Abstract
Modified sweeteners were formulated from mixtures of Black plum syrup and phosphorylated corn starch concentrations of 20, 30, and 40% (w/w). Each mixture was coated on an aluminium plate at 1 mm thickness, dehydrated at 60°, 70°, 80° and 90°C, and evaluated for drying and moisture sorption characteristics. Results showed that the mixtures exhibited falling rate drying characteristics. The rate of dehydration increased in the order 20% > 30% > 40% starch at constant temperature. The incremental choice of 10% starch and drying temperature except at 70° and 80°C had significant effect (p < 0.05) on drying rate. The moisture isotherm curves of the pre-dried mixtures were sigmoid in shape. Their monolayer values or upper critical moisture contents necessary for shelf-stability were significantly different from each other for the samples pre-dried at 60°C. The effect of drying temperatures on equilibrium moisture content was only significant at 90°C (p < 0.05).
INTRODUCTION
Black plum (Vitex doniana) is a tropical tree found in the savannah regions of West Africa, particularly around Kaduna, Kano and Sokoto in Nigeria (Dawtrey, 1978; Keay et al., 1964; Egbekun et al., 1996). The pulp of the drupeceous fruit is sweet and has been utilised in the manufacture of some food products (Abu, 1999; Egbekun et al., 1996). The young tender leaves is used as vegetable while the bark and ash derived from the stem are used as dyes and for soap-making respectively. Syrup from Black plum fruit has potential uses in the food industry as nutritive sweetener in the manufacture of products such as pancakes and bread. However, the syrup like liquid honey is difficult to use. It adheres to containers due to its sticky viscous consistency. This causes substantial losses and inaccuracy of measurements. To overcome handling problems, conversion of the syrup to powder by drying with the aid of starch as a binder could be a feasible alternative.
Vacuum-dried whole fruit and instant fruit juice powders produced from apple, raspberry, black-current and grape fruit using malto-dextrine or starch syrup as a carrier were reported by Lazzio (1992).
Air oven dried honey-starch mixtures with improved handling properties was reported by Yener et al. (1987).
Some basic drying parameters such as drying rates and moisture sorption properties are required to design suitable drier. Knowledge of sorption characteristics is needed for shelf life-critical moisture and water activity predictions in food products that deteriorate mainly by moisture gain (Kats and Labuza, 1981). In this paper, drying rates and moisture adsorption isotherms of Black plum (Vitex doniana) syrup, and syrup-starch mixtures dried at different temperatures were evaluated.
MATERIALS AND METHODS
Drying Experiments
The Black plum syrup and phosphorylated corn starch were produced according to the methods described by Abu (1999). This Black plum syrup contained 25.0% moisture, 51.7% reducing sugars, 12.9% sucrose, 3.8% ask, 2.5% protein and 0.55% fat while the phosphorylated corn starch had a moisture content of 8.5%. Drying experiments were performed according to the modified method of Yener et al. (1987). Black plum syrup and phosphorylated starch were mixed in proper ratio to make homogenous mixtures of 20, 30, and 40% starch. These formulations were uniformly coated on weighed aluminium plates (35 cm × 15 cm, 0.5 mm thick) with the aid of TLC spreader (Shandon, Hungary). The coatings were uniformly maintained at 1.0 mm thick. A laboratory oven (LMIM, Hungary) with internal dimensions of 35 cm × 27 cm × 25 cm was employed for drying samples at 60°, 70°, 80°, and 90°C. Samples data were obtained by weighing the plates with a digital balance (Mettler pc 400, sensitivity 0.01 g) at 10 minutes intervals until constant weights were obtained. Dried samples were scraped from the coated plates with stainless knife, blended and packed in glass bottles. Drying were replicated twice and samples obtained were mixed.
Adsorption Isotherms
The moisture adsorption isotherms were determined gravimetrically by placing the powdered samples in desiccators previously saturated with vapour pressure of molal concentration of sulphuric acid (Raganna, 1977) solutions. Triplicate samples of each mixtures, syrup, and starch were placed inside the desiccators at an ambient temperature of 30° + 20°C. Equilibrium moisture content were determined over water activity (aw) range of 0.11 to 0.97; the time required for the samples to reach equilibrium varied over two weeks. The sorption isotherms were obtained by plotting the moisture content of the samples, expressed as kg moisture/kg of dry matter (DM) versus aw. The equilibrium moisture data were fitted using Branauer-Emmet-Teller (BET) and Henderson equations as described by Arogba (1999a,b).
