K. Observation of fullerene cones. Chem Phys Lett. 1994;220(3–5):192–196.
- Fitzer E, Mueller K, Schaefer W. The chemistry of the pyrolytic conversion of organic compounds to carbon. Chem Phys Carbon. 1971;7(1):237–383.
- Fishbach D. The kinetics and mechanism of graphitization. Chem Phys Carbon. 1971;7:1–106.
- Davydov VA, Rakhmanina AV, Agafonov V, et al. Conversion of polycyclic aromatic hydrocarbons to graphite and diamond at high pressures. Carbon. 2004;42(2):261–269.
- Melikhov IV. Physico-Chemical evolution of solid state. Moscow: BINOM. Laboratory of Knowledge; 2006.
- Jiang Q, Chen ZP. Thermodynamic phase stabilities of nanocarbon. Carbon. 2006;44(1):79–83.
- Vander Wal RL, Tomasek AJ, Street K, et al. Carbon nanostructure examined by lattice fringe analysis of high-resolution transmission electron microscopy images. Appl Spectrosc. 2004;58(2):230–237.
- Khvostantsev LG, Vereshchagin LF, Novikov AP. Device of toroid type for high pressure generation. High Temp- High Pressures. 1977;9:637–639.
- Tonkov EY, Ponyatovsky EG. Phase transformations of elements Under high pressure. London: CRC Press; 2004.
- Bundy FP. Direct conversion of graphite to diamond in static pressure apparatus. Science. 1962;137(3535):1057–1058.
- Digonsky VV, Digonsky SV. Patterns of diamond formation. St. Petersburg: “Nedra” Publishing House; 1992.
- Terrones M, Terrones H. The carbon nanocosmos: novel materials for the twenty-first century. Philos Trans A Math Phys Eng Sci. 2003;361(1813):2789–2806.
- Vereshchagin LF, Ryabinin YN, Semerchan AA, et al. Direct conversion of graphite into diamond at high static pressures. Dokl Akad Nauk SSSR. 1972;206(1):78–79.
- Wentorf RH. The behavior of some carbonaceous materials at very high pressures and high temperatures. J Phys Chem. 1965;69(9):3063–3069.
- Dresselhaus MS, Dresselhaus G, Eklund PC. Science of fullerenes and carbon nanotubes. New York (NY): Academic Press; 1995.
- David WIF, Ibberson RM, Matthewman JC, et al. Crystal structure and bonding of ordered C60. Nature. 1991;353(6340):147–149.
- Heiney PA. Structure, dynamics and ordering transition of solid C60. J Phys Chem Solids. 1992;53(11):1333–1352.
- Heimann RB, Evsvukov SE, Koga Y. Carbon allotropes: a suggested classification scheme based on valence orbital hybridization. Carbon. 1997;35(10–11):1654–1658.
- Burgos E, Halac E, Weht R, et al. New superhard phases for three-dimensional ${C}_{60}$-based fullerites. Phys Rev Lett. 2000;85(11):2328–2331.
- Iwasa Y, Arima T, Fleming RM, et al. New phases of C-60 synthesized at high-pressure. Science. 1994;264(5165):1570–1572.
- Núñez-Regueiro M, Marques L, Hodeau JL, et al. Polymerized fullerite structures. Phys Rev Lett. 1995;74(2):278–281.
- Davydov VA, Kashevarova LS, Rakhmanina AV, et al. Spectroscopic study of pressure-polymerized phases of ${\mathrm{C}}_{60}$. Phys Rev B. 2000;61(18):11936–11945.
- Tang H, Yuan X, Cheng Y, et al. Synthesis of paracrystalline diamond. Nature. 2021;599(7886):605–610.
- Blank VD, Buga SG, Dubitsky GA, et al. High-pressure polymerized phases of C60. Carbon. 1998;36(4):319–343.
