ABSTRACT
Mass transfer during osmotic dehydration of papaya in sucrose solution has been studied. A mathematical model based on Fick's law for unsteady state mass transfer, considering shrinkage, has been used to calculate the moisture and sugars diffusion coefficients. Also, a diffusional model with simultaneous shrinkage was developed using a finite difference method to solve differential equations, assuming moisture content-dependent diffusivities for moisture and sugars. The validity of both proposed models was verified by the good fit between the experimental and calculated curves. The mean percent relative errors were 2.48 and 9.92 for moisture content, and 4.26 and 22.51 for total sugars content, for Model 1 and Model 2, respectively.
INTRODUCTION
Papaya (Carica Papaya L.) is a widely acclaimed temperate zone fruit with commercial production today in Hawaii, tropical Africa, Asia and Australia. There is a widespread but smaller scale production in South Africa and Latin America.Citation[1] One of the main reasons for low production and commercialisation of tropical fruits products like papaya, is the lack of technical processes and adequate equipments for their preservation and transformation into good quality and attractive products.Citation[2]
Osmotic dehydration is a process leading to “intermediate” products. Further processing (i.e., drying, pasteurisation or freezing) are an interesting way of valuation for papaya.Citation[3]
This technique is based on placing foods, such as pieces of fruits or"/>in a hypertonic solution. The solution has a high osmotic pressure, and hence, lowers water activity (aw); so the driving force for water removal is the activity gradient between the osmotic solution and the food, with natural cells acting as a semi-permeable membrane. During this process there is an important flow of water from the inside of the food to the solution, and also solute enters from the solution into the product. Simultaneously, there is some loss of product's solutes affecting its organoleptic properties; but this loss is quantitatively negligible.Citation4-6
Most of the models for mass transfer are either based on the mathematic solutions of Fick's second law for several shapes and boundary conditions (assuming a simple transfer of water), or empirical.Citation7-9 In addition, most of the published models do not often consider the volume reduction and the variation of the diffusion coefficients with moisture content.
The aim of this research was to study the osmotic dehydration of papaya in saturated sucrose solutions and to model the mass transfer applying Fick's model to obtain the diffusion coefficients for water and sugars. Also a finite difference method was applied considering both diffusion coefficients as a function of moisture content.
MATERIALS AND METHODS
Materials
Fresh, ripe papaya fruits obtained from the same plantation were used for processing. The fruits were selected according to the total area of yellow-orange peel colour percentage.Citation[3]
Papayas with 80% yellow-orange peel coloration were hand-peeled (seeds were removed) and cut into slabs (5×3×1 cm). The slabs were then covered with a plastic material on to the major faces, so the diffusion took place only at the 3×1 faces. Saturated solutions were prepared using commercial sucrose, and the concentration was monitored in each experiment. The solution:fruit volume ratio was 10:1 to avoid a medium dilution. Sodium benzoate at a concentration of 700 ppm was added to avoid microbial growth.
Osmotic Dehydration
Samples were weighed and then immersed in the osmotic medium for different time periods (0, 2, 6, 9 and 15 h) at room temperature. Fluid movement was achieved using a metallic stirrer at 60 rpm. The slabs were removed from the solution, wiped with tissue paper, and ten cuts of 5 mm each were then made. Of these, two sets of five were considered. Cuts 1 to 5 were used for moisture content determination and cuts 6 to 10 were taken for sugar content determination.
Shrinkage
Shrinkage coefficient is defined as the relation between the volume at any time and the initial volume.Citation[10] Assuming unidirectional diffusion, shrinkage coefficient was considered as the relation between the larger length at time t and the initial one of the slab. At any time, the length of the slabs was measured before and after each treatment, to obtain a correlation with moisture content. The other dimensions were not considered (they were covered with plastic material) because one direction mass transfer was assumed.
