ABSTRACT
In this review, several chemical quality markers of Atlantic salmon, a widely farmed and consumed fish, are identified and discussed. Lipids, especially omega-3 fatty acids are of special importance as they relate to nutritional benefits. Pigmentation, resulting from the carotenoid deposition and retention in the muscles is one of the main factors that drive the market value of the fish. Both these quality markers are heavily influenced by the diet, and a variety of other important factors. Perhaps less obviously, contaminants and pollutants play a role in determining the overall quality of Atlantic salmon. Monitoring these quality parameters is performed via various analysis methods, many of which are considered industry standard due to many decades of producing reliable and accurate results. However, recent advances in spectroscopic techniques present faster, non-invasive techniques that have considerable potential in fisheries science. The following text outlines influences on the chemical quality markers in Atlantic salmon and compares various analysis techniques that can be used for quantification and monitoring.
Introduction
Atlantic salmon belong to the Salmonidae family, which also includes trout and char. The name “salmon” is applied to two distinct genera: Salmo (Atlantic) and Oncorhyncus (Pacific). Pacific salmon includes a number of species, with the major species including Pink (Oncorhynchus gorbuscha), Coho (Oncorhynchus kisutch), Sockeye (Oncorhynchus nerka), Chinook (Oncorhynchus tshawyscha) and Chum (Oncorhynchus keta). In contrast, Atlantic salmon has only one major species, Atlantic salmon (Salmo salar).[Citation1] Farmed Atlantic salmon are typically harvested from 2.0 kg in weight. They are silver in colour with darker scaling in the dorsal region that becomes lighter towards to the ventral or belly region. Like all salmonids, the flesh is orange in colour because of carotenoid pigments deposited in the flesh. Atlantic salmon hatch from eggs and begin their lives in fresh water. After their juvenile period which may last between 8 to 16 months depending on the farming conditions and is longer again in the wild, migrate or are transferred to salt water. Salmon farms will usually grow the salmon for up to 2 years in saltwater pens before harvesting. In the wild, Atlantic salmon may spend up to 4 years in salt water before returning to fresh water to spawn.[Citation2]
In the wild, Atlantic salmon are found on both sides of the northern Atlantic Ocean. Large-scale Atlantic salmon aquaculture began in Norway in the 1960s, then gradually spread to the UK and north-eastern coast of North America. Atlantic salmon aquaculture in the southern hemisphere began much later in the 1980s with Chile importing salmon eggs from Norway and Scotland in 1980. Australia set up its first salmon farms in Tasmania in 1984 with eggs imported from Canada.[Citation2]
Wild Atlantic salmon are of important cultural and economic value in regions where they are present, and have been for several centuries.[Citation3] More recently, farmed Atlantic salmon has been of enormous economic value to both producers and the associated supply chain businesses.[Citation4,Citation5] It is widely acknowledged that consumption of Atlantic salmon containing high amounts of omega-3 fatty acids helps to reduce the risk of cardiovascular disease and several other chronic illnesses[Citation6,Citation7] and helps to maintain cognitive function,[Citation8] and is widely available in food markets and restaurants across the world.
As with any market fish, it is important to track and maintain the overall quality of the product. In this review, two major quality markers, pigmentation, and lipid content, are discussed in detail. These two markers represent what consumers regard as the most significant attributes of the fish and have the greatest impact on the eating qualities of the fish. Other minor quality makers, such as freshness and pollutants, are also discussed. While these markers are perhaps less obvious, they are still important factors that need to be considered to maintain the overall quality of this highly valued fish.
Commercial significance of Atlantic salmon
In 2020, wild-caught and farmed seafood (including fish, cephalopods, crustaceans, and mollusks) were estimated to be responsible for 17% of the global animal protein consumed. The total amount of fish produced globally in 2020 was a record amount of 178 million tons, with nearly half attributed to aquaculture.[Citation9] Since 2011, total wild-caught production has fluctuated little, averaging approximately 91 million tons per year, while total aquaculture production has increased from 61.8 million tons in 2011 to 80.0 million tons in 2016, equating to approximately 231.6 billion USD worth of product sold. Aquaculture is projected to continue to increase and is growing faster than any other major food production sector, while capture fishing is expected to gradually decline as sustainability issues become increasingly significant.[Citation10]
Atlantic salmon makes up only 4.4% of the total fish produced via aquaculture, however it accounts for 32.6% of all fin fish in marine and coastal aquaculture, and ranks as the 7th most farmed fish overall.[Citation9] The data of the most produced farmed fish are shown in . Due to temperature constraints, Atlantic salmon aquaculture is limited to the coastal regions of cooler countries, such as Norway, Chile, Faroe Islands, UK, Canada, and Australia. Production in Norway and Chile represents nearly 80% of global Atlantic Salmon production.[Citation12] Norway is the largest producer of Atlantic salmon, and in 2020 Norwegian fisheries produced nearly 1.4 million metric tons of Atlantic salmon with an estimated sale value of $9.94 billion AUD ().[Citation13] Salmon production increased substantially after 1992 due to a combination of government restrictions easing, larger pen and farm sizes, breeding, and health programs, as well as feed innovations that decreased production time by half. Production is largely consolidated in a small number of suppliers; it is estimated that 20 companies produce 80% of the farmed salmon.[Citation12]
Figure 1. 2020 production data of the ten most farmed fish globally.[Citation11]
![Figure 1. 2020 production data of the ten most farmed fish globally.[Citation11]](/cms/asset/1a139262-c30d-499b-a458-7f2c1fea416b/lfri_a_2221332_f0001_oc.jpg)
Table 1. Farmed Atlantic salmon production by nation. Values in per 1000 tons live weight.[Citation11]
Production in Australia
Australia’s aquaculture industry produced $1.3 billion (AUD) worth of fish and seafood in 2017–2018, with all states and territories, with the exception of ACT, having aquaculture businesses in operation. Aquaculture production is increasing in all states, even in states with very high wild caught fish production, such as Western Australia. Although the total wild-caught fishing production decreased by 0.4% in 2017, overall, however, wild-caught fishery production is greater than aquaculture production with $1.7 billion worth of fish and seafood.[Citation14] More recently, the gross value of production produced by aquaculture has increased by 10% in 2019–2020 as wild capture decreased by 12%, largely due to the effect of COVID-19 on the wild capture industry.[Citation15]
From the year 2000 to 2017, production of farmed salmonids in Australia increased by 351% to a total of $756 million worth of fish produced, overtaking other popular fish species such as tuna and represents more than half the value of the Australian aquaculture industry. This increase in production is largely due to the expansion of Tasmanian aquaculture over this time period and there continues to be growth in the region.[Citation16] While salmonid production dominates Australian aquaculture, other species produced in Australian include southern bluefin tuna, prawns, oysters both edible and pearl and numerous other species including barramundi (Asian sea bass).[Citation14]
Wild caught versus farmed
Wild caught Atlantic salmon refers to fish that were caught in natural marine environments, whereas farmed Atlantic salmon, as the name implies, are raised and harvested in controlled environment, and due difference in origin, farmed and wild caught salmon differ in several different parameters. Farmed salmon tend to have higher amounts of carotenoid and colour, 86% higher fat content and significantly firmer flesh than their wild counterparts. Growth under farming conditions yields salmon that are also much larger than fish caught in the wild.[Citation17] There are also considerable differences in the contaminants and pollutants found in farmed and wild caught fish. These are discussed in detail in section 5 (Contaminants and pollutants).
Compositional differences
Farmed Atlantic salmon have considerably higher levels of pigments than wild caught salmon. In one study, farmed Atlantic salmon had a total carotenoid pigmentation concentration of 8.43 ± 0.10 mg/kg compared with 6.44 ± 0.27 mg/kg in wild caught Atlantic salmon.[Citation17] Farmed salmon have a higher level of idoxanthin, a yellow pigment that is a metabolite of astaxanthin, in their flesh in comparison to wild salmon. Idoxanthin is found in elevated levels in juvenile farmed salmon that are raised indoors, in a ratio of 90:10 (astaxanthin: idoxanthin). It is thought that increased idoxanthin deposition in the flesh is due to long-day photoperiod that occurs due to their being raised indoors, as once the farmed salmon reach 2 years of age and are transferred to net pens, idoxanthin levels are almost totally depleted.[Citation18]
Although wild caught salmon have substantially less fat, they have dramatically higher proportions of long chain omega-3 fatty acids, DHA and EPA, than farmed Atlantic salmon, and vary as much as 8.9% in farmed Atlantic salmon comparted with 24.1% in wild Atlantic salmon. This difference in fatty acid composition is due to the inclusion of a large amount of lipids of plant origin in salmon feeds over the recent years to increase environmental sustainability of farmed Atlantic salmon.[Citation19,Citation20] Fat content and fatty acid profiles will be discussed in more detail in section 2.
The level of some micronutrients in wild caught Atlantic salmon can differ significantly compared to both farmed Atlantic salmon and other wild Atlantic salmon caught in different geographic regions. Vitamin D3 (cholecalciferol) has been reported to be higher in wild caught Atlantic salmon, though salmon caught in different geographical regions can have significantly different levels, such as wild salmon caught in the Baltic Sea and the North Sea having 18.5 ± 4.6 µg/100 g and 9.4 ± 1.9 µg/100 g of cholecalciferol, respectively. By comparison, the level of cholecalciferol in farmed salmon ranges from 2.3 to 7.3 µg/100 g,[Citation21] whereas, vitamin E has been found to be of similar amounts in both farmed and wild caught fish.[Citation17]
Market perceptions
From the perspective of the consumer, the source of salmon is a somewhat less important attribute. The most important aspects of Atlantic salmon in order of importance to the consumer are whether it is fresh or frozen, flesh colour and price.[Citation22] Historically there was some preference for farmed salmon over wild caught as it was considered to be of higher quality, both in terms of food safety and sensory quality.[Citation22] However, more recently, consumer preference has started to turn away from an inclination towards farmed fish, citing concerns for the environmental sustainability and welfare of fish under farming conditions, and as such, some consumers prefer purchasing wild caught salmon.[Citation23] For consumers that prefer wild caught Atlantic salmon, farmed Atlantic salmon marked as sustainably grown, or organically grown, were considered to be of similar or equal quality.[Citation24]
Lipid profile and concentration
Fats are an important quality of salmon, as they contribute to the texture and overall flavor of the flesh. Apart from the orange pigmentation of Atlantic salmon flesh, the white striations in the salmon flesh are perhaps the next most distinctive feature of the fish. These striations are connective tissue called myosepta. Analysis of the different muscle and tissue types shows that the majority of the muscle lipids are stored within the myosepta.[Citation25] In terms of overall lipid distribution, the belly has the highest levels, while the tail has the lowest level.[Citation26]
From a nutritional perspective, Atlantic salmon are classed as an “oily fish” as they have relatively high amounts of omega-3 fatty acids, which are of particular importance to cognitive and cardiovascular health and contribute much of the nutritional benefits of consuming Atlantic salmon.[Citation27–29] Balancing the two broad types of polyunsaturated fats, omega-3 and 6, is important from a nutritional perspective, as a diet lower in omega-6 and higher in omega-3 is associated with better health outcomes and avoidance of chronic illnesses.[Citation6] Atlantic salmon, both farmed and wild-caught, is known to contain a higher amount of omega-3 fatty acids, in particular eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), compared to other fish.[Citation7,Citation30]
The overall amount of body fat deposition is reliant on the fat concentration in the feed. Fatty acid composition of the feed also heavily influences the resulting composition, much more so than any metabolic effects.[Citation31] Conversely, Atlantic salmon will have a net production of DHA if it is in low amounts in their diet.[Citation32] However, in general, Atlantic salmon take on the fatty acid profile of their feed.[Citation33,Citation34]
Fatty acid composition
Fat content in salmon is considered to be one of the more important characteristics that determines the eating quality of the fish, whether smoked or cooked.[Citation35] Nearly half of all the fatty acids are monosaturated, with saturated and polyunsaturated types contributing similar amounts each. Very generally, the main fatty acids found in farmed salmon are palmitic acid, oleic acid, gadoleic acid, erucic acid and docosahexaenoic acid.[Citation36,Citation37] The typical concentrations of fatty acids in wild caught, farmed, and ocean ranched Atlantic salmon are given in .
Table 2. Fatty acid composition as percentage of total fatty acid in Atlantic salmon from different farming sources.[Citation36,Citation37].