- Brazhkin VV, Lyapin AG. Hard and superhard carbon phases synthesized from fullerites under pressure. J. Superhard Mater. 2012;34(6):400–423.
- Sundqvist B. Carbon under pressure. Phys Rep. 2021;909:1–73.
- Shang Y, Liu Z, Dong J, et al. Ultrahard bulk amorphous carbon from collapsed fullerene. Nature. 2021;599(7886):599–604.
- Chernozatonskii LA, Serebryanaya NR, Mavrin BN. The superhard crystalline three-dimensional polymerized C60 phase. Chem Phys Lett. 2000;316(3–4):199–204.
- De Gennes PG. Scaling concepts in polymer physics. London: Ithaca; 1979.
- Yakovlev EN, Voronov OA. The gibbs energy of fullerite C60 at pressures up to 20 GPa in the temperature range 300-1000 K. High Temper High Pressure. 1994;26:639–643.
- Davydov VA, Shiryaev AA, Rakhmanina AV, et al. Transformations of polyhedral carbon nanoparticles under high pressures and temperatures. Carbon. 2011;49(7):2389–2401.
- Banhart F, Ajayan PM. Carbon onions as nanoscopic pressure cells for diamond formation. Nature. 1996;382(6590):433–435.
- Pierson HO. Handbook of carbon, graphite, diamond, and fullerenes: properties, processing, and applications. Park Ridge (NJ): Noyes Publications; 1993.
- Oberlin A. High-resolution TEM study of carbonization and graphitization. In: Thrower A, editor. Chemistry and physics of carbon . New York (NY): Dekker M; 1989. p. 1–143.
- Whang P, Dachille F, Walker P.Jr Pressure effects on the initial carbonization reactions of anthracene. High Temp-High Press. 1974;6(2):127–136.
- Ayache J, Oberlin A, Inagaki M. Mechanism of carbonization under pressure, part I: influence of aromaticity (polyethylene and anthracene). Carbon. 1990;28(2–3):337–351.
- Yakovlev E, Voronov O, Rakhmanina A. Diamond synthesis from hydrocarbons. Sverkhtverd Mater. 1984;59(4):8–11.
- Onodera A, Suito K. Synthesis of diamond from carbonaceous materials. In: science and technology of high pressure. Hyderabad, India: Universities Press Hyderabad; 2000. p. 875–880.
- Davydov VA, Rakhmanina AV, Boudou JP, et al. Nanosized carbon forms in the processes of pressure-temperature-induced transformations of hydrocarbons. Carbon. 2006;44(10):2015–2020.
- Lambrecht WRL, Lee CH, Segall B, et al. Diamond nucleation by hydrogenation of the edges of graphitic precursors. Nature. 1993;364(6438):607–610.
- Davydov VA, Rakhmanina AV, Agafonov VN, et al. Synergistic effect of fluorine and hydrogen on processes of graphite and diamond formation from Fluorographite-Naphthalene mixtures at high pressures. J Phys Chem C. 2011;115(43):21000–21008.
- Davydov VA, Rakhmanina AV, Agafonov V, et al. On the nature of simultaneous formation of nano- and micron-size diamond fractions under pressure-temperature-induced transformations of binary mixtures of hydrocarbon and fluorocarbon compounds. Carbon. 2015;90:231–233.
- Davydov VA, Agafonov V, Khabashesku VN. Comparative study of condensation routes for formation of nano- and microsized carbon forms in hydrocarbon, fluorocarbon, and Fluoro-Hydrocarbon systems at high pressures and temperatures. J Phys Chem C. 2016;120(51):29498–29509.
- Ekimov E, Shiryaev AA, Grigoriev Y, et al. Size-Dependent thermal stability and optical properties of ultra-small nanodiamonds synthesized under high pressure. Nanomaterials. 2022;12(3):351.
- Aharonovich I, Castelletto S, Simpson DA, et al. Diamond-based single-photon emitters. Rep. Prog. Phys. 2011;74(7):076501.
