ABSTRACT
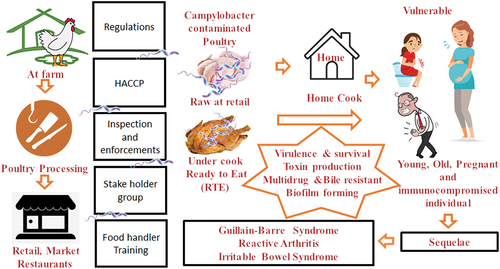
The incident of Campylobacteriosis rate remains high in both the developed and developing world. Recent research showed that the handling, preparation, and consumption of poultry meat is the consequential root of the campylobacteriosis occurrence. The consumers play a crucial role in the last line of defence against Campylobacteriosis. Habitually, consumers handle the food as they please in a domestic setting depending on the probable risk they perceived. Therefore, consumers’ food handling practices are the plausible risk factors that indicate exposure to this foodborne illness. Symptoms are commonly mild and denoted by diarrhoea, fever, nausea, abdominal pain, malaise, and vomiting. However, in some cases campylobacteriosis can be asymptomatic carriage stage yet the infection is associated with long-term sequelae, in particular, Guillain-Barré Syndrome (GBS), Reactive Arthritis (RA) and irritable bowel syndrome (IBS). Thus, the burden of the disease tremendously impacts on economy and victim’s living life with long-term disability. This review presents an updated overview of the global epidemiology, the relevance of official control, the disease associated with food handler and the importance of food safety with respect to Campylobacteriosis.
Introduction
Campylobacter is the most common cause of bacterial gastroenteritis around the globe. WHO[Citation1] estimated that the incidence of Campylobacteriosis is 1 in 4 causes of gastrointestinal disease in both developed and developing world. In Europe, the number of confirmed cases of human campylobacteriosis was 246,158, with an EU notification rate of 64.8 per 100,000 population .[Citation2] The illness represents nearly 70% of all the reported cases at European level since 2005 .[Citation3]
Poultry is considered as one of the most important reservoirs of Campylobacter species and represents a very significant vehicle for the transmission to humans .[Citation4,Citation5] Nonetheless, Campylobacteriosis risk management system is in place at national and international level, so far it has not been possible to cater Campylobacter free poultry to the consumer .[Citation6,Citation7] Numerous studies have been reported that the incident of food-borne illnesses linked to food handlers.[Citation8,Citation9] According to the EFSA Scientific Opinion published in 2010, the risk of human campylobacteriosis linked to poultry handling, preparation and consumption accounts for 20 to 30% of human cases of campylobacteriosis .[Citation10] presents a summary of an overview of human Campylobacteriosis. Therefore, it could be hypothesised that consumer unaware of the severity of the Campylobacteriosis risk or deficient in knowledge of food safety practices .[Citation11] Moreover, it is most likely that consumer disregard the importance of food safety practices at home. Thus, there is a need to explore the factual circumstances of Campylobacteriosis and update the current knowledge of the infection.
Campylobacter
Campylobacter spp is gram-negative microorganisms, non-spore-forming a spirally curved shape, which colonises the mucosal surfaces of the intestinal tracts, oral cavities, or urogenital tracts of most warm-blooded animals .[Citation12] Most of the Campylobacter species is mobile caused by a polar flagellum present on one or both ends of the cell .[Citation13] The species can grow pH between 6.5 and 7.5 and the temperature between 37°C and 42°C .[Citation14] Thus, they are considered thermophile and birds have been widely regarded as natural hosts of these organisms. They are unable to grow the maximum temperature above 55°C or below the temperature of 30°C.[Citation13] Grow well at an optimal water activity of 0.997 and does not grow with water activity less than 0.987 .[Citation15]
Campylobacter species, particularly Campylobacter jejuni and Campylobacter coli are the most common of human bacterial gastroenteritis in the developed world and are responsible for over 95% human campylobacteriosis.[Citation16–18] The organism was first described by German-Austrian paediatrician and a professor at university in Graz and Vienna Theodor Escherich in 1886 from the stools of children suffering from diarrhoea.[Citation16] The genus Campylobacter is commonly found in nature and can contaminate drinking water. Among sporadic human cases, contact with live poultry, consumption of poultry meat, drinking water from untreated water sources, and contact with pets and other animals have been identified as the important sources of infections .[Citation19]
Virulence and survival feature
The onset and infection of Campylobacter require several virulence factors including motility, chemotaxis, adherence, and invasion of the host cell, toxin production, structures of the cell envelope, iron uptake system, multidrug and bile resistance, and mechanisms of responses to stress .[Citation20–22] Additionally, it can also form a biofilm, a polymeric matrix synthesized by aggregates of microbial cells from the same or different microorganisms that are attached to various types of surfaces .[Citation23] These biofilms protect the bacteria from a hostile environment which allows the pathogen to survive. Besides several virulence factors, previous studies reveal that Campylobacter spp are also increasing antimicrobial resistance particularly strains isolated from food.[Citation24–26] Furthermore, it has not been possibly to control the Campylobacter at farm level because it is resistant to various veterinary antibiotics.[Citation16] Thus, it has not been able to provide Campylobacter free poultry to the consumer.
Prevalence of Campylobacter in poultry
Campylobacter spp. have been found in many avian species, both domesticated and wild. Among domesticated birds, a high prevalence of C. jejuni and C. coli is often found in broiler chickens, breeder flocks and egg-laying hens.[Citation13,Citation27,Citation28] Thus, poultry is recognised as the major reservoir of Campylobacter infection for humans.[Citation13,Citation18,Citation27,Citation28] The prevalence of Campylobacter in poultry can lead to a greater exposure risk when consumers mishandle raw poultry in the domestic kitchen.[Citation29] Infection in poultry is predominantly through the oral-faecal route or through vertical transmission from parent flocks.[Citation12] The cross-contamination normally passes on to generation to the next from same farm animal and it is very unusual that cross contaminated from environment to the animal. Bull et al.[Citation30] have estimated that the chicken meat in retail is tainted with C. jejuni up to 98% of cases in the US and from 60% to 80% of cases in Europe.
The highest occurrence of Campylobacter (36.7%) in broiler meat was detected in 2016 reported by some European member state (see ). In 2011 nationwide survey on raw chicken on retail sales in the Republic of Ireland (ROI) revealed Campylobacter was detected in 50.2% of samples using a quantitative method, with bacteria counts ranging from of 10 CFU/g, to of 61,000 CFU/g.[Citation5] represents an account on Prevalence of Campylobacter spp. in raw poultry meat at retail in the various countries. The result of an EU wide baseline study disclosed an Irish prevalence in broiler batches of 83.1% and a prevalence of 98.3% on carcasses at the end of the slaughtering process.[Citation5] A survey conducted in 2008 as part of the FSAI/HSE national microbiological surveillance programme reveals that Campylobacter was detected on 13.2% (104/785) of the external surface of poultry packaging and 10.9% (86/785) of the surface of display cabinets in retail premises.[Citation5] These high levels of bacteria to spread easily in the environment, especially in a domestic setting with mishandling, thus allowing the contamination further.
Table 1. Report on prevalence of Campylobacter spp. in raw poultry meat at retail.
Figure 2. Campylobacter statistics related to major non-RTE food categories reporting EU MS and non-MS, 2019 (Adapted from EFSA, Citation2021[Citation31]).