Typically, farmed Atlantic salmon possess higher amounts of oleic acids as commercial feed often contains significant amounts of terrestrial lipids to reduce the demand for lipid from marine sources, which are available in increasingly limited quantities. The comparative difference in the oleic acid concentration between farmed and wild caught fish is substantial enough that oleic acid concentration can be used to determine if a wild caught Atlantic salmon had originally escaped from a farm.[Citation38] The higher amounts of oleic acid in farmed Atlantic salmon also contribute to a higher omega-6 to omega-3 ratio in farmed versus wild caught Atlantic salmon[Citation19]
Long-chain omega-3 fatty acids and dietary effect on fatty acid composition
Long-chain omega-3 fatty acids are some of the most important fatty acids present in Atlantic salmon. Due to differences in diet, lipid composition differs substantially between wild caught and farmed salmon. Differences due to feed composition are most pronounced for long-chain omega-3 fatty acids (EPA and DHA).
To produce these fatty acids, Atlantic salmon require fish oil to be included in their diet. As the inclusion of fish oil in fish feed presents an environmental concern, some feeding regimes have responded to this by including more vegetable oils in the feed. Atlantic salmon that are fed high vegetable diets result in much lower levels of long-chain omega-3 fatty acids.[Citation39] However, it has also been reported that Atlantic salmon can produce more DHA than what it is usually found from their diet under certain conditions. So long as there remains some fish meal and oil, approximately 5% of both in the feed, fish will produce reasonably high amounts of omega 3 in their muscles.[Citation32,Citation40,Citation41]
Like most muscle foods, the dietary fatty acids have a significant effect on the composition of the final product. As Atlantic salmon are carnivorous, fish meal and oils are a necessary part of their diet. Though the total amount of fat in Atlantic salmon does not change based on the source of dietary fatty acids, fatty acid composition is known to differ, with the fish typically taking on a similar fatty acid profile to that found in their diet. Atlantic salmon, which are fed vegetable oil in place of fish oil show a lower omega-3 to omega-6 ratio, 0.9 compared to a ratio of 6.6, to salmon fed only fish oil, due to a decreased amount of omega-3 fatty acids found in the muscle. A summary of feeding trials with respect to the effects of long chain omega-3 fatty acids is described in .
Table 3. Summary of feeding trials and their effect on polyunsaturated fatty acids.
It should be noted that fish fed vegetable oils have significantly higher amounts of α-Linoleic acid (ALA) than those fed fish oils.[Citation49] While ALA is an omega-3 fatty acid, it is not associated with the same health benefits of long chain omega-3 fatty acids (i.e., EPA and DHA). Several salmon feed studies have shown that different oil types have different effects on fatty acid composition and distribution in salmon muscle. Canola oil and palm oil have both been studied in replacement trials, also achieving similar levels of omega-3 reduction.[Citation42–44] However, the use of finishing diets that contain 100% fish oils can be used to drastically increase omega-3 levels in Atlantic salmon that have been fed diets containing vegetable oils.[Citation44]
Carotenoids and pigmentation
Salmonids are known for the distinctive orange pigmentation of their flesh. As pigmentation intensity and uniformity are important quality indicators for consumers, the aquaculture industry pays a substantial amount of attention to ensuring salmon production processes maximize pigmentation in addition to further researching factors that hinder or enhance pigmentation.
Salmon do not produce the carotenoids responsible for pigmentation as a normal part of their biochemistry and must have it included in their diet. Muscle pigmentation is influenced by a number of factors, including sexual maturity, weight, and genetics.[Citation50,Citation51] In addition, carotenoids must be fed to Atlantic salmon consistently in order to achieve the highest amount of deposition and colouration.[Citation52] The relationship between carotenoid deposition and colouration with the concentration of the carotenoid in the feed is approximately linear; higher concentrations of carotenoids in the diet, generally results in higher levels of pigmentation.[Citation53] However, diets with higher than 60 to 70 mg/kg of astaxanthin concentration show no significant improvement to flesh pigmentation.[Citation50,Citation53]
Astaxanthin is the main carotenoid associated with pigmentation in farmed Atlantic salmon, while historically a combination of canthaxanthin and astaxanthin was commonly used in feeds to achieve the desired pigmentation of the flesh. Both carotenoids will be found in varying levels in wild caught salmon depending on the food sources in the local environment. Carotenoid consumption in wild salmon typically originates from crustaceans, such as krill and shrimp, which contain the pigmentating carotenoids in their carapace,[Citation54] whereas the diet of farmed salmon includes chemically synthesized astaxanthin.[Citation55]
After ingestion, astaxanthin slowly makes its way into the bloodstream as it is integrated into a lipoprotein complex known as a chylomicron. Being a non-polar molecule, the astaxanthin molecules are most likely adhering to the triacyl-glycerols components on the surface of the chylomicron.[Citation56] After a series of enzymatic reactions, the chylomicron is absorbed into the liver where some of the astaxanthin is metabolized into other compounds, such as the carotenoid idoxanthin. The astaxanthin that is not metabolized is reincorporated into very low-density lipoproteins. These lipoproteins are then broken down by lipoprotein lipases, which are thought to mediate the transfer of astaxanthin to albumin circulating in the bloodstream. Albumin then brings the pigment to the muscle cells where it adheres to the surface. Once astaxanthin enters the muscle cell, it forms a complex with actomyosin, the protein complex that forms the basis of skeletal muscles, via hydrogen bonding.[Citation57] This binding is thought to be stronger with astaxanthin than with other carotenoids due to the presence of hydroxyl and keto groups on the β-end of the molecule. Astaxanthin levels in muscles are stable while the salmon is sexually immature and under normal conditions. However, transport of astaxanthin out of the muscle occurs when the salmon reaches sexual maturity to aid in the production of gametes.[Citation56]
Carotenoid chemistry
Carotenoids are a group of naturally occurring pigment compounds that are found in bacteria, algae, fungi, and plants. They exhibit yellow, orange, red and purple colours and are the most widely distributed pigments in nature. Carotenoids compounds fall into one of two categories that are defined by their chemical structure. Carotenes, which include well-known compounds such as lycopene, α-carotene and β-carotene are hydrocarbons. Xanthophylls, such as the compounds lutein, zeaxanthin and fucoxanthin, contain hydroxyl, carbonyl, and various other functional groups as part of their chemical structure. They are approximately 50 types of carotene compounds, while about 800 ×anthophyll compounds have been reported.[Citation58]
Astaxanthin
Astaxanthin is a type of carotenoid belonging to the xanthophyll family, and is the primary pigment used in salmon production as well as for other farmed fish and seafoods. Like other carotenoids, the structure is characterized by a chain of conjugated double carbon bonds, which gives these types of molecules strong light-absorption. As with other carotenoids in the xanthophyll family, astaxanthin molecules have a hydroxyl and other oxygen containing chemical groups and a β-ionone ring structure at either end of the chain.[Citation59] shows the chemical structure of some common carotenoids. shows the chemical structure of some common carotenoids.
Figure 2. Chemical structures of common carotenoids[Citation60].
![Figure 2. Chemical structures of common carotenoids[Citation60].](/cms/asset/0b04e1de-5fa7-4f3c-b883-16c98ca9c455/lfri_a_2221332_f0002_b.gif)
Astaxanthin isomers
Synthetic astaxanthin is most commonly used in Atlantic salmon feed as it is far more cheaply produced than by extracting the compound from natural sources. Synthetic production produces three astaxanthin isomers that differ by the orientation of the hydroxyl group found on each end of the molecule. Each of astaxanthin isomers contributes in varying amounts to pigmentation due to differences in digestibility.[Citation61]
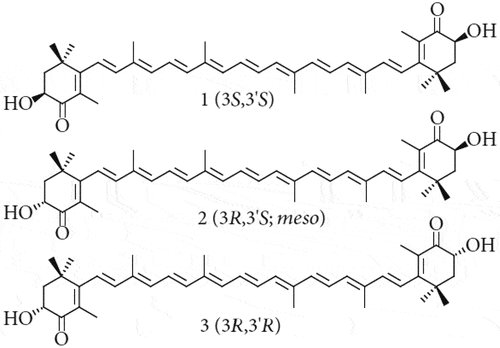
Isomers are molecules that differ in the co-ordinational structural arrangement of atoms but have molecular formulas that are identical. Isomeric compounds can have different physical and chemical properties from one another, despite being similar in structure. Astaxanthin has several isomers, and many which are naturally occurring. Optical isomers are isomers that are structural mirror images, of which astaxanthin has three. These isomers differ by the three-dimensional orientation of the hydroxyl groups at both ends of the molecule. The 3S,3’S form is the most predominant in wild Atlantic salmon, though each of these isomers exists in various ratios. For the synthetic astaxanthin used in farmed Atlantic salmon feed, a racemic mixture of all three optical isomers are present.[Citation62] These optical isomers are shown in .
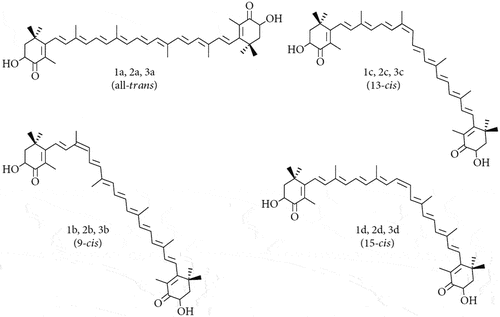
Geometrical isomers, also known as cis-trans isomers, differ by the plane on which functional groups are orientated with respect to each other. Cis isomers have the functional groups on the same plane, while trans isomers have the functional groups on opposing sides. Astaxanthin has four geometrical isomers, each of which differs with respect to the orientation of certain carbon double bonds along the carbon chain that connect each end of the molecule.[Citation62] The four geometric isomers are displayed in .
The apparent digestibility coefficients of astaxanthin geometric isomers show similar levels of digestibility, though for the 9-cis isomer this is lower.[Citation61,Citation63]
Carotenoids accumulation and depletion
Carotenoids in feed
Numerous feed trials have been conducted to better optimize pigmentation in farmed Atlantic salmon, with many factors being identified to contribute towards the digestion, deposition, and retention of carotenoids. A summary of these trials and how they affected colour and flesh carotenoid concentrations are shown in .
Table 4. Summary of feeding trials and their effect on Atlantic salmon pigmentation.