Moisture and Sugar Content
Moisture content of the samples was determined by the gravimetric method in a vacuum oven at 70±1°C for 6 h.Citation[11] Approximately a 2.5 g sample was blended with the same weight of water and placed in a 50 mL centrifuge tube. Then, 30 mL of a heated mixture 80% ethanol and 20% water was added and centrifuged for 15 min at 2500 rpm. Supernatant was decanted and retained, and the extraction process was repeated three times. Resulting supernatants were brought to a certain volume with the ethanol-water mixture and held for analysis by HPLC. A Shimadzu LC-6A liquid chromatograph equipped with Shimadzu SCL–6A controller and a Komla KWK-024–751 differential refraction index detector, was used for the determination of sucrose, fructose and glucose. The mobile phase was aceto nitrile-water (85:15) and the flow rate was 2 mL/min. Also, a NH2 Phenomenex IB-SIL5NH2 column (4.6×250 mm) with a 5 µm particle size was used. All chromatograms were recorded with a C-R3A integrator.Citation12-14
Mathematical Models
Model 1
The first approximation for the mass transfer was based on Fick's second law of unsteady state diffusion.Citation[15] The following assumptions were made: mass transfers take place only in one direction; in the interphase the fruit and the solution are in equilibrium; initial moisture and sugars concentrations are uniform; and shrinkage is linear dependent on moisture content and calculated with Eq. Equation6
Water and sugars diffusivities were calculated using the analytical solution for a slab, in terms of an infinite series given by:Citation[16]
Model 2
Our second mathematical model considered that mass transfer could be calculated in terms of Fick's law and the microscopic mass balance. The differential Equation Equation2 was solved using a finite difference method and the assumptions mentioned earlier.
A computer program was written to estimate the transient moisture and sugar contents at each experimental run. Both diffusivities were considered dependent on moisture content according to Eqs. Equation3 and Equation4:
RESULTS AND DISCUSSION
Shrinkage data were analysed using linear regression and fitted with Eq. Equation6,
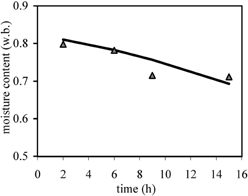
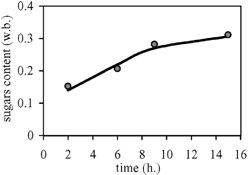
The diffusivity coefficients found using Eq. Equation1 (Fick's model) were 1.3×10−9 m2/s and 3.47×10−9 m2/s for moisture and sugars, respectively. E values obtained indicate the adequacy of the model for reasonably describing the osmotic dehydration of papaya in these experimental conditions. Also, DW and DA obtained are similar to those found for other fruits, in sucrose and with other conditions.Citation18-19
Table shows the moisture contents distribution inside the papayas, as a function of position and time, compared to those values predicted with the finite difference model, involving an average E of 9.92. Table shows the experimental concentration profile of total sugars (fructose, glucose and sucrose) inside the papayas, compared to the calculated values, with average E=22.51. Parameters DW0, DA0, BW and BA of Eqs. Equation3 and Equation4 were found from the fitting of the data, giving:
Table 1. Experimental and Predicted Moisture Contents as a Function of Time and Distance Within the Fruit During Osmotic Dehydration (Model 2) DW=9.3×10−10(1+0.29xw)(m2/s)
Table 2. Experimental and Predicted Sugars Contents as a Function of Time and Distance Within the Fruit During Osmotic Dehydration (Model 2) DA=4.3×10−9(1+0.20xw)(m2/s)
Table 3. Values of Moisture and Total Sugars Diffusion Coefficients (m2/s) for Model 1 and Model 2
CONCLUSIONS
The Fickian unsteady state diffusion model, considering a linear variation of the length of a slab with moisture content, was found to fit well for the osmotic dehydration of papaya. Also, a model that considers the variation of the moisture and sugars diffusion coefficients with the local moisture content was developed. So, the moisture and sugars contents during osmotic dehydration could be predicted with enough accuracy from the equations. These predictions are useful for the application of this process to obtain a good quality fruit product.
NOMENCLATURE
| BA | = |
constant |
| BW | = |
constant |
| DA | = |
sugars diffusivity (m2/s) |
| DA0 | = |
constant |
| DW | = |
moisture diffusivity (m2/s) |
| DW0 | = |
constant |
| i | = |
node number |
| L′ | = |
half thickness of the slab at time t=L/2 (cm) |
| L | = |
length of the slab at time t (cm) |
| L0 | = |
initial length of the slab (cm) |
| M | = |
average moisture content (% dry basis) |
| t | = |
time (min) |
| xa | = |
sugars average content of the slab at time t (wet basis) |
| xa0 | = |
sugars initial content of the slab (wet basis) |
| xae | = |
sugars equilibrium content (wet basis) |
| xexp | = |
experimental moisture content (wet basis) |
| xfit | = |
moisture content calculated with the model (wet basis) |
| xw | = |
moisture average content of the slab at time t (wet basis) |
| xw(i,t) | = |
moisture content at node i at time t (wet basis) |
| xw0 | = |
moisture initial content of the slab at time t (wet basis) |
| xwe | = |
moisture equilibrium content (wet basis) |
| z | = |
variable distance in the diffusion direction (cm) |
Acknowledgments
REFERENCES
- Morton, J.F. Fruits of Warm Climates in www.newcrop.hort.purdue.edu (accessed May, 1999)
- Levi , A. , Gagel , S. and Juven , B. 1983 . Intermediate Moisture Tropical Fruits Products for Developing Countries I: Technological Data on Papaya . Journal of Food Technology , 18 : 667 – 685 .