![Figure 2. Campylobacter statistics related to major non-RTE food categories reporting EU MS and non-MS, 2019 (Adapted from EFSA, Citation2021[Citation31]).](/cms/asset/9d588a54-93f7-447c-accb-1dc3bbd0a17c/lfri_a_1942487_f0002_oc.jpg)
Epidemiology
Campylobacteriosis incident occurs in all age groups[Citation42] with the highest rate of notification reported in the 0–5 year and the percentage of people hospitalised and died was highest among persons aged ≥ 65 years.[Citation13] Common symptoms include mildness such as diarrhoea, abdominal pain, malaise, fever, nausea and vomiting. The illness is self-limiting, the duration of illness lasting from 3 to 6 days to up 10 days. The incubation period is between 2–5 days but may vary from 1 to 11 days.[Citation43] Although diarrhoea is the most common clinical symptom of Campylobacter spp infection, a broad clinical spectrum is associated with this infection, from asymptomatic carriage to systemic illness and bacteraemia to localised infection and association with many more complications. The main recognised secondary consequences of Campylobacteriosis are Guillain-Barré Syndrome (GBS), the Reactive Arthritis (REA) and Irritable Bowel Syndrome (IBS) .[Citation13,Citation26]
Reactive arthritis
Recently, the researchers identified that a small proportion of Campylobacteriosis patient developed reactive arthritis shortly after gastroenteritis.[Citation44] Reactive arthritis was first described several decades ago following gastrointestinal infection with a variable attack rate. There are many enteric microorganisms are associated with the development of post-infectious reactive arthritis, including Salmonella, Shigella, Campylobacter, and Yersinia .[Citation45] There is a wide spectrum of clinical presentation from monoarticular to polyarticular arthritis to slight transient arthropathies to long-standing debilitating arthritis.[Citation44] The persistent of the reactive arthritis symptom is unpredictable. In one of the outbreaks of a Salmonella typhimurium infection, 60% of patients had joint pain after 4–5 months.[Citation46,Citation47] Pope et al.[Citation47] stated that 5% of those who contracted Campylobacteriosis in past developed chronic rheumatoid arthritis or relapsing symptoms a year later.
Guillain-Barre Syndrome (GBS)
Uncommon cases of complication can occur at post campylobacteriosis infection, for instance, an acute immune-mediated inflammation of the peripheral nerves known as GBS often resulting in neuromuscular paralysis .[Citation13] In general, most of the GBS patients can fully recover within a few weeks/months, and do not have any further problems. However, in some cases, it may take longer to recover, and there is a possibility of permanent nerve damage. The incidents of GBS in relation to peripheral nervous system destruction caused by campylobateriosis have been studied globally.[Citation48–50] In some region of the world, GBS data are limited due to improper health system and low-income countries standards. In Ireland, GBS affects 50–100 people every year .[Citation42] It is slightly more common in men than in women. It can affect people of any age, including children.
The likelihood of developing GBS post Campylobacter infection is approximately between 1 per 1000 and 1 per 5000 infections .[Citation44,Citation51,Citation52] The first case of Campylobacter-associated GBS was identified in 1982, subsequently, there have been many researchers confirming the link .[Citation44,Citation53] Approximately, 40% of GBS cases are associated with post Campylobacteriosis infection.[Citation54,Citation55] A case-control study carried out in the UK, n = 103 confirmed 26% GBS cases associated with post Campylobacter jejuni infection .[Citation56] A study in Sweden confirmed that the probability of GBS following C. jejuni infection is 100 times higher than that of those who doesn’t .[Citation57] Similar study carried out in six European countries namely Denmark, the Netherlands, Norway, Poland, Spain and the United Kingdom revealed that Scandinavian countries had the lowest estimated developing GBS post Campylobacter infection of <10 per 100 000 and Spain and Poland had the highest rate of >100 per 100 000.[Citation58]
Post Infective Irritable Bowel Syndrome (PI-IBS)
Numerous studies show that IBS as a consequence of infectious gastroenteritis, a heterogeneous disorder. A study identifies that IBS symptoms were progressed from infective dysentery .[Citation53] A feature of IBS is similar to those of PI-IBS. A large sample size survey of 9,776 individuals in England and Wales about the annual incidence of gastrointestinal infection reveals that approximately 35% of cases caused by viral infections, Campylobacter spp 10% and Salmonella spp 3%, respectively,[Citation59]). However, PI-IBS disorder occurs less frequent in viral gastroenteritis than after infection associated with mucosal ulceration, in particular, Campylobacter jejuni, and Escherichia coli O157: H7. Marshall et al.[Citation60] reported that up to 36% of individuals with acute Campylobacteriosis progressed IBS within 1–2 years. More study established that C jejuni infection was related to a PI-IBS likelihood of 9% to 13% .[Citation61–63]
Campylobacteriosis disease burden
The Foodborne Disease Burden Epidemiology Reference Group (FERG) of the WHO in 2020 estimated that 550 million foodborne illnesses per annum and Campylobacter is 1 of the 4 key global causes of diarrhoeal diseases.[Citation1] FERG also estimated that Campylobacter was the most frequent causes of diarrhoea 166 million, and 31,700 GBS cases per year.[Citation64] These illnesses resulted in 37,600 deaths.[Citation64] It is the third most frequent cause of hospitalization for gastroenteritis following rotavirus and Salmonella species infection, with a hospitalisation rate of 10.8% for all Campylobacter species infections .[Citation65,Citation66]
In a study carried out on US population, it has been estimated that food-borne Campylobacters infect around 2.5 million people each year .[Citation67] According to European Food Safety Authority and European Centre for Disease Prevention and Control[Citation19] summary report and trend, in 2012 were 19,531 reported infections, 4,563 hospitalizations and 68 deaths associated with foodborne diseases. Among these incidents, Campylobacter, in particular, ranked second, after Salmonella, as a cause of food-borne infections. In 2018, were 2,50,384 reported infections in EU/EEA, 21,799 hospitalisations and 60 deaths associated with Campylobacter alone[Citation3] (see ) and it is ranked highest in hospitalisation.
Figure 3. Disease data from ECDC surveillance atlas – campylobacteriosis, adapted from https://ecdc.europa.eu/en/campylobacteriosis/surveillance/atlas[Citation68].
![Figure 3. Disease data from ECDC surveillance atlas – campylobacteriosis, adapted from https://ecdc.europa.eu/en/campylobacteriosis/surveillance/atlas[Citation68].](/cms/asset/fee0743a-4be9-474d-b5d2-644b0fafa30d/lfri_a_1942487_f0003_oc.jpg)
The economic cost of Campylobacteriosis in the UK was estimated at £50 million from 2008 to 2009.[Citation69] In addition, the cost of Campylobacter-related GBS hospitalisation was £1.26 million. In Europe, EFSA estimated the cost to public health systems and to loss of productivity in the EU is around EUR 2.4 billion a year .[Citation13] Numerous studies estimated the economic impact of Campylobacteriosis per case ranges from the US 118.09 USD to the US 8915 USD per case .[Citation70] represents an account of the estimated cost of Campylobacteriosis in various country.
Table 2. Report on estimated cost of Campylobacteriosis.