- Arnault JC. Nanodiamonds: advanced material analysis, properties and applications. Amsterdam, the Netherlands: Elsevier; 2017.
- Bradac C, Gao WB, Forneris J, et al. Quantum nanophotonics with group IV defects in diamond. Nat Commun. 2019;10(1):5625.
- Alkahtani MH, Alghannam F, Jiang L, et al. Fluorescent Nanodiamonds: past, present, and future. Nanophotonics. 2018;7(8):1423–1453.
- Shenderova OA, Shames AI, Nunn NA, et al. Review article: synthesis, properties, and applications of fluorescent diamond particles. J Vac Sci Technol B Nanotechnol Microelectron. 2019;37(3):030802.
- Ekimov E, Kondrin M. High-pressure, high-temperature synthesis and doping of nanodiamonds. Diamond for quantum applications part 1. Semiconductors and semimetals. Amsterdam, the Netherlands: Elsevier Science; 2020. p. 161–199.
- Fehler KG, Ovvyan AP, Antoniuk L, et al. Purcell-enhanced emission from individual SiV − center in nanodiamonds coupled to a Si3N4-based, photonic crystal cavity. Nanophotonics. 2020;9(11):3655–3662.
- Nahra M, Alshamaa D, Deturche R, et al. Single germanium vacancy centers in nanodiamonds with bulk-like spectral stability. AVS Quantum Sci. 2021;3(1):012001.
- Kumar S, Wu C, Komisar D, et al. Fluorescence enhancement of a single germanium vacancy center in a nanodiamond by a plasmonic bragg cavity. J Chem Phys. 2021;154(4):044303.
- Waltrich R, Lubotzky B, Abudayyeh H, et al. High-purity single photons obtained with moderate-NA optics from SiV center in nanodiamonds on a bullseye antenna. New J Phys. 2021;23(11):113022.
- Liu W, Alam MNA, Liu Y, et al. Silicon-Vacancy nanodiamonds as high performance Near-Infrared emitters for Live-Cell Dual-Color imaging and thermometry. Nano Lett. 2022;22(7):2881–2888.
- Mindarava Y, Blinder R, Davydov VA, et al. Core-Shell" diamond nanoparticles with NV- Centers and a highly isotopically enriched C-13 shell as a promising hyperpolarization agent. J Phys Chem C. 2021;125:27647–27653.
- Vlasov II, Shenderova O, Turner S, et al. Nitrogen and luminescent nitrogen-vacancy defects in detonation nanodiamond. Small. 2010;6(5):687–694.
- Mochalin VN, Shenderova O, Ho D, et al. The properties and applications of nanodiamonds. Nat Nanotech. 2012;7(1):11–23.
- Vlasov II, Turner S, Van Tendeloo G, et al. Chapter 9 recent results on characterization of detonation nanodiamonds. In: Shenderova OA, GruenIn DM, editors. Ultananocrystalline diamond. Amsterdam, the Netherlands: Elsevier; 2012. p. 291–326.
- Bergman L, Stoner BR, Turner KF, et al. Microphotoluminescence and Raman-Scattering study of defect formation in diamond films. J Appl Phys. 1993;73(8):3951–3957.
- Turukhin AV, Liu C, Gorokhovsky AA, et al. Picosecond photoluminescence decay of si-doped chemical-vapor-deposited diamond films. Phys Rev B. 1996;54(23):16448–16451.
- Musale D, Sainkar S, Kshirsagar S. Raman, photoluminescence and morphological studies of si-and N-doped diamond films grown on si (100) substrate by hot-filament chemical vapor deposition technique. Diam Relat Mater. 2002;11(1):75–86.
- Balmer R, Brandon J, Clewes S, et al. Chemical vapour deposition synthetic diamond: materials, technology and applications. J Phys Condens Matter. 2009;21:364221.