The carotenoid content in salmon diet appears to have a linear relationship with carotenoid content in the muscle, in that increased dietary carotenoid results in increased pigmentation in the flesh. Even though both astaxanthin and canthaxanthin contribute to the pigmentation, it was originally thought that astaxanthin is deposited in the flesh more readily than canthaxanthin, likely due to its increased digestibility.[Citation75] However, there have been studies that show that canthaxanthin contributes equally to pigmentation[Citation65] or even in some cases contributes more pigmentation than astaxanthin.[Citation64] The higher deposition rate is thought to be attributed at least in part to canthaxanthin being much more digestible and therefore much more readily transferred into the bloodstream than astaxanthin.[Citation76]
Although canthaxanthin seems to be at least deposited as easily in salmon flesh, its potential use has some drawbacks relating to its stability. Raw fillets from canthaxanthin-fed salmon appear to present significantly lower amounts of carotenoid after 6 weeks in frozen storage when compared to raw fillets from astaxanthin fed salmon. The level of carotenoid depletion was also detectable by colour measurement. A similar observation was made with smoked fillets from salmon fed canthaxanthin. Canthaxanthin may have a use in salmon feed, however for it to be effective in maintaining colour to meet consumer expectations, its use would need to be in combination with astaxanthin.[Citation77] An additional benefit of using astaxanthin over canthaxanthin in the diet is that astaxanthin appears to have other positive health effects on the fish in addition to pigmentation. When included in diets, astaxanthin helps with growth performance, survival, reproduction, stress tolerance and disease resistance. These positive effects are primarily due to its function as an antioxidant.[Citation55,Citation78]
The source of the astaxanthin can affect the pigmentation. Atlantic salmon that are fed diets that include red yeast cells (Phaffia rhodozyma) as the astaxanthin source tends to have much higher pigmentation levels then of salmon on diets of feed containing synthetic astaxanthin, due to a higher amount of the pigment being digested and its retention in muscles. It is not clear why astaxanthin within yeast cells are more easily digestible, however the higher retention is because salmon fed synthetic carotenoids pigments had a much higher level of idoxanthin produced, thus depleting astaxanthin stored in muscles. However, to achieve the same level of astaxanthin in a feed formulation, red yeast cells must be used in a much higher percentage of the total formulation as compared to synthetic astaxanthin.[Citation69] A similar study using algal astaxanthin found that it was significantly more digestible source of pigmentation than synthetic astaxanthin.[Citation79] Conversely, astaxanthin from Calanus oil, a type of oil derived from plankton found in the North Sea, did not digest and deposit in fish muscles at the same rate as synthetic astaxanthin. It is suggested that the reduced deposition may be due to esterification of astaxanthin molecules found in the Calanus oil limiting absorption into the blood.[Citation70]
Reduction in feed ration influences astaxanthin digestion, and potentially deposition. Salmon fed to 40% of apparent satiation have higher digestibility of astaxanthin than salmon fed to 100% satiation. Although fish fed to 100% satiation overall digested a higher amount of astaxanthin over all, the ratio between thermal growth coefficient and digested astaxanthin negatively correlated with feed intake, which may cause reduction in muscle retention during rapid growth periods.[Citation61] The lipid content of the feed has a strong influence on pigmentation, with higher energy feeds yielding fish with significantly higher levels of pigmentation.[Citation67,Citation80]
Seasonal effects on pigmentation and carotenoid depletion
Deposition and retention of astaxanthin does appear to be affected by seasonal changes such as temperature and lighting conditions. Under continuous light conditions, salmon retain less astaxanthin than with salmon grown under simulated natural light during the initial stages of fish development. After 4 months of growth, the carotenoid levels in fish grown under continuous light increase beyond the levels found simulated natural light grown fish. The initial difference between the two levels is suspected to be due to the higher growth rate of fish grown under continuous light, which affects carotenoid deposition.[Citation72,Citation81]
Water temperature affects the uptake of astaxanthin. Atlantic salmon will have their digestibility of astaxanthin reduced by 10% while at 8°C, as opposed to 12°C. This suggests that salmon should be maintained at the warmer end of their preferred temperature scale to best optimize pigment deposition.[Citation82] Pigment depletion in salmon can occur prior to slaughter resulting in suboptimum fillets. There have been a number of explanations put forth as to how this occurs. Oxidative stress is thought to be one cause of depletion, although there are some observations that show that oxidative stress may not be the cause of depletion, or at least is not the only factor involved.[Citation83,Citation84] Starvation of salmon has been shown to increase color depletion. This effect is exacerbated by increased temperature, which is common during summer months, and during the starvation period, which is routinely performed prior to harvesting. The biochemistry of how this occurs is not yet understood.[Citation85]
In a study that evaluated the effect of using increased levels of α-tocopherol in the feed and the use of both astaxanthin and canthaxanthin showed that pigment depletion still occurred when fish were put under oxidative stress by increased temperature. The pigment depletion was also restricted to certain areas of the fish, primarily the front dorsal region and a middle region known as the Norwegian Quality Cut.[Citation83] A similar study looked at the effect of Atlantic salmon growth at elevated temperatures versus control temperatures with varying dietary fatty acid composition, this variation being high versus low fish oil levels. The NQC regions of Atlantic salmon grown at elevated temperatures showed higher levels of astaxanthin than control temperature salmon, however the anterior dorsal region showed a lower amount of astaxanthin and thus carotenoid distribution was heterogeneous in fish at elevated temperature. Differences in the amount of dietary fish oil had no effect on pigmentation levels, nor were chemical indicators of oxidative stress or carotenoid metabolism detected, thus suggesting the heterogeneous distribution could be the result of differences in the myofibrillar proteins’ ability to bind to astaxanthin at higher temperatures.[Citation86]
More recent research into this area has found that pigmentation and fatty acid content is different flesh regions are linked, as it appears fatty acid content, particularly LC-n3 and other PUFA, are also affected at higher water temperatures. This suggests a metabolic response to the increase water temperature.[Citation87]Citation88
Chemical indicators of freshness
The shelf-life of food is determined by a number of parameters, including changes in microbial profile, chemical composition, and sensory attributes. While microbial spoilage is determined by classical microbiological techniques, chemical changes can also affect spoilage or sensory acceptability and can indicate time since slaughter. As Atlantic salmon presents additional risks compared to other fish as it is very commonly consumed raw (e.g., sashimi or cold-smoked salmon), maintaining storage conditions and determining freshness is of particular importance.
Total Volatile Basic Nitrogen
Degradation of seafood during storage causes several chemical and physical changes over time. Many of the chemical products from this degradation are nitrogen-containing compounds that originate from proteins breaking down. Spoilage bacteria are known to produce trimethylamine, which can be used as a spoilage indicator and can be determined by measuring the total volatile basic nitrogen (TVB-N), along with other similar compounds that occur from proteolytic enzymes that remain active post-slaughter.[Citation89] Fresh fish typically have TVB-N levels of between 5 and 20 mg N/100 g. TVB-N levels above 30 to 35 mg N/100 g are considered to be sensorially unacceptable for ice stored cold-water fish.[Citation90] For Atlantic salmon sold in the European Union, the levels of TVB-N cannot exceed 35 mg of nitrogen/100 g in the flesh.[Citation91] However, considering that trimethylamine is not present in significant quantities until after or late into the shelf life of ice stored fish, measurement of TVB-N may not be a good measure of spoilage. In one such example, TVB-N did not increase in European sea bass stored on ice until after 20 days of storage.[Citation92]
Histamine and other biogenic amines
Histamine is a naturally occurring basic compound that is found in fish and a variety of other foods and beverages, and is similarly related to other biogenic amine compounds such as tryptamine, cadaverine and putrescine in that they form from the enzymatic decarboxylation of free amino acids.[Citation93] While histamine is produced as a normal part of human biochemistry and performs several different functions within the body, high levels of ingestion can cause histamine intoxication. Histamine intoxication is a somewhat common occurrence, though it is frequently misdiagnosed as an allergic response due to histamine’s involvement with immune responses.[Citation94]
Atlantic salmon is not recognized as a species of concern for histamine poison as it has not been conclusively linked to an outbreak event.[Citation95] However, there have been some surveys on market fish that reported cases of histamine levels in Atlantic salmon with concerning amounts that could present health risks if consumed.[Citation96–98]
In fish, histamine is produced from the amino acid histidine, which can be found at higher concentrations when proteolysis begins to occur. The enzyme responsible for the conversion of histidine to histamine, histidine carboxylase, is bacterial in origin and is produced by a wide variety of species, including Escherichia, Salmonella, Clostridium, Bacillus and Lactobacillus, though bacteria that also have proteolytic activity in addition to histidine carboxylase production, such as Lactobacillus, tend to produce more histamine.[Citation93]
Fresh fish typically only have small amounts of histamine, though amounts can increase quickly if adequate storage temperatures (<5°C) are not maintained. Additionally, gutting and removing the head as quickly as possible post-mortem can reduce the likelihood of histamine production, as much of the bacteria capable of histamine production can be found in the gills and gut. Storage at 18°C will also halt the production of histamine.[Citation99] In other salmon products such as cold-smoked salmon, where salt concentrations are higher than fresh fish or fillets and 18°C would not be ideal, histamine production is still possible. However, this risk can be mitigated by maintaining storage temperatures under 5°C.[Citation100]
Microbiology
Although this review has focussed on chemical measurements of quality, and while microorganisms such as bacteria are certainly not chemicals or single molecules; their methods of detection and identification are fast becoming more based on biochemistry and molecular techniques rather than traditional microbiological media-based analysis, and should be briefly discussed considering this context.[Citation101,Citation102]
There are a considerable number of different bacterial species that can indicate potential spoilage in Atlantic salmon. Whole Atlantic salmon stored at 2°C over a period of 10 days have steadily increasing counts of hydrogen sulfide producing bacteria, lactic acid bacteria, Pseudomonas spp., Brochothrix thermosphacta and Photobacterium spp. Growth rates were similar in the flesh and on the skin of the fish. Enterobacteriaceae spp where present in the fish by day 10, but at significantly lower amounts, (1.5 log10 CFU/cm2 compared with 5.9 log10 CFU/cm2 lactic acid bacteria).[Citation103] Under MAP, Photobacterium phosphoreum seems to be the organism that proliferates the most, producing compounds such as acetic acid that strongly correlate to salmon bacterial spoilage.[Citation104]
Post-slaughter processing is often a source of microbial contamination, although some species of bacteria can be found in Atlantic salmon pre-slaughter and remain present on the fish throughout processing. In Norwegian processing plants, high levels of Pseudomonas spp. and Shewanella spp. can be found in fillets early in a processing shift, compared to fillets processed later in the shift. Photobacterium spp. could be found in fillets processed throughout the shift as these species of bacteria can be found on the live fish and in seawater.[Citation105]
Microbial production of spoilage chemicals
Microorganisms produce a large array of metabolic compounds in the foods they inhabit. Which types of compounds are produced is dependent on the species of microorganism, the packaging, storage temperature and treatment of the food, i.e., raw versus cooked. In raw, aerobically stored Atlantic salmon fillets, compounds such as trimethylamine, ethanol, 3-methyl-1-butanol, acetoin and acetic acid are potentially the main chemical markers for spoilage at storage temperatures of 4, 10 and 21°C as these compounds correlate to results of bacteriological enumeration.[Citation106] When salmon fillets are stored under modified atmosphere packaging (MAP), several spoilage compounds produced by bacteria can be detected, with dimethyl sulfide, hydrogen sulphide and ethanol typically being detected. Alcohols and ketones can be detected in packaging under air, while under high oxygen packaging only 3-methylbutanal could be detected.[Citation107]
Contaminants and pollutants
Chemical contaminants and pollutants in food can be categorized into two main groups, heavy metals and organic compounds. Heavy metals that are of concern include arsenic, lead, cadmium, and mercury, which are toxic when ingested even at low concentrations. Organic-based contaminants and pollutants are much more diverse and include compounds such as pesticides, polycyclic aromatic hydrocarbons, polychlorinated biphenols (PCBs), dioxins, perfluorninated compounds, pharmaceutical productions, and plastics, many of which fall under the category of persistent organic pollutants (POPs) as these compounds are unable to fully break down in the environment. Both heavy metals and POPs can bioaccumulate, which can cause long-term exposure problems to individuals who consume comminuted food regularly.[Citation108]
Both farmed and wild caught Atlantic salmon are affected by pollutants, though the sources of contamination and concentrations can differ. Differences in pollutants and contaminants between wild and farmed Atlantic salmon does translate into differing health risks depending on the source of the fish. From a nutritional perspective, the high amounts polyunsaturated fatty acid, specifically omega-3 and − 6 fatty acids, that Atlantic salmon typically carries many health benefits. As the concentration of these fatty acids in salmon muscle differs between sources, along with the varying levels of contaminants, so too does the health-benefit trade-off vary by source. Analyzing the health-benefits for each source shows that overall, farmed Atlantic salmon have lower carcinogenic and non-carcinogenic risks than wild Atlantic salmon. However, certain locations of farmed salmon may have similar risks to wild salmon due to higher exposure to potentially carcinogenic contaminants.[Citation109]
The source of pollutants in farmed Atlantic salmon originates in the feed ingredients. Depending on the source of these ingredients, compounds such as polychlorinated biphenyls, dioxins and furans, OCPs and polybrominated diphenyl ethers, along with an array of heavy metals can be detected in the feed.[Citation110] Switching to feeds that include higher amounts of terrestrial lipids has been shown to reduce the amount of certain contaminants as it is the marine oil component of the feed that was considered the main source of aforementioned pollutants.[Citation110,Citation111] However, the terrestrial lipids are not free from contaminants and may introduce other contaminants, such as polyaromatic hydrocarbons, which are typically not found in Atlantic salmon fed marine diets.[Citation112] Contaminants in wild caught salmon are due to sources in the environment and food chain.[Citation113,Citation114]
Recent analytical data of heavy metals and POP levels in farmed Atlantic salmon show an overall decline in concentration over more than 10 years, which is primarily attributed to a move away from fish metal and oils in salmon feed. However, wild Atlantic salmon still show much higher levels of contaminants than farmed salmon, although still within safe limits.[Citation19,Citation115,Citation116]
Heavy metals
The concentration of heavy metals in the tissues of farmed and wild-caught salmon from the same region is often varied. When farmed Atlantic salmon from the US and Canada were compared with wild-caught salmon, organic arsenic is significantly lower in farmed salmon, while cobalt, copper and cadmium are higher in wild-caught salmon. However, all metal concentrations detected were below regulatory levels.[Citation117]
Mercury analysis performed in a study on farmed and wild Atlantic salmon from British Columbia showed that 97.1% of the total mercury is in the form of the organic compound methylmercury (MeHg) form, which is more toxic than the inorganic form. Compared with other toxic heavy metals, such as arsenic, cadmium and lead, MeHg appears to undergo slight amounts of biomagnification, possible due to a high dietary uptake efficiency and is not eliminated quickly from salmon tissues. Concentrations of total mercury in both farmed and wild salmon were in a similar range of approximately 0.05 µg/g wet weight, although wild Atlantic salmon had a statistically significant higher amount of total mercury. While the mercury levels reported from both sources of Atlantic salmon were under regulatory levels, they were considerably higher than in other farmed protein sources, such as chicken, pork and beef, which had levels that were all below detectable limits.[Citation118]
A comparative study looking at mercury levels in farmed and wild caught Atlantic salmon from Northern America showed similar results in the previously mentioned study, however the mercury levels of farmed Atlantic salmon were only approximately a third of the amount of mercury found in wild Atlantic salmon. In addition to this, mercury levels were higher in salmon flesh that were lipid extracted, which suggests that mercury is found primarily within muscle tissue rather than fat deposits.[Citation119]
Organic compounds
Persistent organic pollutants have been detected in both wild and farmed Atlantic salmon from numerous locations, including Norway, Sweden, UK and North America. The exact concentration is location dependent, with large variations in concentration in fish from farms that were closely situated to each other.[Citation120–122] A study comparing the concentration of organochlorine pesticides (OCP) in Atlantic salmon from the British Columbia region of Canada found that farmed Atlantic salmon had higher amounts of OCP in their flesh than wild caught salmon, and positively correlated with fish size. However, the OCP concentrations by lipid weight in wild-caught Atlantic salmon were higher than those of farmed salmon.[Citation123]
Other more recent studies comparing contaminant levels in wild caught Atlantic salmon to farmed salmon have shown that wild salmon have concentrations that are several times higher for PCBs, dioxins and OCPs. However, all levels of contaminants were still below regulatory limits.[Citation19,Citation116]
As with other non-polar compounds, persistent organic pollutants tend to be found in lipid deposits. Removal of the skin and the accompanying subcutaneous fat layers is thought to reduce the amount of pollutants that the consumer ingests. In a study examining the effect of skin removal on the levels POPs in Atlantic salmon, results showed some highly variable reduction in compounds such as PCBs, DDT but not so in other compounds like hexachlorocyclohexane isomers (HCH) and hexachlorobenzene (HCB) where the concentration increased with skin removal.[Citation121]
Analytical methods for fish composition and quality parameters
As mentioned throughout this review, there are various quality parameters that are important to monitor during the production of Atlantic salmon and other fish. As with any measurement technique, there are several ways in which to perform the analysis each with its own advantages and disadvantages. Conventional chemical analysis is still very much considered the “gold standard,” offering highly accurate and reproducible results. These techniques often involve the use of technologies such as High-Pressure Liquid Chromatography (HPLC) and Gas Chromatography (GC), which while being robust and reliable analysis methods, however, are more time consuming, costly to set up and run and have larger laboratory footprints than comparative techniques offered by vibrational spectroscopic methods. As vibrational spectroscopy gains more widespread adoption throughout food production industries, more techniques that have specific applications to aquaculture and fish production are being developed.