Campylobacteriosis control relevant to poultry
The control of Campylobacter in the food chain is a tremendous challenge for every stakeholder. It is both the pervasive and the low infective dose required for illness. It is often difficult to detect the origin of exposure to Campylobacter, because of its sporadic nature of the infection and the important role of cross-contamination. Indeed, there is no single control measure approach that can completely eliminate Campylobacter from the food chain, or even reduce to the safe level, without affecting product attributes. Consumers’ acceptability of the decontamination method of the poultry meats varies from country to country.
Thus, over the past decade, many countries have put in place, monitor and amended a number of important control measures time to time in order to mitigate this food-borne infections.[Citation77] In EU, recently amended EU Regulation 2073/2005 has included the maximum permitted level of 1000 CFU/g at the slaughter for the broiler (Commission Regulation (EU) 2017/1495). FAO and WHO joint committee Codex Alimentarius urge the authorities around the world to establish preventive precautionary measures to control the Campylobacter in poultry meat due to its possibility of vertical transmission.[Citation78] presents detailed policies and control measures laid out for poultry meat to mitigate Campylobacter transmission.
Table 3. Policies and control measures relevant to Campylobacteriosis.
Since 2005, Campylobacter has been the causative agent of the most common zoonosis in Europe. Under the EU legislation, all member states have a well-established food safety official control system. The national food official control system in all member state is routinely audited by the European Commission. However, still quite unable to provide Campylobacter-free poultry to the consumer.[Citation5,Citation6,Citation10] Although recently amended EU Regulation 2073/2005 has included the maximum permitted level of 1000 CFU/g at the slaughter for the broiler (Commission Regulation (EU) 2017/1495), the infective dose is as low as 500 cells .[Citation35,Citation79,Citation80]
Campylobacteriosis associated with food handler
Owing to neither cultural, nor religious restrictions, a high nutritional content (high protein, low-fat, low-sodium, low-cholesterol), [Citation81] ease of accessibility and consequent affordability, poultry consumption is the most popularly consumed muscle-based food globally.[Citation82] Undeniably, the current increase in reported campylobacteriosis cases globally mirrored the increased consumption. Higher consumption most likely caused elevation of campylobacteriosis. Currently, it has not been possible to provide Campylobacter free poultry to the consumer.[Citation6,Citation7] A number of food-borne illnesses have been linked to food handlers.[Citation8,Citation9,Citation83] represents the domestic Campylobacteriosis cases associated with consuming inappropriately prepared poultry meat.
Table 4. Report on Campylobacteriosis cases associated with poultry meat/ handling/ consuming.
The most plausible route of spread could be during the handling and preparation of foods in the domestic kitchen Campylobacter would simply spread, quickly contaminating other foods and surfaces. According to the EFSA Scientific Opinion on the risk of human campylobacteriosis linked to poultry, published in 2010, it is likely that handling, preparation and consumption of broiler meat accounts for 20 to 30% of human cases of campylobacteriosis.[Citation10] Available Campylobacteriosis data by country on Atlas.ecdc.europa.eu. (2021)[Citation68] shown the overall notification cases and the notification N/10, 000 in domestic cases in 2019 ().
Figure 4. Campylobacteriosis data by country in EU on atlas.ecdc.europa.eu.[Citation68].
![Figure 4. Campylobacteriosis data by country in EU on atlas.ecdc.europa.eu.[Citation68].](/cms/asset/5e820d6e-8bcd-43d9-a18c-125f8b7dbab8/lfri_a_1942487_f0004_oc.jpg)
There has been a substantial amount of study carried out in the past to identify some risk factors for sporadic campylobacteriosis, particularly the handling of raw poultry.[Citation20,Citation93,Citation94] The main factors which lead to infection include ignorance or oversight the thoroughly cooking the poultry meat in the domestic kitchen, which in turn lead to consumption of undercook or raw poultry. A case-control study identified that the association of campylobacteriosis and eating undercooked poultry.[Citation95–99] During 2016, there were five notified outbreaks which included cases of campylobacteriosis, four of which were in private houses.[Citation100] On the same report, there was one VTEC/Campylobacter outbreak which involved 32 confirmed Campylobacter cases; the reported mode of transmission was foodborne, and person-to-person spread.
Domestic poultry handling practices
The increase in the number of Campylobacteriosis incidence raises the question of how they handle the poultry in their own home. Unmistakably, the domestic environment is the final stage in the farm-to-fork chain. The consumers play a crucial role in the last line of defence against Campylobacteriosis.[Citation11] The consumers handle the food as they please in domestic setting depend on the probable risk they perceived. Therefore, consumers’ food handling practices could be the risk factors that indicate exposure to certain foodborne pathogens. Safe food handling behaviour in the domestic environment remains just as important as the microbial safety of the raw ingredients at the retail or food service stage in the farm-to-fork chain.
According to the EFSA report in 2009, the most common set of exposure for verified outbreaks was the private household (37%), followed by restaurants and cafe´s (28.6%).[Citation101] Extensive research has been identified the consumption of undercooked poultry (chicken liver pate) is the major risk factor for Campylobacter infections.[Citation101] Myintzaw, Moran and Jaiswal[Citation11] highlighted that Campylobacteriosis risk related to poultry meat handling practices in domestic setting by surveying the general public residing in Ireland. Result reveals that a substantial number of consumers still lacks awareness of Campylobacteriosis. gives an overview of the number of studies reported domestic poultry handling practices worldwide. This demonstrated that public awareness on appropriate handling of raw chicken meat products in domestic setting were principally disregarded.
Table 5. Report on domestic poultry handling practices.
Food safety training
Despite, the fact that food safety and nutrition is part of the curriculum of primary and secondary school in the most developed countries, example, in Ireland ‘Social, Personal and Health Education’ (SPHE) programme in the curriculum in all Irish primary schools since 2002. In United States, food safety program in the curriculum as well as additional educational strategies have been employed to improve food safety in elementary and middle school students such as electronic game, Kitchen Ninja to the Rescue .[Citation109] However, the current foodborne illness reports indicate that food handlers’ knowledge remained low. In most cases, the knowledge was not always translated into practice. This leads us to suggest that only risk perception and perceived behavioural control are the only make differences in food safety practices.
Food businesses across the globe are legally required to have a food safety management system based on the principles of Hazard Analysis & Critical Control Point (HACCP) and have trained the food handlers since 1998 .[Citation99] HACCP trainings are mandatory and improved food handler’s knowledge and hygiene practices .[Citation110] The training alone is not elixir, [Citation111] the food handlers’ knowledge, attitude and practices are required to monitored by Environmental Health Officer (EHO) on their routine inspection of the food businesses .[Citation112] Refresher training is also required to provide upon the EHO’s recommendation and or periodically otherwise food safety knowledge of the trained food handlers decline significantly.[Citation113]
Future perspective and conclusion
In conclusion, poultry is the main contributor to human campylobacteriosis and is still one of the most pandemic infectious diseases that is a foreseeable threat to consumers in the years ahead. Countless studies demonstrated that an absolute elimination of Campylobacter in the poultry production chain is not feasible. The Campylobacter tainted poultry remain cross-contaminate from farm to fork. While all the food businesses require legitimately to implement HACCP to mitigate the incidents, it is almost impossible to control particularly in the domestic setting. Which lead to suggest that the integrated effort from all stakeholders in terms of biosecurity at the farm level, effective HACCP at processing along with distribution and ultimately inform the consumer about the risk associated with it. Accordingly, the incident of campylobacteriosis perhaps lowered in human’s population. This review provides well-grounded and updated useful information for food industries, health services and public food safety authorities with regards to stepping up the risk communication effort to consumer and advice appropriate mitigation measures. It is noticeable that in order to lower the Campylobacteriosis incident, the development of the strategy to combat is necessary. Such a strategy will establish the appropriate measure to control the Campylobacter from farm to fork. Further research is needed to identify the most efficient channel in risk communication to the consumer.