RESULTS AND DISCUSSION
Drying Rates
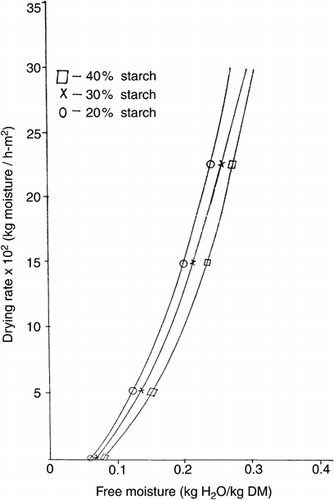
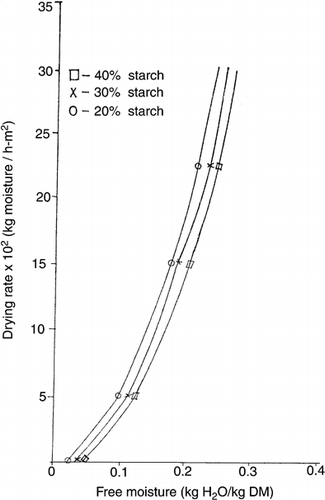
The rate of dehydration of 1 mm film thick of Black plum syrup-starch mixtures at 60° and 90°C are shown in Figures & . The shapes of the curves were similar at all drying temperatures (70° & 80°C not shown). In all cases, only falling rate drying period could be observed. The low initial moisture contents of the un-dried mixtures ranged between 19.6–23.5% wet basis. Consequently, only falling rate phase, which usually characterizes later stages of drying in foods could be supported. This phase is very common in food materials such as starch and gum where water is associated by hydrogen bonding and liquid diffusion mechanism predominate (karathanos and Belessiotis, 1997; Geankoplis, 1993). Similar pattern of drying rate curve was observed in dehydration of honey-starch mixtures (Yener et al., 1987).
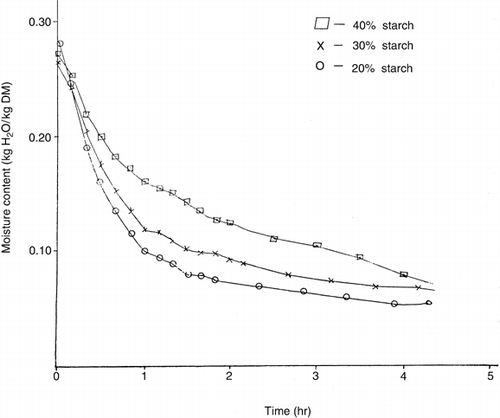
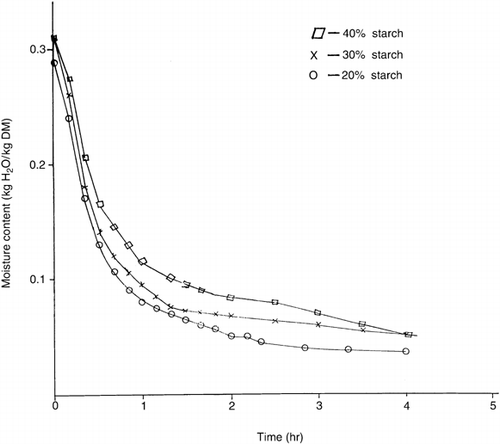
At a given drying rate (kg moisture/h-m2), the moisture content (kg H20/kg dry matter) of syrup-starch mixtures decreased from samples containing 20% starch to 40% starch. However, the gradients (not shown) of the drying rate curves over the range 5 to 25 × 102 kg moisture/h-m2, for all samples were not significantly (p < 0.05) different. This implies that the rate of dehydration of the mixtures increased in the order 20% > 30% > 40% > starch at constant temperature. Probably, the water molecules in the syrup-starch mixtures were bound to the hydroxyl groups of starch by hydrogen bonding. Slower breaking of this bond in the mixtures with high starch contents could explain the decreased rate of dehydration. The drying curves of syrup-starch mixtures obtained by plotting moisture contents (on drying basis) versus time (hr) are shown in Figures. and for temperatures of 60° and 90°C respectively. It is also apparent from the similar curves (that for 70° and 80°C not shown) that the drying of syrup-starch mixture is faster for 20% starch than 30% or 40% starch at constant temperature. The percentage of initial moisture lost during 2 hrs-about half the total drying period, shown in Table were 74.16% and 54.97% for samples containing 20% and 40% starch respectively at 60°C, 79.87% and 67.97% at 70°C, 80.83% and 68.53% at 80°C, and 83.17% and 73.39% at 90°C. Statistical analysis of the data thereof by the least significant difference test showed that the drying rates of all the samples were significantly different (p < 0.05) and greater for 20% starch than 30% or 40% starch at constant temperature. Thus the incremental choice of 10% starch had significant effect on the drying rate. At a given starch content only 70° and 80°C drying temperatures were not significantly different from each another in drying rate.