Lipid and fatty acid analysis
Gas Chromatography and fatty acid profiles
Fatty acid methyl esterification, or FAMEs, is a very widely used method for quantitating individual fatty acids in animal tissues. The principle of the method is the addition of methanol to the fatty acid ester functional group, known as transesterification. After this chemical conversion, the fatty acids are much more volatile, and can be analyzed using gas chromatography.
Synthesis of FAMEs can be performed a variety of ways. Classically, it has been performed using boron trifluoride as a catalyst, however this compound is highly toxic.[Citation124] Direct FAME synthesis can be performed using sulfuric acid, which is still a dangerous chemical, but it is much safer and less costly than boron trifluoride.[Citation125] Once synthesis is complete, the FAMEs are extracted using hexane prior to conducting chromatographic analysis. Using appropriate standards, individual fatty acids from a sample can be separated, identified, and quantified, which is extremely useful in determining fatty acid profiles.[Citation126–128]
Spectroscopic methods for estimating lipids
Total fat quantitation has been performed using several types of spectroscopic techniques. Initial work performed on ground salmon showed that several Raman spectral peaks from lipid compounds could be used to accurately determine total lipid concentration using a statistical method called partial least squares regressions, which can find correlations between two complex variables. Both pre-processed full spectra and selected lipid spectral regions showed similar levels of prediction accuracy, however the selected spectral regions were more accurate overall due to it containing more specific data relating to lipids.[Citation129]
Differentiation of fatty acids from complex spectra is a difficult task, however a method to determine the iodine value, (a measure of the level of unsaturated fatty acids) in salmon flesh has been developed.[Citation130] The technique was able to identify variations across a nominal range of values commonly found in Atlantic salmon. However, the higher prediction error with measurements on intact salmon muscle compared with ground salmon shows that sample heterogeneity plays a role in accuracy. Following from this, iodine value predication from Atlantic salmon flesh was also found to be possible with Spatially Offset Raman Spectroscopy (SORS), which allows for sub-surface measurements through the skin.[Citation131] Defocused Raman spectroscopy can also be used for sub-surface measurement to determine lipid concentration, as well as accurately measure lipid type using PLS regression of the spectra.[Citation132]
Some other more specialized spectroscopic work has focussed on quantitating individual fatty acids in salmon and fish products. Using PLSR analysis on FT-IR, NIR and Raman data, a method to accurately predict total omega-3 fatty acids as well as DHA and EPA with low amounts of error was developed.[Citation133] A similar project was able to identify several individual fatty acids and lipid class from salmon oil using NIR and PLS.[Citation134] Whilst not performed on Atlantic salmon, a very thorough project involving lipid prediction on more than 260 rainbow trout, we were able to develop a predictive method of fatty acid composition on the visceral adipose tissue using a Raman microscope. This method had a remarkably high predictive value for both long-chain omega-3 fatty acids.[Citation135]
Carotenoid and pigment analysis
Hplc
Carotenoids are typically quantitated using normal phase HPLC,[Citation136] after undergoing a vigorous extraction process involving a number of solvents.[Citation137] The precise parameters for extraction, such as solvent used, differ depending on the carotenoid of interest and the food type. Typically, for fish, carotenoid extraction involves homogenizing freeze-dried fish flesh with methanol, followed by centrifugation and separation of the solvent from solid material. Subsequent extractions on the remaining solid material use tetrahydrofuran (THF) to remove any additional carotenoids. All solvents used in the extraction are collected and evaporated under nitrogen gas at 40°C. After evaporation, the solid material is resuspended in ethanol.[Citation138]
For differentiation and quantification of a large array of carotenoids, a system consisting of photodiode array detectors coupled with a standard normal phase C30 column with an isocratic mobile phase for analyte elution is a common choice. Detection wavelength is usually approximately 470 nm but can vary slightly depending on the composition of the mobile phase.
If HPLC analysis is required to differentiate carotenoid isomers, such as astaxanthin isomers found in wild caught Atlantic salmon, the analysis becomes increasingly complex, involving the use of highly specific reverse-phase chirally sensitive columns and sometimes the use of solvent gradients to optimize the procedure.[Citation139,Citation140] In situations where carotenoid identification is the purpose of the analysis, HPLC coupled with atmospheric pressure chemical ionization mass spectroscopy can identify carotenoids based on their mass/charge fragmentation profiles.[Citation141]
Colour analysis
Colour measurement has been performed by using a chromameter, standardized visual colour evaluation and other similar methods. Analysis using a colorimeter can record colour measurement in a few different outputs though L*a*b* is the most common for food. The different aspects of color are represented by the three parameters of the measurement. L* represents the lightness of the measured color, a* is a measure of the green/red value and b* follows blue/yellow values.[Citation142]
For measurements of salmon flesh color, all three colorimetric values are affected by astaxanthin concentration, though the relationship is slightly different for each value. Both a* and b* values have a non-linear relationship, where each value increases along with astaxanthin concentration. The L* value also has a non-linear correlation to astaxanthin concentration, however this value decreases as concentration increases.[Citation143]
Determining color using visual evaluation requires the use of standardized color cards that can be used to visually compare against the color of a salmon fillet. This process is time consuming and prone to errors and additionally requires the use of a controlled light environment and trained personal as visual evaluation is highly subjective. A color card system based on the Natural Color System was found to match salmon fillet color and can accurately determine astaxanthin concentration,[Citation144] and as such a color card visual evaluation system is considered the industry standard for salmon color measurement according to Norwegian Standard 9402 (1994).
Automation of colour analysis is possible using computer vision to capture images of the fillet and evaluate and sort them based on color. Computer imaging, or vision, works using an algorithm to assign values or classify based on images, in this case, of salmon fillets. Using the standard colour measurement outlined in NS 9402 (1994), a classification algorithm can accurately assess colour levels in salmon fillets as well as human operators.[Citation145,Citation146]
Vibrational spectroscopy techniques for assessing pigmentation
Early works to establish carotenoid detection using Raman spectroscopy were performed on ground salmon samples. The resulting spectra showed concentration-dependent carotenoid peaks, which could be used to determine astaxanthin concentration. The PLS regression on both full spectra and the carotenoid peak regions showed high R2 values after pre-processing the spectral data, though the peak region spectra offered a slightly higher R2 value.[Citation129] Another early work was performed using resonance Raman spectroscopy on acetone extracts of salmon flesh. The Raman spectroscopy spectra displayed the same peaks as found in astaxanthin, with peak intensity increasing in line with concentration using resonance Raman spectroscopy.[Citation147]
Astaxanthin quantitation using Raman spectroscopy on intact Atlantic salmon fillets has been attempted by taking multiple measurements over a 2 mm square to overcome the issue to the heterogeneity. This method worked for astaxanthin quantitation across a number of different salmon species included coho and sockeye salmon., as well across fillets from different regions of Atlantic salmon.[Citation148]
The use of SORS to detect astaxanthin has been found to be possible, as all major peaks can be detected through subsurface measurements through both the dark and light regions of salmon skin. It is worth noting that this work was performed on salmon flesh that had been homogenized, and not on intact fish or fillets.[Citation131] Though skin quantization of astaxanthin can also be reasonably well performed using defocused Raman spectroscopy, however unlike SORS, this type of spectroscopy cannot penetrate through dark skin areas of Atlantic salmon skin.[Citation132]
Total Volatile Basic Nitrogen and other related compounds
Titrimetric determination of TVB-N
As previously mentioned, the TVB-N of Atlantic salmon and other seafood items have maximum limits to which have been legislated by a number of governments and are routinely monitored by regional food authorities. In situations where a quality parameter has legal and safety implications, a standard method of analysis is sometimes included in the legislation, as is the case with EU Commission Regulation 2074/2005 that sets the limits for TVB-N in various seafood commodities in the European Union. The method outlined in the legislation involves digesting a homogenized sample in perchloric acid solution, whereupon after filtration, a sodium hydroxide solution is added to the sample digest which is then steam distilled. Once 100 mL of distillate is collected into a boric acid solution, a pH chemical indicator is added and titrated to endpoint with a hydrochloric acid solution.[Citation91]
Conclusion
Atlantic salmon is a commercially significant fish that is farmed in vast quantities in a number of different regions across the globe. Lipid profiles and pigmentation are of particular importance for maintaining the market value of the fish, with both parameters being primarily affected by diet. Numerous feeding trials have shown that Atlantic salmon tend to have a lipid profile that is very similar to that of their feed, which is an important consideration when using vegetable and other terrestrial oils in conjunction with attempts to produce fish with substantial omega-3 levels. Fish oil-based finishing diets in the weeks leading up to processing as well as including small amounts of fish meal and oil in the regular feed may help to reduce the overall usage of fish oils whilst still producing fish with the desired amount of omega-3 fatty acids. Pigmentation on the other hand is controlled by several factors, with not only the carotenoid compound itself affecting muscle deposition, the source (synthetic versus natural sources) but also plays a role in the deposition rate. Lighting conditions during early growth and seasonality have an influence on the final color values in the muscles. Increased ocean temperature impacts pigmentation by poor carotenoid retention and heterogenous pigmentation, due to genetic factors that control the stress response in individual fish. Several differences exist between wild caught and farmed Atlantic salmon, the most pronounced being lipid profiles and the types of contaminants. There are numerous analytical techniques available to assess all the chemical markers detailed throughout this review, with promise of late shown with Raman spectroscopic techniques that could provide speedy, non-destructive information on fish quality.