Notes on contributions
“Conceptualization, P.M., S.J. and A.J.; writing—original draft preparation, P.M.; writing—review and editing, P.M., S.J. and A.J.; supervision, A.J. All authors have read and agreed to the published version of the manuscript.
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
The authors would like to acknowledge the support and facilities provided by TU Dublin – City Campus, Dublin, Ireland.
Additional information
Funding
References
- World Health Organization (WHO). Campylobacter, World Health Organisation web page, 2020. Available at: https://www.who.int/news-room/fact-sheets/detail/campylobacter. [Accessed: 22 May 2021].
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food‐borne Outbreaks in 2017. EFSA J. 2018, 16(12), e05500. doi:10.2903/j.efsa.2018.5500.
- European Food Safety Authority (EFSA). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food‐borne Outbreaks in 2016. EFSA J. 2017, 15(12), e05077.
- Hwang, H.; Singer, R. S. Survey of the U.S. Broiler Industry regarding Pre- and Postharvest Interventions Targeted to Mitigate Campylobacter Contamination on Broiler Chicken Products. J. Food Prot. 2020, 83(7), 1137–1148. DOI: 10.4315/JFP-19-527.
- Food Safety Authority Ireland (FSAI). Survey to Determine the Prevalence of Campylobacter and Salmonella on Raw Chicken on Retail Sale in Ireland in 2011 (11NS2). 2016, 21.
- Bearth, A.; Cousin, M.-E.; Siegrist, M. Poultry Consumers’ Behaviour, Risk Perception and Knowledge Related to Campylobacteriosis and Domestic Food Safety. Food Control. 2014, 44, 166–176. doi:10.1016/j.foodcont.2014.03.055
- MacRitchie, L. A.; Hunter, C. J.; Strachan, N. J. C. Consumer Acceptability of Interventions to Reduce Campylobacter in the Poultry Food Chain. Food Control. 2014, 35(1), 260–266. DOI: 10.1016/j.foodcont.2013.06.005.
- Howes, M.; McEwen, S.; Griffiths, M.; Harris, L. Food Handler Certification by Home Study: Measuring Changes in Knowledge and Behavior. Dairy, Food Environ. Sanit. A Publ. Int. Assoc. Milk. Food Environ. Sanit. (USA). 1996, 16(11), 737–744.
- Greig, J. D.; Todd, E. C. D.; Bartleson, C. A.; Michaels, B. S. Outbreaks Where Food Workers Have Been Implicated in the Spread of Foodborne Disease. Part 1. Description of the Problem, Methods, and Agents Involved. J. Food Prot. 2007, 70(7), 1752–1761. DOI: 10.4315/0362-028X-70.7.1752.
- European Commission. Overview Report on the Mitigation Measures in Place for Campylobacter Spp. in Poultry; Luxembourg, 2017. Luxembourg10.2875/894648. https://op.europa.eu/en/publication-detail/-/publication/54ce4034-f5b8-11e7-b8f5-01aa75ed71a1/language-en/format-PDF
- Myintzaw, P.; Moran, F.; Jaiswal, A. K. Campylobacteriosis, Consumer’s Risk Perception, and Knowledge Associated with Domestic Poultry Handling in Ireland. J. Food Saf. 2020, 40(4), e12799. DOI: 10.1111/jfs.12799.
- Humphrey, T.; O’Brien, S.; Madsen, M. Campylobacters as Zoonotic Pathogens: A Food Production Perspective. Int. J. Food Microbiol. 2007, 117(3), 237–257. DOI: 10.1016/j.ijfoodmicro.2007.01.006.
- Facciolà, A.; Riso, R.; Avventuroso, E.; Visalli, G.; Delia, S. A.; Laganà, P. Campylobacter: From Microbiology to Prevention. J. Prevent. Med. Hyg. 2017, 58, 2. DOI: 10.15167/2421-4248/jpmh2017.58.2.672.
- Silva, J.; Leite, D.; Fernandes, M.; Mena, C.; Gibbs, P. A.; Teixeira, P. Campylobacter Spp. As A Foodborne Pathogen: A Review. Front. Microbiol. 2011, 2(2), 200. DOI: 10.3389/fmicb.2011.00200.
- De Cesare, A.; Sheldon, B. W.; Smith, K. S.; Jaykus, L. A. Survival and Persistence of Campylobacter and Salmonella Species under Various Organic Loads on Food Contact Surfaces. J. Food Prot. 2003, 66(9), 1587–1594. DOI: 10.4315/0362-028X-66.9.1587.
- García-Sánchez, L.; Melero, B.; Rovira, J. Campylobacter in the Food Chain. In Advances in Food and Nutrition Research; Cambridge, MA: Academic Press Inc., 2018, Vol. 86, pp 215–252. DOI:10.1016/bs.afnr.2018.04.005.
- Sheppard, S. K.; Maiden, M. C. J. The Evolution of Campylobacter Jejuni and Campylobacter Coli. Cold Spring Harb. Perspect. Biol. 2015, 7(8), 1–13. DOI: 10.1101/cshperspect.a018119.
- Park, S. F.;. The Physiology of Campylobacter Species and Its Relevance to Their Role as Foodborne Pathogens. Int. J. Food Microbiol. 2002, 74(3), 177–188. DOI: 10.1016/S0168-1605(01)00678-X.
- European Food Safety Authority (EFSA). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2011 Has Been Published. Eurosurveillance. 2013, 18(15), 20449. DOI:10.2807/ese.18.15.20449-en.
- Bolton, D.; Meredith, H.; Walsh, D.; Mcdowell, D. Poultry Food Safety Control Interventions in the Domestic Kitchen. J. Food Saf. 2014, 34(1), 34–41. DOI: 10.1111/jfs.12092.
- Mehat, J. W.; Park, S. F.; van Vliet, A. H. M.; La Ragione, R. M. CapC, a Novel Autotransporter and Virulence Factor of Campylobacter Jejuni. Appl. Environ. Microbiol. 2018, 84(16), 16. DOI: 10.1128/AEM.01032-18.
- Johnson, D. I.;. Bacterial Virulence Factors. In Bacterial Pathogens and Their Virulence Factors; Cham, Switzerland: Springer International Publishing: 2018; pp 1–38. doi:10.1007/978-3-319-67651-7_1
- Teh, A. H. T.; Lee, S. M.; Dykes, G. A. Does Campylobacter Jejuni Form Biofilms in Food-Related Environments? Appl. Env. Microbiol. 2014, 80(17), 5154–5160. DOI: 10.1128/AEM.01493-14.
- Gupta, A.; Nelson, J. M.; Barrett, T. J.; Tauxe, R. V.; Rossiter, S. P.; Friedman, C. R.; Joyce, K. W.; Smith, K. E.; Jones, T. F.; Hawkins, M. A.;, et al. Antimicrobial Resistance among Campylobacter Strains, United States, 1997-2001. Emerg. Infect. Dis. 2004, 10(6), 1102–1109. DOI: 10.3201/eid1006.030635.