Table 1. Percentage Moisture Lost During 2 hr Drying of Black Plum Syrup-Starch Mixtures at Different Temperatures
Moisture Adsorption Isotherm
The isotherm curves of the Black plum syrup, the phosphorylated corn starch used as a binder, and the starch-syrup mixtures are shown in Figure . The starch-syrup samples were pre-dried at 60°C, and the isotherm studies were conducted later at the ambient temperature of 30 + 2°C. All isotherm curves obtained were sigmoid in shape.
Figure 5. Effect of composition on moisture sorption isotherms of Black plum syrup-starch mixtures pre-dried at 60°C.
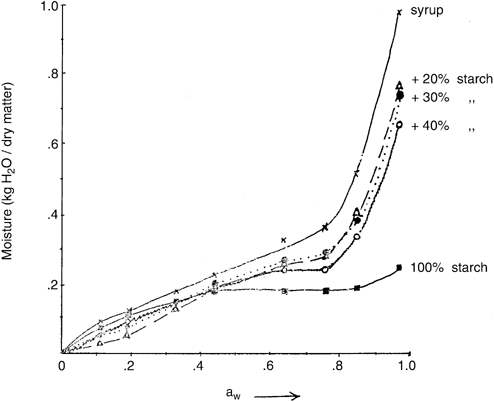
The syrup had the highest equilibrium moisture content (EMC) at all the water activity (aw) values studied compared with those of the starch, and the mixtures. The EMC of the syrup increased steadily with increasing aw up to about aw 0.76, and there-after increased sharply to 0.98 kg H20/kg dry matter content at aw 0.97. The isotherms indicate that the syrup was hygroscopic. Furthermore, the sharp rise in EMC at aw 0.76 for the syrup and the dried starch syrup mixtures signifies a higher rate of water re-absorption by the amorphous sugars. The reducing sugars, and sucrose contents of the syrup, reported elsewhere (Abu, 1999), are 51.7% and 12.9% (wet weight basis) respectively. The Black plum syrup appeared to have similar moisture adsorption behaviour with Jerusalem artichoke flour containing significantly more fructose than glucose (Mazza, 1984). The inference coupled with the negative correlation observed on the syrup with 40% starch is of practical significance. It implies that the use of 30% starch base is a compromise between economic gain and attainment of stable shelf-life of a powdery syrup-starch product. The economic gain could be viewed in terms of cost of starch needed, and the quantity of heat to be applied in drying the mixture.
The moisture adsorption isotherm data were fitted into BET, and Henderson equations. The characteristics of the syrup, syrup-starch mixtures, and the reference starch base were computed and presented in Table . The mean relative deviation (%E) between the experimental and predicted moisture values varied between 2.0 and 8.0 using both models. As with Peleg, and GAB equations (not analysed), %E of up to 7.0 was considered a good description of an isotherm (Palou et al., 1997). The isotherm of 100% syrup sample was best predicted by the Henderson model with %E of 2.1.
Table 2. Parameter Values of the BET and Henderson Models to Describe the Isotherm of Starch-Black Plum Syrup Mixture Previously Dried at 60 °C
The BET, and Henderson models have the practical significance of identifying critical lower monolayer and upper moisture content for attainment of prolonged shelf-life of a product (Arogba, 1999b). The choice of the syrup with 30% starch for practical application is further justified by the statistical relationships between the syrup with 40% starch, as well as the monlayer (Mo) values of the syrup with 30% starch and 100% syrup (Table ).
Except for the inexplicable and anomalous characteristics of the syrup with 20% starch recorded, the monolayer values of the other samples studied ranged from 11.6% to 14.4% (Table ). This range is within the expected range of 3.2% to 16.0% for starchy foods (Palou et al., 1997).
The aw values in Table were extrapolated from Figure using the calculated monolayer values from BET equation and the moisture values from Henderson equation. Statistically, it was inferred that moisture values were more sensitive and serve as better indicators of shelf-stability than aw values.