Acknowledgments
The authors wish to acknowledge the support of RMIT University.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Schoffmann, J. Trout and salmon of the genus Salmo; Bethesda, Maryland: American Fisheries Society, 2021.
- FAO. Salmo Salar. Cultured Aquatic Species Information Programme [Fact Sheet]; Rome: FAO, 2022.
- Myrvold, K. M.; Mawle, G. W.; Andersen, O.; Aas, Ø. The Social, Economic and Cultural Values of Wild Atlantic Salmon; Norwegian Institute for Nature Research: Lillehammer, 2019.
- Misund, B.; Nygård, R. Big Fish: Valuation of the World’s Largest Salmon Farming Companies. Mar. Resour. Econ. 2018, 33(3), 245–261. DOI: 10.1086/698447.
- Dahl, R. E.; Oglend, A.; Yahya, M. Salmon Stock Market Prices Revealing Salmon Price Information. Mar. Resour. Econ. 2021, 36(2), 173–190. DOI: 10.1086/713769.
- Simopoulos, A. P. The Importance of the Omega-6/omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Experiment. Biol. Med. 2008, 233(6), 674–688. DOI: 10.3181/0711-MR-311.
- Wall, R.; Ross, R. P.; Fitzgerald, G. F.; Stanton, C. Fatty Acids from Fish: The Anti-Inflammatory Potential of Long-Chain Omega-3 Fatty Acids. Nutr. Rev. 2010, 68(5), 280–289. DOI: 10.1111/j.1753-4887.2010.00287.x.
- Swanson, D.; Block, R.; Mousa, S. A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3(1), 1–7. DOI: 10.3945/an.111.000893.
- FAO. The State of World Fisheries and Aquaculture 2022; Rome, Italy: FAO, 2022.
- FAO. The State of World Fisheries and Aquaculture 2018 - Meeting the Sustainable Development Goals; Rome: FAO, 2018.
- FAOSTAT. FAO Global Fishery and Aquaculture Production Statistics; Rome: FAO Fisheries and Aquaculture Division & FAO Statistics and Information Branch, 2022.
- Asche, F.; Roll, K. H.; Sandvold, H. N.; Sørvig, A.; Zhang, D. Salmon Aquaculture: Larger Companies and Increased Production. Aquac. Econ. Manag. 2013, 17(3), 322–339. DOI: 10.1080/13657305.2013.812156.
- Fauske, M. Key Figures from Norwegian Aquaculture Industry 2020; Editor., S. Department. Directorate of Fisheries: Nowary, 2021 p. 28.
- Mobsby, D. Australian Fisheries and Aquaculture Statistics 2017; A.B.o.A.a.R.E.a. Sciences. Editor.; Australian Bureau of Agricultural and Resource Economics and Sciences: Canberra, 2018.
- Steven, A. H.; Dylewski, M.; Curtotti, R. Australian disheries and aquaculture statistics 2020. ABARES: Canberra, 2021. DOI: 10.25814/0wzy-re76.
- Mobsby, D.; Curtotti, R. Snapshot of Australia‘s commerical fisheries and aquaculture. ABARES: Canberra, 2018.
- Johnston, I. A.; Li, X.; Vieira, V. L. A.; Nickell, D.; Dingwall, A.; Alderson, R.; Campbell, P.; Bickerdike, R. Muscle and Flesh Quality Traits in Wild and Farmed Atlantic Salmon. Aquaculture. 2006, 256(1–4), 323–336. DOI: 10.1016/j.aquaculture.2006.02.048.
- Schiedt, K.; Foss, P.; Storebakken, T.; Liaane-Jensen, S. Metabolism of Carotenoids in Salmon-I. Idoxanthin, a Metabolite of Astaxanthin in the Flesh of Atlanic Salmon (Salmo Salar, L.) Under Varying External Conditions. Comp. Biochem. Physiol. B: Comp. Biochem. 1989, 92(2), 277–281. DOI: 10.1016/0305-0491(89)90278-2.
- Jensen, I. J.; Eilertsen, K. E.; Otnaes, C. H. A.; Maehre, H. K.; Elvevoll, E. O. An Update on the Content of Fatty Acids, Dioxins, PCBs and Heavy Metals in Farmed, Escaped and Wild Atlantic Salmon (Salmo Salar L.) in Norway. Foods. 2020, 9(12), 1901. DOI: 10.3390/foods9121901.
- Sprague, M.; Fawcett, S.; Betancor, M. B.; Struthers, W.; Tocher, D. R. Variation in the Nutritional Composition of Farmed Atlantic Salmon (Salmo Salar L.) Fillets with Emphasis on EPA and DHA Contents. J. Food Compost. Anal. 2020, 94, 94. DOI: 10.1016/j.jfca.2020.103618.
- Jakobsen, J.; Smith, C.; Bysted, A.; Cashman, K. D. Vitamin D in Wild and Farmed Atlantic Salmon (Salmo Salar)-What Do We Know? Nutrients. 2019, 11(5), 982. DOI: 10.3390/nu11050982.
- Sylvia, G.; Morrissey, M. T.; Graham, T.; Garcia, S. Changing Trends in Seafood Markets. J. Food Prod. Mark. 1996, 3(2), 49–63. DOI: 10.1300/J038v03n02_05.
- Pulcini, D.; Franceschini, S.; Buttazzoni, L.; Giannetti, C.; Capoccioni, F. Consumer Preferences for Farmed Seafood: An Italian Case Study. J. Aquat. Food Prod. Technol. 2020, 29(5), 445–460. DOI: 10.1080/10498850.2020.1749201.
- Bronnmann, J.; Asche, F. Sustainable Seafood from Aquaculture and Wild Fisheries: Insights from a Discrete Choice Experiment in Germany. Ecol. Econ. 2017, 142, 113–119. DOI: 10.1016/j.ecolecon.2017.06.005.
- Zhou, S.; Ackman, R. G.; Morrison, C. Storage of Lipids in the Myosepta of Atlantic Salmon. Fish Physiol. Biochem. 1995, 14(2), 171–178. DOI: 10.1007/BF00002460.
- Segtnan, V. H.; Høy, M.; Lundby, F.; Narum, B.; Wold, J. P. Fat Distribution Analysis in Salmon Fillets Using Non-Contact Near Infrared Interactance Imaging: A Sampling and Calibration Strategy. J. Near Infrared Spectrosc. 2009, 17(5), 247–253. DOI: 10.1255/jnirs.851.
- Dinicolantonio, J. J.; O’Keefe, J. H. The Importance of Marine Omega-3s for Brain Development and the Prevention and Treatment of Behavior, Mood, and Other Brain Disorders. Nutrients. 2020, 12(8), 2333. DOI: 10.3390/nu12082333.
- Satizabal, C. L.; Himali, J. J.; Beiser, A. S.; Ramachandran, V.; Melo van Lent, D.; Himali, D.; Aparicio, H. J.; Maillard, P.; DeCarli, C. S.; Harris, W., et al. Association of Red Blood Cell Omega-3 Fatty Acids with MRI Markers and Cognitive Function in Midlife: The Framingham Heart Study; Neurology, 2022. DOI:10.1212/WNL.0000000000201296.
- Khan, S. U.; Lone, A. N.; Khan, M. S.; Virani, S. S.; Blumenthal, R. S.; Nasir, K.; Miller, M.; Michos, E. D.; Ballantyne, C. M.; Boden, W. E., et al. Effect of Omega-3 Fatty Acids on Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. eClinicalmedicine. 2021, 38, 100997. DOI: 10.1016/j.eclinm.2021.100997.
- Taşbozan, O.; Gökçe, M. A. Fatty Acids in Fish. In Fatty Acids; A. Catala, Ed.; InTech, 2017.
- Jobling, M.; Larsen, A. V.; Andreassen, B.; Sigholt, T.; Olsen, R. L. Influence of a Dietary Shift on Temporal Changes in Fat Deposition and Fatty Acid Composition of Atlantic Salmon Post-Smolt During the Early Phase of Seawater Rearing. Aquacult. Res. 2002, 33(11), 875–889. DOI: 10.1046/j.1365-2109.2002.00727.x.
- Sanden, M.; Stubhaug, I.; Berntssen, M. H.; Lie, O.; Torstensen, B. E. Atlantic Salmon (Salmo Salar L.) as a Net Producer of Long-Chain Marine Omega-3 Fatty Acids. J. Agric. Food Chem. 2011, 59(23), 12697–12706. DOI: 10.1021/jf203289s.
- Mock, T. S.; Francis, D. S.; Drumm, D. W.; Versace, V. L.; Glencross, B. D.; Smullen, R. P.; Jago, M. K.; Turchini, G. M. A Systematic Review and Analysis of Long-Term Growth Trials on the Effect of Diet on Omega-3 Fatty Acid Levels in the Fillet Tissue of Post-Smolt Atlantic Salmon. Aquaculture. 2020, 516, 734643. DOI: 10.1016/j.aquaculture.2019.734643.
- Glencross, B.; Carr, I.; Santigosa, E. Distribution, Deposition, and Modelling of Lipid and Long-Chain Polyunsaturated Fatty Acids in Atlantic Salmon Fillets. Rev. Fish. Sci. Aquac. 2022, 1–22. doi: 10.1080/23308249.2022.2090831.
- Robb, D. H. F.; Kestin, S. C.; Warriss, P. D.; Nute, G. R. Muscle Lipid Content Determines the Eating Quality of Smoked and Cooked Atlantic Salmon (Salmo Salar). Aquaculture. 2002, 205(3–4), 345–358. DOI: 10.1016/S0044-8486(01)00710-4.
- Jónsson, Á.; Pálmadóttir, H.; Kristbergsson, K. Fatty Acid Composition in Ocean-Ranched Atlantic Salmon (Salmo Salar). International Journal Of Food Science & Technology. 1997, 32(6), 547–551. DOI: 10.1111/j.1365-2621.1997.tb02130.x.
- Molversmyr, E.; Devle, H. M.; Naess-Andresen, C. F.; Ekeberg, D. Identification and Quantification of Lipids in Wild and Farmed Atlantic Salmon (Salmo Salar), and Salmon Feed by GC-MS. Food Science & Nutrition. 2022, 10(9), 3117–3127. DOI: 10.1002/fsn3.2911.
- Skilbrei, O. T.; Normann, E.; Meier, S.; Olsen, R. E. Use of Fatty Acid Profiles to Monitor the Escape History of Farmed Atlantic Salmon. Aquac. Environ. Interact. 2015, 7(1), 1–13. DOI: 10.3354/aei00132.
- Turchini, G. M.; Torstensen, B. E.; Ng, W.-K. Fish Oil Replacement in Finfish Nutrition. Rev. Aquac. 2009, 1(1), 10–57. DOI: 10.1111/j.1753-5131.2008.01001.x.
- Rosenlund, G.; Torstensen, B. E.; Stubhaug, I.; Usman, N.; Sissener, N. H. Atlantic Salmon Require Long-Chain N-3 Fatty Acids for Optimal Growth Throughout the Seawater Period. J. Nutr. Sci. 2016, 5, e19. DOI: 10.1017/jns.2016.10.
- Foroutani, M. B.; Parrish, C. C.; Wells, J.; Taylor, R. G.; Rise, M. L.; Shahidi, F. Minimizing Marine Ingredients in Diets of Farmed Atlantic Salmon (Salmo Solar): Effects on Growth Performance and Muscle Lipid and Fatty Acid Composition. PLoS One. 2018, 13(9), e0198538. DOI: 10.1371/journal.pone.0198538.
- Bell, J. G.; McEvoy, J.; Tocher, D. R.; McGhee, F.; Campbell, P. J.; Sargent, J. R. Replacement of Fish Oil with Rapeseed Oil in Diets of Atlandtic Salmon Affects Tissue Lipid Compositions and Hepatocyte Fatty Acid Metabolism. J. Nutr. Metab. 2001, 131(5), 1535–1543. DOI: 10.1093/jn/131.5.1535.