- Thai, J.; Med, V.; Saengthongpinit, C. Prevalence and Antimicrobial Resistance of Salmonella and Campylobacter Species Isolated from Laying Duck Flocks in Confinement and Free-Grazing Systems. Thai J. Vet. Med. 2015, 45(3), 341.
- Silva, W. C.; Targino, B. N.; Gonçalves, A. G.; Silva, M. R.; Hungaro, H. M. Campylobacter: An Important Food Safety Issue. In Food Safety and Preservation; Grumezescu A. M.; Holban A. M. (Eds.). Academic Press, Cambridge, MA, 2018; pp 391–430. doi:10.1016/b978-0-12-814956-0.00013-5
- García-Sánchez, L.; Melero, B.; Diez, A. M.; Jaime, I.; Canepa, A.; Rovira, J. Genotyping, Virulence Genes and Antimicrobial Resistance of Campylobacter Spp.Isolated during Two Seasonal Periods in Spanish Poultry Farms. Prev. Vet. Med. 2020, 176, 104935. DOI: 10.1016/j.prevetmed.2020.104935.
- Skarp, C. P. A.; Hänninen, M. L.; Rautelin, H. I. K. Campylobacteriosis: The Role of Poultry Meat. Clin. Microbiol. Infect. 2016, 22(2), 103–109. DOI: 10.1016/j.cmi.2015.11.019.
- Fullerton, K. E.; Ingram, L. A.; Jones, T. F.; Anderson, B. J.; McCarthy, P. V.; Hurd, S.; Shiferaw, B.; Vugia, D.; Haubert, N.; Hayes, T.;, et al. Sporadic Campylobacter Infection in Infants: A Population-Based Surveillance Case-Control Study. Pediatr. Infect. Dis. J. 2007, 26(1), 19–24. DOI: 10.1097/01.inf.0000247137.43495.34.
- Bull, S. A.; Allen, V. M.; Domingue, G.; Jørgensen, F.; Frost, J. A.; Ure, R.; Whyte, R.; Tinker, D.; Corry, J. E. L.; Gillard-King, J.;, et al. Sources of Campylobacter Spp. Colonizing Housed Broiler Flocks during Rearing. Appl. Environ. Microbiol. 2006, 72(1), 645–652. DOI: 10.1128/AEM.72.1.645-652.2006.
- European Food Safety Authority (EFSA). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19(2). DOI: 10.2903/j.efsa.2021.6406.
- Stella, S.; Soncini, G.; Ziino, G.; Panebianco, A.; Pedonese, F.; Nuvoloni, R.; Di Giannatale, E.; Colavita, G.; Alberghini, L.; Giaccone, V. Prevalence and Quantification of Thermophilic Campylobacter Spp. In Italian Retail Poultry Meat: Analysis of Influencing Factors. Food Microbiol. 2017, 62, 232–238. DOI: 10.1016/j.fm.2016.10.028.
- Guyard-Nicodème, M.; Rivoal, K.; Houard, E.; Rose, V.; Quesne, S.; Mourand, G.; Rouxel, S.; Kempf, I.; Guillier, L.; Gauchard, F.;, et al. Prevalence and Characterization of Campylobacter Jejuni from Chicken Meat Sold in French Retail Outlets. Int. J. Food Microbiol. 2015, 203, 8–14. DOI: 10.1016/j.ijfoodmicro.2015.02.013.
- EFSA. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food‐borne Outbreaks in 2015. EFSA J. 2016, 14(12). DOI: 10.2903/j.efsa.2016.4634.
- Korsak, D.; Maćkiw, E.; Rozynek, E.; Zyłowska, M. Prevalence of Campylobacter Spp. In Retail Chicken, Turkey, Pork, and Beef Meat in Poland between 2009 and 2013. J. Food Prot. 2015, 78(5), 1024–1028. DOI: 10.4315/0362-028X.JFP-14-353.
- Cook, A.; Odumeru, J.; Lee, S.; Pollari, F. Campylobacter, Salmonella, Listeria Monocytogenes,Verotoxigenic Escherichia Coli, and Escherichia Coli Prevalence, Enumeration, and Subtypes on Retail Chicken Breasts with and without Skin. J. Food Prot. 2012, 75(1), 34–40. DOI: 10.4315/0362-028X.JFP-11-206.
- Wei, B.; Cha, S. Y.; Yoon, R. H.; Kang, M.; Roh, J. H.; Seo, H. S.; Lee, J. A.; Jang, H. K. Prevalence and Antimicrobial Resistance of Campylobacter Spp. Isolated from Retail Chicken and Duck Meat in South Korea. Food Control. 2016, 62, 63–68. DOI: 10.1016/j.foodcont.2015.10.013.
- Ma, L.; Wang, Y.; Shen, J.; Zhang, Q.; Wu, C. Tracking Campylobacter Contamination along a Broiler Chicken Production Chain from the Farm Level to Retail in China. Int. J. Food Microbiol. 2014, 181, 77–84. DOI: 10.1016/j.ijfoodmicro.2014.04.023.
- Huang, J.; Zong, Q.; Zhao, F.; Zhu, J.; Jiao, X. Quantitative Surveys of Salmonella and Campylobacter on Retail Raw Chicken in Yangzhou, China. Food Control. 2016, 59, 68–73. DOI: 10.1016/j.foodcont.2015.05.009.
- Furukawa, I.; Ishihara, T.; Teranishi, H.; Saito, S.; Yatsuyanagi, J.; Wada, E.; Kumagai, Y.; Takahashi, S.; Konno, T.; Kashio, H.;, et al. Advance Publication by J-STAGE Japanese Journal of Infectious Diseases Prevalence and Characteristics of Salmonella and Campylobacter in Retail Poultry Meat in Japan. Jpn J. Infect. Dis. 2016. DOI: 10.7883/yoken.JJID.2016.164.
- Mäesaar, M.; Praakle, K.; Meremäe, K.; Kramarenko, T.; Sõgel, J.; Viltrop, A.; Muutra, K.; Kovalenko, K.; Matt, D.; Hörman, A.;, et al. Prevalence and Counts of Campylobacter Spp. In Poultry Meat at Retail Level in Estonia. Food Control 2014, 44, 72–77. DOI: 10.1016/j.foodcont.2014.03.044.
- Health Service Executive(HSE). Campylobacter Infection in Ireland, 2017 Available at: https://www.hpsc.ie/a-z/gastroenteric/campylobacter/publications/annualreportsoncampylobacteriosis/Campy/Annual/Report/2017/FINAL.pdf [Accessed: 22 May 2021].
- Cresci, G. A. M.; Mayor, P. C.; Thompson, S. A. Effect of Butyrate and Lactobacillus GG on a Butyrate Receptor and Transporter during Campylobacter Jejuni Exposure. FEMS Microbiol. Lett. 2017, 364(6), 46. DOI: 10.1093/femsle/fnx046.
- Connor, B. A.;. Post-Infectious Sequelae of Travelers’ Diarrhea: Reactive Arthritis, Guillain-Barré Syndrome, and Irritable Bowel Syndrome. Cur. Tropical Med. Rep. 2016, 3(3), 102–107. DOI: 10.1007/s40475-016-0080-4.
- Rohekar, S.; Tsui, F. W. L.; Tsui, H. W.; Xi, N.; Riarh, R.; Bilotta, R.; Inman, R. D. Symptomatic Acute Reactive Arthritis after an Outbreak of Salmonella. J. Rheumatol. 2008, 35(8), 1599–1602.