The constants K1, N1 and K2, N2 described by the Henderson equation define two intersecting curves that are usually identified with several food systems such as Bologna (Igbeka and Blaisdell, 1982), freeze-dried banana (Lima & Cal-Vidal, 1983), Garcinia kola and Cola nitida kernels (Arogba, 1999b), dried Locust bean and soyabean condiments (Arogba, 1999c,d). In this study, only the 100% starch sample conformed while the syrup with 30% starch, and 100% syrup samples showed singular curves (Table ). Consequently, this features could assist to identify extents of adulteration of the Black plum syrup or the phosphorylated corn starch, from consumer perspective.
CONCLUSION
The study has indicated possible competitive preference for the 100% Black plum syrup because of its longer shelf-stability or the powdery syrup with 30% starch for the convenience in packaging and transportation. Furthermore, the level of added starch in a syrup-starch mixture has more influence on the shelf-stability of the dried product than drying temperatures of less than 90°C.
ACKNOWLEDGEMENT
One of the authors, J. D. Abu is grateful to the Federal Polytechnic, Idah, Nigeria for the research grant awarded during the post-graduate programme.
REFERENCES
- Abu , J. D. 1999 . Development of a Sweetener from Black Plum (Vitex doniana) Fruit . International Journal of Food Properties , (submitted)
- Arogba , S. S. in press . (1999a). Studies on Kolanut and Cashew Kernel: Moisture Adsorption Isotherm, Proximate Composition, and Functional Properties . Food Chemistry ,
- Arogba , S. S. in press . (1999b). Comparative Analyses of the Moisture Isotherms, Proximate Compositions, Physical and Functional Properties of Dried cola nitida and Garcinia Kola kernels . J. Food Comp. Anal. ,
- Arogba , S. S. (1999c). Shelf Characteristics of African Locust Bean (Parkis filicoidea) (unpublished)
- Arogba , S. S. (1999d). Moisture Adsorption Behaviour and Chemical Properties of Stored Soyabean (Glycine max) Condiment Powders (unpublished)
- Dawtrey , B. 1978 . Some Free Bearing Edible Fruits and Their Igala Names . Elaeis. J. Igala-land , 1 ( 2 ) : 19
- Egbekun , M. K. , Akowe , J. I. and James , R. E. 1996 . Physico-Chemical and Sensory Properties of Formulated Syrup from Black Plum (Vitex doniana) Fruit . Plants Food Hum. Nutr. , 49 : 301 – 306 .
- Geankoplis , C. J. 1993 . Transport Processes and Unit Operations , 3rd Ed. Englewood Cliffs, N.J. : Prentice Hall .
- Igbeka , J. C. and Blaisdell , J. L. 1982 . Moisture Isotherms of a Processed Meat Product – Bologna . J. Food Technol. , 17 ( 1 ) : 37 – 46 .
- Karathanos , V. T. and Belessiotis , V. G. 1997 . Sun and Artificial Air Drying Kinetics of Some Agricultural Products . J. Food Engr. , 31 : 47 – 57 .
- Katz , E. E. and Labuza , J. P. 1981 . Effect of Water Activity on Sensory Crispness and Mechanical Deformation of Snack Food Products . J. Food Sci. , 46 : 403 – 409 .
- Keay , R. W.J. , Onochie , C. F.A. and Standiold , D. E. 1964 . Nigerian Trees Vol. II , Ibadan : Federal Department of Forestry Research .
- Laszlo , P. 1992 . Production of Whole Fruit Juice Powders . Food Technol. , 50 : 141 – 143 .
- Lima , A. W.O. and Cal-Vidal , J. 1983 . Hydroscopic Behaviour of Freeze Dried Bananas . J. Food Technol. , 18 : 687 – 696 .
- Mazza , G. 1982 . Moisture Sorption Isotherms of Potato Slices . J. Food Technol. , 17 : 47 – 50 .
- Mazza , G. 1984 . Sorption Isotherm and Drying Rates of Jerusalem Artichoke (Helianthus tuberosus L.) . J. Food Sci. , 49 : 384 – 388 .
- Palou , E , Lopez-Malo , A. and Argaiz , A. 1997 . Effect of Temperature on Moisture Sorption Isotherms of Some Cookies and Snacks . J. Food Eng. , 31 : 85 – 94 .
- Raganna , S. 1997 . Manual of Analysis of Fruit and Vegetable Products New Delhi : Tat McGraw-Hill .
- Yener , E. , Ungan , S. and Ozilgen , M. 1987 . Drying Behaviour of Hone-Starch Mixtures . J. Food Sci. , 52 ( 4 ) : 1054 – 1058 .