- Heng , K. , Gilbert , S. and Cuq , J.L. 1990 . Osmotic Dehydration of Papaya: Influence of Process Variables on the Product Quality . Sciences des Aliments , 10 : 831 – 848 .
- Salvatori , D. and Alzamora , S.M. 2000 . Structural Changes and Mass Transfer During Glucose Diffusion of Apples as Affected by Blanching and Process Variables . Drying Technology , 18 ( 1&2 ) : 361 – 382 .
- Lenart , A. 1996 . Osmo-Convective Drying of Fruits and Vegetables: Technology and Applications . Drying Technology , 14 ( 2 ) : 391 – 413 .
- Lerici , C.R. , Pinnavaia , G. , Dalla Rosa , M. and Bartolucci , L. 1985 . Osmotic Dehydration of Fruit: Influence of Osmotic Agents on Drying Behaviour and Product Quality . Journal of Food Science , 50 ( 4 ) : 1217 – 1219 .
- Rastogi , N.K. and Niranjan , K. 1998 . Enhanced Mass Transfer During Osmotic Dehydration of High-Pressure Treated Pineapple . Journal of Food Science , 63 ( 3 ) : 508 – 511 .
- Waliszewski , K.N. , Texon , N.I. , Salgado , M.A. and García , M.A . 1997 . Mass Transfer in Banana Chips During Osmotic Dehydration . Drying Technology , 15 ( 10 ) : 2597 – 2607 .
- Biswal , R.N. , Bozorgmehr , K. , Thompkins , F.D. and Liu , X. 1991 . Osmotic Concentration of Green Beans Prior to Freezing . Journal of Food Science , 56 ( 4 ) : 1008 – 1012 .
- Ramallo , L.A. , Pokolenko , J.J. , Balmaceda , G.Z. and Schmalko , M.E. 2001 . Moisture Diffusivity, Shrinkage, and Apparent Density Variation During Drying of Leaves at High Temperatures . International Journal of Food Properties , 4 ( 1 ) : 163 – 170 .
- Cunnif , Patricia , ed. 1996 . “ AOAC INTERNATIONAL, Fruits & Fruit Products ” . In Official Methods of Analysis , 16 Vol. 2, 4 , Washington D.C., , USA : AOAC INTERNATIONAL .
- Giangiacomo , R. , Torregiani , D. and Abbo , E. 1987 . Osmotic Dehydration: Part 1. Sugars Exchange Between Fruit and Extracting Syrups . Journal of Processing and Preservation , : 183 – 195 .
- Zhu , S. , Mount , J.R. and Collins , J.L. 1992 . Sugar and Soluble Solids Changes in Refrigerated Sweet Corn (Zea mays L) . Journal of Food Science , 57 ( 2 ) : 454 – 457 .
- Cortes Sanchez Mata , M. , Peñuela-Teruel , M.J. , Cámara-Hurtado , M. , Díez-Marque´s , C. and Torija-Isasa , M.E. 1998 . Determination of Mono-, Di-, and Oligosaccharides in Legumes by High-Performance Liquid Chromatography Using an Amino-Bonded Silica Column. . Journal of Agricultural and Food Chemistry , 46 ( 9 ) : 3648 – 3652 .
- Nsonzi , F. and Ramaswamy , H.S. 1998 . Osmotic Dehydration Kinetics of Blueberries . Drying Technology , 16 ( 3–5 ) : 725 – 741 .
- Crank , J. 1975 . Mathematics of Diffusion , 2 24 – 25 . Oxford : Clarendon Press .
- Simal , S. , Mulet , A. , Cañellas , J. and Roselló , C. 1996 . Moving Boundary Model for Simulating Moisture Movement in Grapes . Journal of Food Science , 61 ( 1 ) : 157 – 160 .
- Lazarides , H.N. , Gekas , V. and Mavroudis , N. 1997 . Apparent Mass Diffusivities in Fruit and Vegetable Tissues Undergoing Osmotic Processing . Journal of Food Engineering , 31 : 315 – 324 .
- Rastogi , N.K. , Raghavarao , K.S.M.S. and Niranjan , K. 1997 . Mass Transfer During Osmotic Dehydration of Banana: Fickian Diffusion in Cylindrical Configuration . Journal of Food Engineering , 31 : 423 – 432 .