- Ehuwa, O.; Jaiswal, A. K.; Jaiswal, S. Salmonella, Food Safety and Food Handling Practices. Foods. 2021, 10(5), 907. DOI: 10.3390/foods10050907.s.
- Pope, J. E.; Krizova, A.; Garg, A. X.; Thiessen-Philbrook, H.; Ouimet, J. M. Campylobacter Reactive Arthritis: A Systematic Review. Semin. Arthritis Rheum. 2007, 37(1), 48–55. DOI: 10.1016/j.semarthrit.2006.12.006.
- Zhang, M.; Li, Q.; He, L.; Meng, F.; Gu, Y.; Zheng, M.; Gong, Y.; Wang, P.; Ruan, F.; Zhou, L.;, et al. Association Study between an Outbreak of Guillain-Barre Syndrome in Jilin, China, and Preceding Campylobacter Jejuni Infection. Foodborne Pathog. Dis. 2010, 7(8), 913–919. DOI: 10.1089/fpd.2009.0493.
- Takahashi, M.; Koga, M.; Yokoyama, K.; Yuki, N. Epidemiology of Campylobacter Jejuni Isolated from Patients with Guillain-Barré and Fisher Syndromes in Japan. J. Clin. Microbiol. 2005, 43(1), 335–339. DOI: 10.1128/JCM.43.1.335-339.2005.
- Islam, Z.; Jacobs, B. C.; Islam, M. B.; Mohammad, Q. D.; Diorditsa, S.; Endtz, H. P. High Incidence of Guillain-Barré Syndrome in Children, Bangladesh. Emerg. Infect. Dis. 2011, 17(7), 1317. DOI: 10.3201/eid1707.101999.
- O’Brien, S. J.;. The Consequences of Campylobacter Infection. Cur. Opin. Gastroenterol. 2017, 33(1), 14–20. DOI: 10.1097/MOG.0000000000000329.
- Jasti, A. K.; Selmi, C.; Sarmiento-Monroy, J. C.; Vega, D. A.; Anaya, J. M.; Gershwin, M. E. Guillain-Barré Syndrome: Causes, Immunopathogenic Mechanisms and Treatment. Expert Rev. Clin Immunol. 2016, 12(11), 1175–1189. DOI: 10.1080/1744666X.2016.1193006.
- Stern, N. J.; Hiett, K. L.; Cox, N. A.; Alfredsson, G. A.; Kristinsson, K. G.; Line, J. E. Recent Developments Pertaining to Campylobacter. Irish J. Agricul. Food Res. 2000, 39, 183–187.
- Rhodes, K. M.; Tattersfield, A. E. Guillain-Barre Syndrome Associated with Campylobacter Infection. Br. Med. J. 1982, 285(6336), 173–174. DOI: 10.1136/bmj.285.6336.173.
- Heikema, A. P.; Islam, Z.; Horst-Kreft, D.; Huizinga, R.; Jacobs, B. C.; Wagenaar, J. A.; Poly, F.; Guerry, P.; van Belkum, A.; Parker, C. T.;, et al. Campylobacter Jejuni Capsular Genotypes are Related to Guillain-Barré Syndrome. Clin. Microbiol. Infect.2015, 21(9), 852.e1–852.e9. DOI: 10.1016/j.cmi.2015.05.031.
- Rees, J. H.; Soudain, S. E.; Gregson, N. A.; Hughes, R. A. C. Campylobacter Jejuni Infection and Guillain-Barré Syndrome. N. Engl. J. Med. 1995, 333(21), 1374–1379. DOI: 10.1056/NEJM199511233332102.
- McCarthy, N.; Giesecke, J. Incidence of Guillain-Barré Syndrome following Infection with Campylobacter Jejuni. Am. J. Epidemiol. 2001, 153(6), 610–614. DOI: 10.1093/aje/153.6.610.
- Mangen, M.-J.-J.; Havelaar, A. H.; Haagsma, J. A.; Kretzschmar, M. E. E. The Burden of Campylobacter-Associated Disease in Six European Countries. Microb. Risk Anal. 2016, 2–3(3), 48–52. DOI: 10.1016/j.mran.2016.04.001.
- Spiller, R.; Garsed, K. Postinfectious Irritable Bowel Syndrome. Gastroenterol. 2009, 136(6), 1979–1988. DOI: 10.1053/j.gastro.2009.02.074.
- Marshall, J. K.; Thabane, M.; Garg, A. X.; Clark, W. F.; Salvadori, M.; Collins, S. M. Incidence and Epidemiology of Irritable Bowel Syndrome after a Large Waterborne Outbreak of Bacterial Dysentery. Gastroenterol. 2006, 131(2), 445–450. DOI: 10.1053/j.gastro.2006.05.053.
- Dunlop, S. P.; Jenkins, D.; Neal, K. R.; Spiller, R. C. Relative Importance of Enterochromaffin Cell Hyperplasia, Anxiety, and Depression in Postinfectious IBS. Gastroenterol. 2003, 125(6), 1651–1659. DOI: 10.1053/j.gastro.2003.09.028.
- Thornley, J. P.; Jenkins, D.; Neal, K.; Wright, T.; Brough, J.; Spiller, R. C. Relationship of Campylobacter Toxigenicity in Vitro to the Development of Postinfectious Irritable Bowel Syndrome. J. Infect. Dis. 2001, 184(5), 606–609. DOI: 10.1086/322845.
- Pimentel, M.; Morales, W.; Pokkunuri, V.; Brikos, C.; Kim, S. M.; Kim, S. E.; Triantafyllou, K.; Weitsman, S.; Marsh, Z.; Marsh, E.;, et al. Autoimmunity Links Vinculin to the Pathophysiology of Chronic Functional Bowel Changes following Campylobacter Jejuni Infection in a Rat Model. Dig. Dis. Sci. 2015, 60(5), 1195–1205. DOI: 10.1007/s10620-014-3435-5.
- Havelaar, A. H.; Kirk, M. D.; Torgerson, P. R.; Gibb, H. J.; Hald, T.; Lake, R. J.; Praet, N.; Bellinger, D. C.; de Silva, N. R.; Gargouri, N.;, et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12(12), e10019231001923. DOI: 10.1371/journal.pmed.1001923.
- Helms, M.; Simonsen, J.; Mølbak, K. Foodborne Bacterial Infection and Hospitalization: A Registry-Based Study. Clin. Infect. Dis. 2006, 42(4), 498–506. DOI: 10.1086/499813.
- Wheeler, J. G.; Sethi, D.; Cowden, J. M.; Wall, P. G.; Rodrigues, L. C.; Tompkins, D. S.; Hudson, M. J.; Roderick, P. J. Study of Infectious Intestinal Disease in England: Rates in the Community, Presenting to General Practice, and Reported to National Surveillance. Bmj. 1999, 318(7190), 1046–1050. DOI: 10.1136/bmj.318.7190.1046.
- Mead, P. S.; Slutsker, L.; Dietz, V.; Mccaig, L. F.; Bresee, J. S.; Shapiro, C.; Griffin, P. M.; Tauxe, R. V. Food-Related Illness and Death in the United States. Emerg. Infect. Dis. 1999, 5(5), 607–625. DOI: 10.3201/eid0505.990502.