- Grudinkin SA, Feoktistov NA, Medvedev AV, et al. Luminescent isolated diamond particles with controllably embedded silicon-vacancy colour centres. J Phys D Appl Phys. 2012;45(6):062001.
- Bolshakov A, Ralchenko V, Sedov VS, et al. Photoluminescence of SiV centers in single crystal CVD diamond in situ doped with si from silane. Phys Status Solidi A. 2015;212(11):2525–2532.
- Vavilov VS, Gippius AA, Zaitsev BV, et al. Study of cathodoluminescence of epitaxial diamond films. Sov Phys Semicond. 1980;14:1078.
- Tchernij SD, Luhmann T, Herzig T, et al. Single-photon emitters in lead-implanted single-crystal diamond. Acs Photonics. 2018;5(12):4864–4871.
- Boudou JP, Tisler J, Reuter R, et al. Fluorescent nanodiamonds derived from HPHT with a size of less than 10 nm. Diam Relat Mater. 2013;37:80–86.
- Neu E, Arend C, Gross E, et al. Narrowband fluorescent nanodiamonds produced from chemical vapor deposition films. Appl Phys Lett. 2011;98(24):243107–243107.
- Sittas G, Kanda H, Kiflawi I, et al. Growth and characterization of si-doped diamond single crystals grown by the HTHP method. Diam Relat Mater. 1996;5(6–8):866–869.
- Clark CD, Kanda H, Kiflawi I, et al. Silicon defects in diamond. Phys Rev B. 1995;51(23):16681–16688.
- Nadolinny VA, Komarovskikh A, Palyanov YN, et al. EPR study of si‐ and ge‐related defects in HPHT diamonds synthesized from mg‐based solvent‐catalysts. Phys Status Solidi A. 2016;213(10):2623–2628.
- Rogers L, Wang O, Liu Y, et al. Single SiV- centers in low-strain nanodiamonds with bulk-like spectral properties and nano-manipulation capabilities. Phys Rev Appl. 2018;11:024073.
- Jantzen U, Kurz AB, Rudnicki DS, et al. Nanodiamonds carrying silicon-vacancy quantum emitters with almost lifetime-limited linewidths. New J Phys. 2016;18(7):073036.
- Uskoković V. Earthicle and its discontents: a historical critical review of iron (oxide) particles singly and doubly shelled with silica and/or carbon. ACS Earth Space Chem. 2020;4(10):1843–1877.
- Elihn K. Synthesis of carbon-covered iron nanoparticles by photolysis of ferrocene. Uppsala, Sweden: Uppsala Universitet; 2002.
- Elihn K, Otten F, Boman M. Size distributions and synthesis of nanoparticles by photolytic dissociation of ferrocene. Appl Phys A. 2001;72(1):29–34.
- Elihn K, Larsson K. A theoretical study of the thermal fragmentation of ferrocene. Thin Solid Films. 2004;458(1–2):325–329.
- Elihn K, Landström L, Alm O, et al. Size and structure of nanoparticles formed via ultraviolet photolysis of ferrocene. J Appl Phys. 2007;101(3):034311–034311.
- Bagramov RH, Blank VD, Serebryanaya NR, et al. High pressures synthesis of iron carbide nanoparticles covered with onion-like carbon shells. Fuller Nanotub Carbon Nanostruct. 2012;20(1):41–48.
- Baskakov A, Lyubutin I, Starchikov S, et al. Mechanism of transformation of ferrocene into carbon-encapsulated iron carbide nanoparticles at high pressures and temperatures. Inorg Chem. 2018;57(23):14895–14903.
- Starchikov S, Zayakhanov VA, Vasiliev AL, et al. Core@shell nanocomposites Fe7C3/FexOy/C obtained by high pressure-high temperature treatment of ferrocene fe(C5H5)2. Carbon. 2021;178:708–717.
- Harris PJF. Carbon nanotubes and related structures: new materials for the twenty-first century. Cambridge: Cambridge University Press; 1999.