- Bell, J. G.; Henderson, R. J.; Tocher, D. R.; McGhee, F.; Dick, J. R.; Porter, A.; Smullen, R., P, and Sargent, J. R. Substituting Fish Oil with Crude Palm Oil in the Diet of Atlantic Salmon Affect Muscle Fatty Acid Composition and Hepatic Fatty Acid Metabolism. J. Nutr. Requirements. 2002, 132(2), 222–230. DOI: 10.1093/jn/132.2.222.
- Torstensen, B. E.; Bell, J. G.; Rosenlund, G.; Henderson, R. J.; Graff, I. E.; Tocher, D. R.; Lie, O.; Sargent, J. R. Tailoring of Atlantic Salmon (Salmo Salar L.) Flesh Lipid Composition and Sensory Quality by Replacing Fish Oil with a Vegetable Oil Blend. J. Agric. Food Chem. 2005, 53(26), 10166–10178. DOI: 10.1021/jf051308i.
- Pratoomyot, J.; Bendiksen, E. A.; Bell, J. G.; Tocher, D. R. Comparison of Effects of Vegetable Oils Blended with Southern Hemisphere Fish Oil and Decontaminated Northern Hemisphere Fish Oil on Growth Performance, Composition and Gene Expression in Atlantic Salmon (Salmo Salar L.). Aquaculture. 2008, 280(1–4), 170–178. DOI: 10.1016/j.aquaculture.2008.04.028.
- Pratoomyot, J.; Bendiksen, E. A.; Bell, J. G.; Tocher, D. R. Effects of Increasing Replacement of Dietary Fishmeal with Plant Protein Sources on Growth Performance and Body Lipid Composition of Atlantic Salmon (Salmo Salar L.). Aquaculture. 2010, 305(1–4), 124–132. DOI: 10.1016/j.aquaculture.2010.04.019.
- Codabaccus, M. B.; Bridle, A. R.; Nichols, P. D.; Carter, C. G. Effect of Feeding Atlantic Salmon (Salmo Salar L.) a Diet Enriched with Stearidonic Acid from Parr to Smolt on Growth and N-3 Long-Chain PUFA Biosynthesis. Br. J. Nutr. 2011, 105(12), 1772–1782. DOI: 10.1017/S0007114510005714.
- Hatlen, B.; Larsson, T.; Østbye, T. K.; Romarheim, O. H.; Rubio, L. M.; Ruyter, B. Improved Fillet Quality in Harvest-Size Atlantic Salmon Fed High N-3 Canola Oil as aDHA-Source. Aquac. 2022, 560. DOI: 10.1016/j.aquaculture.2022.738555.
- Nanton, D. A.; Vegusdal, A.; Rørå, A. M. B.; Ruyter, B.; Baeverfjord, G.; Torstensen, B. E. Muscle Lipid Storage Pattern, Composition, and Adipocyte Distribution in Different Parts of Atlantic Salmon (Salmo Salar) Fed Fish Oil and Vegetable Oil. Aquaculture. 2007, 265(1–4), 230–243. DOI: 10.1016/j.aquaculture.2006.03.053.
- Torrissen, O. J.; Naevdal, G. Pigmentation of Salmonids — Variation in Flesh Carotenoids of Atlantic Salmon. Aquaculture. 1988, 68(4), 305–310. DOI: 10.1016/0044-8486(88)90244-X.
- Helgeland, H.; Sodeland, M.; Zoric, N.; Torgersen, J. S.; Grammes, F.; von Lintig, J.; Moen, T.; Kjøglum, S.; Lien, S.; Våge, D. I. Genomic and Functional Gene Studies Suggest a Key Role of Beta-Carotene Oxygenase 1 Like (Bco1l) Gene in Salmon Flesh Color. Sci. Rep. 2019, 9(1), 20061. DOI: 10.1038/s41598-019-56438-3.
- Wathne, E.; Bjerkeng, B.; Storebakken, T.; Vassvik, V.; Odland, A. B. Pigmentation of Atlantic Salmon (Salmo Salar) Fed Astaxanthin in All Meals or in Alternating Meals. Aquaculture. 1998, 159(3–4), 217–231. DOI: 10.1016/S0044-8486(97)00218-4.
- March, B. E.; Macmillan, C. Muscle Pigmentation and Plasma Concentrations of Astaxanthin in Rainbow Trout, Chinook Salmon, and Atlantic Salmon in Response to Different Dietary Levels of Astaxanthin. Prog. Fish-Cult. 1996, 58(3), 178–186. DOI: 10.1577/1548-8640(1996)058<0178:MPAPCO>2.3.CO;2.
- Jacobsen, J. Feeding Habits of Wild and Escaped Farmed Atlantic Salmon, Salmo Salar L., in the Northeast Atlantic. ICES J. Mar. Sci. 2001, 58(4), 916–933. DOI: 10.1006/jmsc.2001.1084.
- Lim, K. C.; Yusoff, F. M.; Shariff, M.; Kamarudin, M. S. Astaxanthin as Feed Supplement in Aquatic Animals. Rev. Aquac. 2018, 10(3), 738–773. DOI: 10.1111/raq.12200.
- Rajasingh, H.; Oyehaug, L.; Vage, D. I.; Omholt, S. W. Carotenoid Dynamics in Atlantic Salmon. BMC Biol. 2006, 4(1), 10. DOI: 10.1186/1741-7007-4-10.
- Matthews, S. J.; Ross, N. W.; Lall, S. P.; Gill, T. A. Astaxanthin Binding Protein in Atlantic Salmon. Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 2006, 144(2), 206–214. DOI: 10.1016/j.cbpb.2006.02.007.
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74(1), 1–16. DOI: 10.1007/s11418-019-01364-x.
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F. M. Astaxanthin: A Review of Its Chemistry and Applications. Crit. Rev. Food Sci. Nutr. 2006, 46(2), 185–196. DOI: 10.1080/10408690590957188.
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella Zofingiensis as an Alternative Microalgal Producer of Astaxanthin: Biology and Industrial Potential. Mar. Drugs. 2014, 12(6), 3487–3515. DOI: 10.3390/md12063487.
- Ytrestøyl, T.; Struksnæs, G.; Rørvik, K. A.; Koppe, W.; Bjerkeng, B. Astaxanthin Digestibility as Affected by Ration Levels for Atlantic Salmon. Salmo salar. Aquaculture. 2006, 261(1), 215–224. DOI: 10.1016/j.aquaculture.2006.06.046.
- Brotosudarmo, T. H. P.; Limantara, L.; Setiyono, E.; Heriyanto. Structures of Astaxanthin and Their Consequences for Therapeutic Application. Int. J. Food Sci. 2020, 2020, 2156582. DOI: 10.1155/2020/2156582.
- Bjerkeng, B.; Berge, G. M. Apparent Digestibility Coefficients and Accumulation of Astaxanthin E/Z Isomers in Atlantic Salmon (Salmo Salar L.) and Atlantic Halibut (Hippoglossus Hippoglossus L.). Comp. Biochem. Physiol. B. 2000, 127(3), 423–432. DOI: 10.1016/S0305-0491(00)00278-9.
- Buttle, L. G.; Crampton, V. O.; Williams, P. D. The Effect of Feed Pigment Type on Flesh Pigment Deposition and Colour in Farmed Atlantic Salmon, Salmo Salar L. Salmo Salar L. Aquac. Res. 2001, 32(2), 103–111. DOI: 10.1046/j.1365-2109.2001.00536.x.
- Baker, R. T. M.; Pfeiffer, A. M.; Schoner, F. J.; Smith-Lemmon, L. Pigmenting Efficacy of Astaxanthin and Canthanxanthin in Fresh-Water Reaered Atlantic Salmon, Salmo Salar. Animal Feed Science And Technology. 2002, 99(1–4), 97–106. DOI: 10.1016/S0377-8401(02)00116-5.
- Ytrestoyl, T.; Coral-Hinostroza, G.; Hatlen, B.; Robb, D. H.; Bjerkeng, B. Carotenoid and Lipid Content in Muscle of Atlantic Salmon, Salmo Salar, Transferred to Seawater as 0+ or 1+ Smolts. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 138(1), 29–40. DOI: 10.1016/j.cbpc.2004.01.011.
- Young, A.; Morris, P. C.; Huntingford, F. A.; Sinnott, R. Replacing Fish Oil with Pre-Extruded Carbohydrate in Diets for Atlantic Salmon, Salmo Salar, During Their Entire Marine Grow-Out Phase: Effects on Growth, Composition and Colour. Aquaculture. 2006, 253(1–4), 531–546. DOI: 10.1016/j.aquaculture.2005.08.006.
- Olsen, R. E.; Baker, R. T. M. Lutein Does Not Influence Flesh Astaxanthin Pigmentation in the Atlantic Salmon (Salmo Salar L.). Aquaculture. 2006, 258(1–4), 558–564. DOI: 10.1016/j.aquaculture.2006.03.031.
- Bjerkeng, B.; Peisker, M.; von Schwartzenberg, K.; Ytrestøyl, T.; Åsgård, T. Digestibility and Muscle Retention of Astaxanthin in Atlantic Salmon, Salmo Salar, Fed Diets with the Red Yeast Phaffia rhodozyma in Comparison with Synthetic Formulated Astaxanthin. Aquaculture. 2007, 269(1–4), 476–489. DOI: 10.1016/j.aquaculture.2007.04.070.
- Hynes, N.; Egeland, E. S.; Koppe, W.; Baardsen, G.; Kiron, V. Calanusoil as a Natural Source for Flesh Pigmentation in Atlantic Salmon (Salmo salarL.). Aquac. Nutr. 2009, 15(2), 202–208. DOI: 10.1111/j.1365-2095.2008.00584.x.
- Johnsen, C. A.; Hagen, O.; Adler, M.; Jonsson, E.; Kling, P.; Bickerdike, R.; Solberg, C.; Bjornsson, B. T.; Bendiksen, E. A. Effects of Feed, Feeding Regime and Growth Rate on Flesh Quality, Connective Plasma Hormones in Farmed Atlantic Salmon (Salmo Salar L.). Aquaculture. 2011, 318(3–4), 343–354. DOI: 10.1016/j.aquaculture.2011.05.040.
- Johnsen, C. A.; Hagen, Ø.; Solberg, C.; Björnsson, B. T. H.; Jönsson, E.; Johansen, S. J. S.; Bendiksen, E. Å. Seasonal Changes in Muscle Structure and Flesh Quality of 0+ and 1+ Atlantic Salmon (Salmo salarL.): Impact of Feeding Regime and Possible Roles of Ghrelin. Aquac. Nutr. 2013, 19(1), 15–34. DOI: 10.1111/j.1365-2095.2011.00927.x.
- Albrektsen, S.; Ostbye, T. K.; Pedersen, M.; Ytteborg, E.; Ruyter, B.; Ytrestoyl, T. Dietary Impacts of Sulphuric Acid Extracted Fish Bone Compounds on Astaxanthin Utilization and Muscle Quality in Atlantic Salmon (Salmo Salar). Aquaculture. 2018, 495, 255–266. DOI: 10.1016/j.aquaculture.2018.05.047.
- Lutfi, E.; Berge, G. M.; Bæverfjord, G.; Sigholt, T.; Bou, M.; Larsson, T.; Mørkøre, T.; Evensen, Ø.; Sissener, N. H.; Rosenlund, G., et al. Increasing Dietary Levels of the N-3 Long-Chain PUFA, EPA and DHA, Improves the Growth, Welfare, Robustness and Fillet Quality of Atlantic Salmon in Sea Cages. Br. J. Nutr. 2022, 129(1), 1–19. DOI: 10.1017/S0007114522000642.
- Storebakken, T.; Foss, P.; Schiedt, K.; Austreng, E.; Liaane-Jensen, S.; Manz, U. Carotenoids in Diets for Salmonids IV. Pigmentation of Atlantic Salmon with Astaxanthin, Astaxanthin Dipalmitate and Canthaxanthin. Aquac. 1987, 65, 279–292.
- Kiessling, A.; Olsen, R. E.; Buttle, L. Given the Same Dietary Carotenoid Inclusion, Atlantic Salmon,salmo Salar(l.) Display Higher Blood Levels of Canthaxanthin Than Astaxanthin. Aquac. Nutr. 2003, 9(4), 253–261. DOI: 10.1046/j.1365-2095.2003.00251.x.