- European Centre for Disease Prevention and Control (ECDC). Disease Data from ECDC Surveillance Atlas - Campylobacteriosis. Available at: https://www.ecdc.europa.eu/en/campylobacteriosis/surveillance/atlas.[ Accessed: Jun 8, 2021].
- Tam, C. C.; O’Brien, S. J. Economic Cost of Campylobacter, Norovirus and Rotavirus Disease in the United Kingdom. PLoS One. 2016, 11(2), 2. DOI: 10.1371/journal.pone.0138526.
- Devleesschauwer, B.; Bouwknegt, M.; Mangen, M. J. J.; Havelaar, A. H. Health and Economic Burden of Campylobacter. In Campylobacter: Features, Detection, and Prevention of Foodborne Disease; Klein, G. (Ed.) Academic Press, Cambridge, MA, 2017; pp 27–40. doi:10.1016/B978-0-12-803623-5.00002-2
- Roberts, J. A.; Cumberland, P.; Sockett, P. N.; Wheeler, J.; Rodrigues, L. C.; Sethi, D. The Study of Infectious Intestinal Disease in England: Socio-Economic Impact. Epidemiol. Infect. 2003, 103(1), 1–11. DOI: 10.1017/S0950268802007690.
- Buzby, J. C.; Allos, M.; Roberts, T. The Economic Burden of Campylobacter-Associated Guillain-Barré Syndrome. J. Infect. Dis. 1997, 176(s2), 192–197. DOI: 10.1086/513785.
- Scott, W. G.; Scott, H. M.; Lake, R. J.; Baker, M. G. Economic Cost to New Zealand of Foodborne Infectious Disease. N. Z. Med. J. 2000, 113(1113), 281–284.
- Ruzante, J. M.; Davidson, V. J.; Caswell, J.; Fazil, A.; Cranfield, J. A. L.; Henson, S. J.; Anders, S. M.; Schmidt, C.; Farber, J. M.; Multifactorial Risk, A. Prioritization Framework for Foodborne Pathogens. Risk Anal. 2010, 30(5), 724–742. DOI: 10.1111/j.1539-6924.2009.01278.x.
- Collier, S. A.; St O C Km, L. J.; Hicks, L. A.; Garrison, L. E.; Ho, F. J. Z.; Ea, D. M. J. B. Direct Healthcare Costs of Selected Diseases Primarily or Partially Transmitted by Water. Epidemiol. Infect. 2012, 140(11), 2003–2013. DOI: 10.1017/S0950268811002858.
- Mangen, M. J. J.; Bouwknegt, M.; Friesema, I. H. M.; Haagsma, J. A.; Kortbeek, L. M.; Tariq, L.; Wilson, M.; van Pelt, W.; Havelaar, A. H. Cost-of-Illness and Disease Burden of Food-Related Pathogens in the Netherlands, 2011. Int. J. Food Microbiol. 2015, 196, 84–93. DOI: 10.1016/j.ijfoodmicro.2014.11.022.
- Wagenaar, J. A.; French, N. P.; Havelaar, A. H. Preventing Campylobacter at the Source: Why Is It so Difficult? Clin. Infect. Dis. 2013, 57(11), 1600–1606. DOI: 10.1093/cid/cit555.
- Food and Agriculture Organization/ World Health Organisation (FAO/WHO). Salmonella and Campylobacter in Chicken Meat; Rome. 2009. ISBN978-92-5-106411-5(FAO).
- Blackall, P. J.;. Campylobacter and Poultry. 16th Chulalongkorn Univ. Vet. Conf. 2017, 2017, Bangkok, Thailand.
- Casey, E.; Fitzgerald, E.; Lucey, B. Towards Understanding Clinical Campylobacter Infection and Its Transmission: Time for a Different Approach? Brit. J. Biomed. Sci. 2017, 74(2), 53–64. DOI: 10.1080/09674845.2017.1291205.
- Sohaib, M.; Butt, M. S.; Shabbir, M. A.; Shahid, M. Lipid Stability, Antioxidant Potential and Fatty Acid Composition of Broilers Breast Meat as Influenced by Quercetin in Combination with α-Tocopherol Enriched Diets. Lipids Health Dis. 2015, 14(1), 1–15. DOI: 10.1186/s12944-015-0058-6.
- Daghir, N.; Diab-El-Harake, M.; Kharroubi, S. Poultry Production and Its Effects on Food Security in the Middle Eastern and North African Region. J. Appl. Poult. Res. 2021, 30(1), 100110. DOI: 10.1016/j.japr.2020.10.009.
- MacDonald, E.; White, R.; Mexia, R.; Bruun, T.; Kapperud, G.; Lange, H.; Nygard, K.; Vold, L. Risk Factors for Sporadic Domestically Acquired Campylobacter Infections in Norway 2010-2011: A National Prospective Case-Control Study. PLoS ONE. 2015, 10(10), e0139636. DOI: 10.1371/journal.pone.0139636.
- Kosa, K. M.; Cates, S. C.; Bradley, S.; Chambers, E.; Godwin, S. Consumer-Reported Handling of Raw Poultry Products at Home: Results from a National Survey. J. Food Prot. 2015, 78(1), 180–186. DOI: 10.4315/0362-028X.JFP-14-231.
- Danis, K.; Di Renzi, M.; O’Neill, W.; Smyth, B.; McKeown, P.; Foley, B.; Tohani, V.; Devine, M. Risk Factors for Sporadic Campylobacter Infection: An All-Ireland Case-Control Study. Euro Surveill. 2009, 14(7), 19123. DOI: 10.2807/ese.14.07.19123-en.
- Signorini, M. L.; Zbrun, M. V.; Romero-Scharpen, A.; Olivero, C.; Bongiovanni, F.; Soto, L. P.; Frizzo, L. S.; Rosmini, M. R. Quantitative Risk Assessment of Human Campylobacteriosis by Consumption of Salad Cross-Contaminated with Thermophilic Campylobacter Spp. From Broiler Meat in Argentina. Prev. Vet. Med. 2013, 109(1–2), 37–46. DOI: 10.1016/j.prevetmed.2012.09.011.
- Gaardbo Kuhn, K.; Møller Nielsen, E.; Mølbak, K.; Ethelberg, S. Determinants of Sporadic Campylobacter Infections in Denmark: A Nationwide Case-Control Study among Children and Young Adults. Clin. Epidemiol. 2018, 10, 10–1695. DOI: 10.2147/CLEP.S177141.
- Lahti, E.; Löfdahl, M.; Ågren, J.; Hansson, I.; Olsson Engvall, E. Confirmation of a Campylobacteriosis Outbreak Associated with Chicken Liver Pâté Using PFGE and WGS. Zoonoses Public Health. 2017, 64(1), 14–20. DOI: 10.1111/zph.12272.
- Wensley, A.; Coole, L. Cohort Study of a Dual-Pathogen Point Source Outbreak Associated with the Consumption of Chicken Liver Pâté, UK, October 2009. J. Public Heal. (UK). 2013, 35(4), 585–589. DOI: 10.1093/pubmed/fdt020.
- Calciati, E.; Lafuente, S.; De Simó, M.; Balfagon, P.; Bartolomé, R.; Caylà, J.; Campylobacter, A. Outbreak in a Barcelona School. Enferm. Infecc. Microbiol. Clin. 2012, 30(5), 243–245. DOI: 10.1016/j.eimc.2011.10.004.