- Sheehan, E. M.; O’Connor, T. P.; Sheehy, P. J. A.; Buckley, D. J.; FitzGerald, R. Stability of Astaxanthin and Canthaxanthin in Raw and Smoked Atlantic Salmon (Salmo Salar) During Frozem Storage. Food Chem. 1998, 63(3), 313–317. DOI: 10.1016/S0308-8146(98)00048-X.
- Schmeisser, J.; Verlhac-Trichet, V.; Madaro, A.; Lall, S. P.; Torrissen, O.; Olsen, R. E. Molecular Mechanism Involved in Carotenoid Metabolism in Post-Smolt Atlantic Salmon: Astaxanthin Metabolism During Flesh Pigmentation and Its Antioxidant Properties. Mar. Biotechnol. 2021, 23(4), 653–670. DOI: 10.1007/s10126-021-10055-2.
- Courtot, E.; Musson, D.; Stratford, C.; Blyth, D.; Bourne, N. A.; Rombenso, A. N.; Simon, C. J.; Wu, X.; Wade, N. Dietary Fatty Acid Composition Affects the Apparent Digestibility of Algal Carotenoids in Diets for Atlantic Salmon, Salmo salar. Aquacult. Res. 2022, 53(6), 2343–2353. DOI: 10.1111/are.15753.
- Johnsen, C. A.; Hagen, Ø.; Bendiksen, E. Å. Long-Term Effects of High-Energy, Low-Fishmeal Feeds on Growth and Flesh Characteristics of Atlantic Salmon (Salmo Salar L.). Aquaculture. 2011, 312(1–4), 109–116. DOI: 10.1016/j.aquaculture.2010.12.012.
- Nordgarden, U.; Ornsrud, R.; Hansen, T.; Hemre, G. I. Seasonal Changes in Selected Muscle Quality Parameters in Atlantic Salmon (Salmo Salar L.) Reared Under Natural and Continuous Light. Aquac. Nutr. 2003, 9(3), 161–168. DOI: 10.1046/j.1365-2095.2003.00236.x.
- Ytrestoyl, T.; Struksnaes, G.; Koppe, W.; Bjerkeng, B. Effects of Temperature and Feed Intake on Astaxanthin Digestibility and Metabolism in Atlantic Salmon, Salmo Salar. Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 2005, 142(4), 445–455. DOI: 10.1016/j.cbpb.2005.09.004.
- Grunenwald, M.; Adams, M. B.; Carter, C. G.; Nichols, D. S.; Koppe, W.; Verlhac-Trichet, V.; Schierle, J.; Adams, L. R. Pigment-Depletion in Atlantic Salmon (Salmo Salar) Post-Smolt Starved at Elevated Temperature is Not Influenced by Dietary Carotenoid Type and Increasing Alpha-Tocopherol Level. Food Chem. 2019, 299, 125140. DOI: 10.1016/j.foodchem.2019.125140.
- Hamre, K.; Micallef, G.; Hillestad, M.; Johansen, J.; Remø, S.; Zhang, W.; Ødegård, E.; Araujo, P.; Prabhu Philip, A. J.; Waagbø, R. Changes in Daylength and Temperature from April Until August for Atlantic Salmon (Salmo Salar) Reared in Sea Cages, Increase Growth, and May Cause Consumption of Antioxidants, Onset of Cataracts and Increased Oxidation of Fillet Astaxanthin. Aquaculture. 2022, 551, 737950. DOI: 10.1016/j.aquaculture.2022.737950.
- Grunenwald, M. Factors affecting pigmentation quality in Atlantic salmon (Salmo salar L.) at evelated temperature. University of Tasmania, 2018. DOI: 10.25959/23238263.v1.
- Grünenwald, M.; Carter, C. G.; Nichols, D. S.; Adams, M. B.; Adams, L. R. Heterogeneous Astaxanthin Distribution in the Fillet of Atlantic Salmon Post-Smolt at Elevated Temperature is Not Affected by Dietary Fatty Acid Composition, Metabolic Conversion of Astaxanthin to Idoxanthin, or Oxidative Stress. Aquaculture. 2020, 521, 735096. DOI: 10.1016/j.aquaculture.2020.735096.
- Vo T.; T. Tran, G.; Amoroso, T.; Ventura, A., Elizur. Analysis of carotenoids and fatty acid compositions in Atlantic salmon exposed to elevated temperatures and displaying flesh color loss. Food Chemistry, 2023,417, 135867. DOI: 10.1016/j.foodchem.2023.135867.
- Vo, T.; G.; Amoroso, T.; Ventura, A.; Elizur. Histological and transcriptomic analysis of muscular atrophy associated with depleted flesh pigmentation in Atlantic salmon (Salmo salar) exposed to elevated seawater temperatures. Sci Rep. 2023, 13(1), DOI: 10.1038/s41598-023-31242-2.
- Bekhit, A. E.-D. A.; Holman, B. W. B.; Giteru, S. G.; Hopkins, D. L. Total Volatile Basic Nitrogen (TVB-N) and Its Role in Meat Spoilage: A Review. Trends Food Sci. Technol. 2021, 109, 280–302. DOI: 10.1016/j.tifs.2021.01.006.
- Claudia Ruiz-Capillas, F. J.-C.; Fidel, T. Biogenic Amines in Seafood Products. In Handbook of Seafood and Seafood Products Analysis; L. Nollet and F. Toldrá, Eds.; Taylor & Francis Group: Boca Raton, FL, 2010; pp. 743–760. DOI:10.1201/EBK1439848173-30.
- European Commision, COMMISSION REGULATION (EC) No 2074/2005. 2005.
- Castro, P.; Padrón, J. C. P.; Cansino, M. J. C.; Velázquez, E. S.; Larriva, R. M. D. Total Volatile Base Nitrogen and Its Use to Assess Freshness in European Sea Bass Stored in Ice. Food Control. 2006, 17(4), 245–248. DOI: 10.1016/j.foodcont.2004.10.015.
- Halasz, A.; Barath, A.; Simon-Sarkadi, L.; Holzapfel, W. Biogenic Amines and Their Production by Microorganisms in Food. Trends Food Sci. Technol. 1994, 5(2), 42–49. DOI: 10.1016/0924-2244(94)90070-1.
- Attaran, R. R.; Probst, F. Histamine Fish Poisoning: A Common but Frequently Misdiagnosed Condition. Emerg Med J. 2002, 19(5), 474–475. DOI: 10.1136/emj.19.5.474.
- Goulding, I. Histamine in Salmonids. FAO WHO: Rome, 2018.
- Buňka, F.; Budinský, P.; Zimáková, B.; Merhaut, M.; Flasarová, R.; Pachlová, V.; Kubáň, V.; Buňková, L. Biogenic Amines Occurrence in Fish Meat Sampled from Restaurants in Region of Czech Republic. Food Control. 2013, 31(1), 49–52. DOI: 10.1016/j.foodcont.2012.09.044.
- Garmienė, G.; Zaborskienė, G.; Šalaševičienė, A. Fish Product Safety in Lithuania: Assessment of Histamine Levels. Annals Food Sci. Technol. 2014, 15(2), 353–360.
- Pawul-Gruba, M.; Osek, J. Identification of Histamine in Fish and Fish Products in Poland During 2014-2018. J. Vet. Res. 2021, 65(4), 483–486. DOI: 10.2478/jvetres-2021-0066.
- Visciano, P.; Schirone, M.; Tofalo, R.; Suzzi, G. Histamine Poisoning and Control Measures in Fish and Fishery Products. Front. Microbiol. 2014, 5, 500. DOI: 10.3389/fmicb.2014.00500.
- Lovdal, T. The Microbiology of Cold Smoked Salmon. Food Control. 2015, 54, 360–373. DOI: 10.1016/j.foodcont.2015.02.025.
- Franco-Duarte, R.; Cernakova, L.; Kadam, S.; Kaushik, K. S.; Salehi, B.; Bevilacqua, A.; Corbo, M. R.; Antolak, H.; Dybka-Stepien, K.; Leszczewicz, M., et al. Advances in Chemical and Biological Methods to Identify Microorganisms-From Past to Present. Microorganisms. 2019, 7(5), 130. DOI: 10.3390/microorganisms7050130.
- Tito, N. B.; Rodemann, T.; Powell, S. M. Use of Near Infrared Spectroscopy to Predict Microbial Numbers on Atlantic Salmon. Food Microbiol. 2012, 32(2), 431–436. DOI: 10.1016/j.fm.2012.07.009.
- Fogarty, C.; Whyte, P.; Brunton, N.; Lyng, J.; Smyth, C.; Fagan, J.; Bolton, D. Spoilage Indicator Bacteria in Farmed Atlantic Salmon (Salmo Salar) Stored on Ice for 10 Days. Food Microbiol. 2019, 77, 38–42. DOI: 10.1016/j.fm.2018.08.001.
- Sveinsdottir, K.; Martinsdottir, E.; Hyldig, G.; Jorgensen, B.; Kristbergsson, K. Application of Quality Index Method (QIM) Scheme in Shelf-Life Study of Farmed Atlantic Salmon (Salmo Salar). J. Food Sci. 2002, 67(4), 1570–1579. DOI: 10.1111/j.1365-2621.2002.tb10324.x.
- Moretro, T.; Moen, B.; Heir, E.; Hansen, A. A.; Langsrud, S. Contamination of Salmon Fillets and Processing Plants with Spoilage Bacteria. Int. J. Food Microbiol. 2016, 237, 98–108. DOI: 10.1016/j.ijfoodmicro.2016.08.016.
- Miks-Krajnik, M.; Yoon, Y. J.; Ukuku, D. O.; Yuk, H. G. Volatile Chemical Spoilage Indexes of Raw Atlantic Salmon (Salmo Salar) Stored Under Aerobic Condition in Relation to Microbiological and Sensory Shelf Lives. Food Microbiol. 2016, 53(Pt B), 182–191. DOI: 10.1016/j.fm.2015.10.001.
- Kuuliala, L.; Sader, M.; Solimeo, A.; Perez-Fernandez, R.; Vanderroost, M.; De Baets, B.; De Meulenaer, B.; Ragaert, P.; Devlieghere, F. Spoilage Evaluation of Raw Atlantic Salmon (Salmo Salar) Stored Under Modified Atmospheres by Multivariate Statistics and Augmented Ordinal Regression. Int. J. Food Microbiol. 2019, 303, 46–57. DOI: 10.1016/j.ijfoodmicro.2019.04.011.
- Thompson, L. A.; Darwish, W. S. Environmental Chemical Contaminants in Food: Review of a Global Problem. Journal Of Toxicology. 2019, 2019, 1–14. DOI: 10.1155/2019/2345283.
- Foran, J. A.; Good, D. H.; Carpenter, D. O.; Hamilton, M. C.; Knuth, B. A.; Schwager, S. J. Quantitative Analysis of the Benefits and Risks of Consuming Farmed and Wild Salmon. J. Nutr. 2005, 135(11), 2639–2643. DOI: 10.1093/jn/135.11.2639.
- Easton, M. D. L.; Luszniak, D.; Von der Geest, E. Preliminary Examination of Contaminant Loadings in Farmed Salmon, Wild Salmon and Commercial Salmon Feed. Chemosphere. 2002, 46(7), 1053–1074. DOI: 10.1016/S0045-6535(01)00136-9.
- Friesen, E. N.; Ikonomou, M. G.; Higgs, D. A.; Ang, K. P.; Dubetz, C. Use of Terrestrial Based Lipids in Aquaculture Feeds and the Effects on Flesh Organohalogen and Fatty Acid Concentrations in Farmed Atlantic Salmon. Environ. Sci. Technol. 2008, 42(10), 3519–3523. DOI: 10.1021/es0714843.
- Berntssen, M. H.; Julshamn, K.; Lundebye, A. K. Chemical Contaminants in Aquafeeds and Atlantic Salmon (Salmo Salar) Following the Use of Traditional- versus Alternative Feed Ingredients. Chemosphere. 2010, 78(6), 637–646. DOI: 10.1016/j.chemosphere.2009.12.021.