- Parry, A.; Fearnley, E.; Denehy, E. ‘Surprise’: Outbreak of Campylobacter Infection Associated with Chicken Liver Pâté at a Surprise Birthday Party, Adelaide, Australia, 2012. West. Pacific Surveill. Response J. 2012, 3(4), 16–19. DOI: 10.5365/wpsar.2012.3.4.011.
- Gormley, F. J.; Rawal, N.; Little, C. L. Choose Your Menu Wisely: Cuisine-Associated Food-Poisoning Risks in Restaurants in England and Wales. Epidemiol. Infect. 2012, 140(6), 997–1007. DOI: 10.1017/S0950268811001567.
- Norkrans, G.; Svedhem, A. Epidemiological Aspects of Campylobacter Jejuni Enteritis. Epidemiol. Infect. 1982, 89(1), 163–170.
- Hopkins, R. S.; Scott, A. S. Handling Raw Chicken as a Source for Sporadic Campylobacter Jejuni Infections. J. Infect. Dis. 1983, 148(4), 770. DOI: 10.1093/infdis/148.4.770.
- Kapperud, G.; Skjerve, E.; Vik, L.; Hauge, K.; Lysaker, A.; Aalmen, I.; Ostroff, S. M.; Potter, M. Epidemiological Investigation of Risk Factors for Campylobacter Colonization in Norwegian Broiler Flocks. Epidemiol. Infect. 1993, 111(2), 245–256. DOI: 10.1017/S0950268800056958.
- Harris, N. V.; Weiss, N. S.; Nolan, C. M. The Role of Poultry and Meats in the Etiology of Campylobacter Jejuni/Coli Enteritis. Am. J. Public Health. 1986, 76(4), 407–411. DOI: 10.2105/AJPH.76.4.407.
- Deming, M. S.; Tauxe, R. V.; Blake, P. A.; Dixon, S. E.; Fowler, B. S.; Jones, T. S.; Lockamy, E. A.; Patton, C. M.; Sikes, R. O. Campylobacter Enteritis at a University: Transmission from Eatingchicken and from Cats. Am. J. Epidemiol. 1987, 126(3), 526–534. DOI: 10.1093/oxfordjournals.aje.a114685.
- Glashower, D.; Snyder, J.; Welch, D.; McCarthy, S. Notes from the Field: Outbreak of Campylobacter Jejuni Associated with Consuming Undercooked Chicken Liver Mousse — Clark County, Washington, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2017, 66(38), 1027. DOI: 10.15585/mmwr.mm6638a4.
- Jones, A. K.; Rigby, D.; Burton, M.; Millman, C.; Williams, N. J.; Jones, T. R.; Wigley, P.; O’Brien, S. J.; Cross, P. Restaurant Cooking Trends and Increased Risk for Campylobacter Infection. Emerg. Infect. Dis. 2016, 22(7), 1208–1215. DOI: 10.3201/eid2207.151775.
- Health Protection Surveillance Centre (HPSC). Campylobacter infection in Ireland, 2017. Annual Epidemiological Report2017. Available at: https://www.hpsc.ie/a-z/gastroenteric/campylobacter/publications/annualreportsoncampylobacteriosis/Campy%20Annual%20Report%202017%20FINAL.pdf [Accessed: 22 May 2021]
- European Food Safety Authority (EFSA). The Community Summary Report on Food‐Borne Outbreaks in the European Union in 2007. EFSA J. 2009, 7(5), 271r. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2009.271r?src=getftr
- Mazengia, E.; Fisk, C.; Liao, G.; Huang, H.; Meschke, J. Direct Observational Study of the Risk of Cross-Contamination during Raw Poultry Handling: Practices in Private Home. Food Prot. Trends. 2015, 35, 8–23.
- Sampers, I.; Berkvens, D.; Jacxsens, L.; Ciocci, M. C.; Dumoulin, A.; Uyttendaele, M. Survey of Belgian Consumption Patterns and Consumer Behaviour of Poultry Meat to Provide Insight in Risk Factors for Campylobacteriosis. Food Control. 2012, 26(2), 293–299. DOI: 10.1016/j.foodcont.2012.01.054.
- Kennedy, J.; Nolan, A.; Gibney, S.; O’brien, S.; Mcmahon, M. A. S.; Mckenzie, K.; Healy, B.; Mcdowell, D.; Fanning, S.; Wall, P. G. Deteminants of Cross-Contamination during Home Food Preparation. Br. Food J. 2011, 113(2), 280–297. DOI: 10.1108/00070701111105349.
- Gilbert, S. E.; Whyte, R.; Bayne, G.; Paulin, S. M.; Lake, R. J.; van der Logt, P. Survey of Domestic Food Handling Practices in New Zealand. Int. J. Food Microbiol. 2007, 117(3), 306–311. DOI: 10.1016/j.ijfoodmicro.2007.05.004.
- Van Asselt, E.; Fischer, A.; De Jong, A. E. I.; Nauta, M. J.; De Jonge, R. Cooking Practices in the Kitchen - Observed versus Predicted Behavior. Risk Anal. 2009, 29(4), 533–540. DOI: 10.1111/j.1539-6924.2008.01189.x.
- Hoelzl, C.; Mayerhofer, U.; Steininger, M.; Brüller, W.; Hofstädter, D.; Aldrian, U. Observational Trial of Safe Food Handling Behavior during Food Preparation Using the Example of Campylobacter Spp. J. Food Prot. 2013, 76(3), 482–489. DOI: 10.4315/0362-028X.JFP-12-231.
- Kosa, K. M.; Cates, S. C.; Bradley, S.; Chambers Iv, E.; Godwin, S. Consumer-Reported Handling of Raw Poultry Products at Home: Results from a National Survey. J. Food Prot. 2015; 78(1): 180–186. DOI: 10.4315/0362-028X.JFP-14-231.
- Barrett, T.; Feng, Y. Evaluation of Food Safety Curriculum Effectiveness: A Longitudinal Study of High-School-Aged Youths’ Knowledge Retention, Risk-Perception, and Perceived Behavioral Control. Food Control. 2021, 121, 107587. DOI: 10.1016/j.foodcont.2020.107587.
- Malavi, D. N.; Abong, G. O.; Muzhingi, T. Effect of Food Safety Training on Behavior Change of Food Handlers: A Case of Orange-Fleshed Sweetpotato Purée Processing in Kenya. Food Control. 2021, 119, 107500. DOI: 10.1016/j.foodcont.2020.107500.
- Harris, K.; Taylor, S.; DiPietro, R. B. Antecedents and Outcomes of Restaurant Employees’ Food Safety Intervention Behaviors. Int. J. Hosp. Manag. 2021, 94, 102858. DOI: 10.1016/j.ijhm.2021.102858.
- Lynch, K.; What Food Safety Certification Really Means - Safe Food & Water. Michigan State University Extension. 2018, Available at: https://www.canr.msu.edu/news/what-food-safety-certification-really-means [Accessed: 22 May 2021].
- Nik Husain, N. R.; Wan Muda, W. M.; Noor Jamil, N. I.; Nik Hanafi, N. N.; Abdul Rahman, R. Effect of Food Safety Training on Food Handlers’ Knowledge and Practices: A Randomized Controlled Trial. Br. Food J. 2016, 118(4), 795–808. DOI: 10.1108/BFJ-08-2015-0294.