- Looser, R.; Froescheis, O.; Cailliet, G. M.; Jarman, W. M.; Ballschmiter, K. The Deep-Sea as a Final Global Sink of Semivolatile Persistent Organic Pollutants? Part II: Organochlorine Pesticides in Surface and Deep-Sea Dwelling Fish of the North and South Atlantic and the Monterey Bay Canyon (California). Chemosphere. 2000, 40(6), 661–670. DOI: 10.1016/S0045-6535(99)00462-2.
- Froescheis, O.; Looser, R.; Cailliet, G. M.; Jarman, W. M.; Ballschmiter, K. The Deep-Sea as a Final Global Sink of Semivolatile Persistent Organic Pollutants? Part I: PCBs in Surface and Deep-Sea Dwelling Fish of the North and South Atlantic and the Monterey Bay Canyon (California). Chemosphere. 2000, 40(6), 651–660. DOI: 10.1016/S0045-6535(99)00461-0.
- Nostbakken, O. J.; Hove, H. T.; Duinker, A.; Lundebye, A. K.; Berntssen, M. H.; Hannisdal, R.; Lunestad, B. T.; Maage, A.; Madsen, L.; Torstensen, B. E., et al. Contaminant Levels in Norwegian Farmed Atlantic Salmon (Salmo Salar) in the 13-Year Period from 1999 to 2011. Environ. Int. 2015, 74, 274–280. DOI: 10.1016/j.envint.2014.10.008.
- Lundebye, A. K.; Lock, E. J.; Rasinger, J. D.; Nostbakken, O. J.; Hannisdal, R.; Karlsbakk, E.; Wennevik, V.; Madhun, A. S.; Madsen, L.; Graff, I. E., et al. Lower Levels of Persistent Organic Pollutants, Metals and the Marine Omega 3-Fatty Acid DHA in Farmed Compared to Wild Atlantic Salmon (Salmo Salar). Environ. Res. 2017, 155, 49–59. DOI: 10.1016/j.envres.2017.01.026.
- Foran, J. A.; Hites, R. A.; Carpenter, D. O.; Hamilton, M. C.; Mathews-Amos, A.; Schwager, S. J. A Survey of Metals in Tissues of Farmed Atlantic and Wild Pacific Salmon. Environ. Toxicol. Chem. 2004, 23(9), 2108–2110. DOI: 10.1897/04-72.
- Kelly, B. C.; Ikonomou, M. G.; Higgs, D. A.; Oakes, J.; Dubetz, C. Mercury and Other Trace Elements in Farmed and Wild Salmon from British Columbia, Canada. Environ. Toxicol. Chem. 2008, 27(6), 1361–1370. DOI: 10.1897/07-527.1.
- Jardine, L. B.; Burt, M. D. B.; Arp, P. A.; Diamond, A. W. Mercury Comparisons Between Farmed and Wild Atlantic Salmon (Salmo salarL.) and Atlantic Cod (Gadus morhuaL.). Aquacult. Res. 2009, 40(10), 1148–1159. DOI: 10.1111/j.1365-2109.2009.02211.x.
- Svendsen, T. C.; Vorkamp, K.; Ronsholdt, B.; Frier, J. O. Organochlorines and Polybrominated Diphenyl Ethers in Four Geographically Separated Populations of Atlantic Salmon (Salmo Salar). J. Environ. Monit. 2007, 9(11), 1213–1219. DOI: 10.1039/b707658d.
- Shaw, S. D.; Brenner, D.; Berger, M. L.; Carpenter, D. O.; Hong, C. S.; Kannan, K. PCBs, PCDD/Fs, and Organochlorine Pesticides in Farmed Atlantic Salmon from Maine, Eastern Canada, and Norway, and Wild Salmon from Alaska. Environ. Sci. Technol. 2006, 40(17), 5347–5354. DOI: 10.1021/es061006c.
- Shaw, S. D.; Berger, M. L.; Brenner, D.; Carpenter, D. O.; Tao, L.; Hong, C. S.; Kannan, K. Polybrominated Diphenyl Ethers (PBDEs) in Farmed and Wild Salmon Marketed in the Northeastern United States. Chemosphere. 2008, 71(8), 1422–1431. DOI: 10.1016/j.chemosphere.2008.01.030.
- Kelly, B. C.; Ikonomou, M. G.; Higgs, D. A.; Oakes, J.; Dubetz, C. Flesh Residue Concentrations of Organochlorine Pesticides in Farmed and Wild Salmon from British Columbia, Canada. Environ. Toxicol. Chem. 2011, 30(11), 2456–2464. DOI: 10.1002/etc.662.
- Morrison, W. R.; Smith, L. M. Preparation of Fatty Acid Methyl Esters and Dimethylacetals from Lipids with Boron Fluoride–Methanol. Journal Of Lipid Research. 1964, 5(4), 600–608. DOI: 10.1016/S0022-2275(20)40190-7.
- O’Fallon, J. V.; Busboom, J. R.; Nelson, M. L.; Gaskins, C. T. A Direct Method for Fatty Acid Methyl Ester Synthesis: Application to Wet Meat Tissues, Oils, and Feedstuffs. J. Anim. Sci. 2007, 85(6), 1511–1521. DOI: 10.2527/jas.2006-491.
- Indarti, E.; Majid, M. I. A.; Hashim, R.; Chong, A. Direct FAME Synthesis for Rapid Total Lipid Analysis from Fish Oil and Cod Liver Oil. J. Food Compost. Anal. 2005, 18(2–3), 161–170. DOI: 10.1016/j.jfca.2003.12.007.
- Shantha, N. C.; Napolitano, G. E. Gas Chromatography of Fatty Acids. J. Chromatography. A. 1992, 624(1–2), 37–51. DOI: 10.1016/0021-9673(92)85673-H.
- Seppänen-Laakso, T.; Laakso, I.; Hiltunen, R. Analysis of Fatty Acids by Gas Chromatography, and Its Relevance to Research on Health and Nutrition. Analytica Chimica Acta. 2002, 465(1–2), 39–62. DOI: 10.1016/S0003-2670(02)00397-5.
- Wold, J. P.; Marquardt, B. J.; Dable, B. K.; Robb, D.; Hatlen, B. Rapid Quantification of Carotenoids and Fat in Atlantic Salmon (Salmo Salar L.) by Raman Spectroscopy and Chemometrics. Applied Spectroscopy. 2004, 58(4), 395–403. DOI: 10.1366/000370204773580220.
- Afseth, N. K.; Wold, J. P.; Segtnan, V. H. The Potential of Raman Spectroscopy for Characterisation of the Fatty Acid Unsaturation of Salmon. Analytica Chimica Acta. 2006, 572(1), 85–92. DOI: 10.1016/j.aca.2006.05.013.
- Afseth, N. K.; Bloomfield, M.; Wold, J. P.; Matousek, P. A Novel Approach for Subsurface Through-Skin Analysis of Salmon Using Spatially Offset Raman Spectroscopy (SORS). Applied Spectroscopy. Applied Spectroscopy. 2014, 68(2), 255–262. DOI: 10.1366/13-07215.
- Landry, J. D.; Torley, P. J.; Blanch, E. W. Quantitation of Carotenoids and Fatty Acids from Atlantic Salmon Using a Portable Raman Device. Analyst. 2022, 147(19), 4379–4388. DOI: 10.1039/D2AN01140A.
- Bekhit, M. Y.; Grung, B.; Mjos, S. A. Determination of Omega-3 Fatty Acids in Fish Oil Supplements Using Vibrational Spectroscopy and Chemometric Methods. Applied Spectroscopy. 2014, 68(10), 1190–1200. DOI: 10.1366/13-07210.
- Cascant, M. M.; Breil, C.; Fabiano-Tixier, A. S.; Chemat, F.; Garrigues, S.; de la Guardia, M. Determination of Fatty Acids and Lipid Classes in Salmon Oil by Near Infrared Spectroscopy. Food Chem. 2018, 239, 865–871. DOI: 10.1016/j.foodchem.2017.06.158.
- Prado, E.; Eklouh-Molinier, C.; Enez, F.; Causeur, D.; Blay, C.; Dupont-Nivet, M.; Labbe, L.; Petit, V.; Moreac, A.; Taupier, G., et al. Prediction of Fatty Acids Composition in the Rainbow Trout Oncorhynchus Mykiss by Using Raman Micro-Spectroscopy. Analytica Chimica Acta. 2022, 1191, 339212. DOI: 10.1016/j.aca.2021.339212.
- Amorim-Carrilho, K. T.; Cepeda, A.; Fente, C.; Regal, P. Review of Methods for Analysis of Carotenoids. TrAc Trends Anal. Chem. 2014, 56, 49–73. DOI: 10.1016/j.trac.2013.12.011.
- Saini, R. K.; Keum, Y. S. Carotenoid Extraction Methods: A Review of Recent Developments. Food Chem. 2018, 240, 90–103. DOI: 10.1016/j.foodchem.2017.07.099.
- Rasmussen, H. M.; Muzhingi, T.; Eggert, E. M. R.; Johnson, E. J. Lutein, Zeaxanthin, Meso-Zeaxanthin Content in Egg Yolk and Their Absence in Fish and Seafood. J. Food Compost. Anal. 2012, 27(2), 139–144. DOI: 10.1016/j.jfca.2012.04.009.
- Moretti, V. M.; Mentasti, T.; Bellagamba, F.; Luzzana, U.; Caprino, F.; Turchini, G. M.; Giani, I.; Valfre, F. Determination of Astaxanthin Stereoisomers and Colour Attributes in Flesh of Rainbow Trout (Oncorhynchus Mykiss) as a Tool to Distinguish the Dietary Pigmentation Source. Food Addit. Contam. 2006, 23(11), 1056–1063. DOI: 10.1080/02652030600838399.
- Rufer, C. E.; Moeseneder, J.; Briviba, K.; Rechkemmer, G.; Bub, A. Bioavailability of Astaxanthin Stereoisomers from Wild (Oncorhynchus Spp.) and Aquacultured (Salmo Salar) Salmon in Healthy Men: A Randomised, Double-Blind Study. Br. J. Nutr. 2008, 99(5), 1048–1054. DOI: 10.1017/S0007114507845521.
- Rivera, S. M.; Christou, P.; Canela-Garayoa, R. Identification of Carotenoids Using Mass Spectrometry. Mass Spectrom. Rev. 2014, 33(5), 353–372. DOI: 10.1002/mas.21390.
- Pathare, P. B.; Opara, U. L.; Al-Said, F. A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess. Technol. 2012, 6(1), 36–60. DOI: 10.1007/s11947-012-0867-9.
- Christiansen, R.; Struksnæs, G.; Estermann, R.; Torrissen, O. J. Asssessment of Flesh Colour in Atlantic Salmon. Salmo salar L. Aquac. Res. 1995, 26(5), 311–321. DOI: 10.1111/j.1365-2109.1995.tb00919.x.
- Skrede, G.; Risvik, E.; Huber, M.; Enersen, G.; Blümlein, L. Developing a Color Card for Raw Flesh of Astaxanthin-Fed Salmon. J. Food Sci. 1990, 55(2), 361–363. DOI: 10.1111/j.1365-2621.1990.tb06763.x.
- Misimi, E.; Mathiassen, J. R.; Erikson, U. Computer Vision-Based Sorting of Atlantic Salmon (Salmo Salar) Fillets According to Their Color Level. J. Food Sci. 2007, 72(1), S030–5. DOI: 10.1111/j.1750-3841.2006.00241.x.
- Quevedo, R. A.; Aguilera, J. M.; Pedreschi, F. Color of Salmon Fillets by Computer Vision and Sensory Panel. Food Bioprocess. Technol. 2008, 3(5), 637–643. DOI: 10.1007/s11947-008-0106-6.
- Ermakov, I. V.; Kollias, N.; Ermakova, M. R.; Zeng, H.; Choi, B.; Gellermann, W.; Malek, R. S.; Wong, B. J.; Ilgner, J. F. R.; Trowers, E. A., 2006 Photonic Therapeutics and Diagnostics II, SPIE BiOS San Jose, California, United States, 22 February 2006 doi:10.1117/12.644783.
- Hikima, J. I.; Ando, M.; Hamaguchi, H. O.; Sakai, M.; Maita, M.; Yazawa, K.; Takeyama, H.; Aoki, T. On-Site Direct Detection of Astaxanthin from Salmon Fillet Using Raman Spectroscopy. Mar. Biotechnol. 2017, 19(2), 157–163. DOI: 10.1007/s10126-017-9739-7.