Abstract
Bacteriocins are antimicrobial peptides acting on certain pathogens and spoilage microorganisms. They have recently been considered as natural alternative to food preservatives. The objective of this work was to study the inhibition spectrum of a bacteriocin produced by Lactobacillus buchneri, isolated from a Mexican sausage, against Listeria monocytogenes and several lactic acid bacteria, and the influence of several extrinsic parameters on bacteriocin activity. Culture media (MRS or APT) had no significant effect on bacteriocin production (p < 0.060) although higher activity was observed in MRS (220 AU/mL). Conversely, temperature significant affected production (p > 0.001). Bacteriocin activity was also increased by 100% nitrogen atmosphere (180 AU/mL) as compared to N2/CO2 (50:50) (150 AU/mL) and 100% CO2 (60 AU/mL). Maximum activity occurred at the end of the exponential growth phase. This bacteriocin inhibited some Listeria monocytogens strains and several lactic acid bacteria, mainly Lactobacillus sp.
Introduction
Lactic acid bacteria (LAB) have been traditionally used in fermented foods due to their preservative effect.Citation[1] Their main metabolites are lactic acid, diacetyl, acetaldehyde, and other antimicrobial compounds such as bacteriocins. These compounds have been described as antagonistic peptides against Gram positive bacteria genetically related to the producing strain.Citation[2] Citation[3] However, some bacteriocins are active against pathogens such as Bacillus cereus, Clostridium perfringens, Listeria monocytogenes, and Staphylococcus aureus. Citation[4]
These characteristics, together with extrinsic factors affecting antimicrobial activity and stability, determine their efficiency as possible food preservatives. Several authors reported the use of bacteriocins as microbial inhibitors in muscle foods, inoculating bacteriocin-producing strains in the meat substrate. Cutter and SiragusaCitation[5] studied bacteriocins as beef preservatives whereas Listeria sp. inhibition by sakacine in beef was reported by Schillinger et al.Citation[6] and Hugas et al.,Citation[7] and by pediocin in sausages by Foegeding et al.Citation[8]
Bacteriocins can also be produced in vitro and added as bacterial inhibitors to meat substrates.Citation[9] In vitro production parameters have been reported by several authorsCitation10–13 stressing the effect of culture media, gas atmosphere, surfactants (Tween 80), pH, and temperature. pH deeply affects bacteriocin production as demonstrated by Parente and RicciardiCitation[13] who reported that a Lactococcus lactis sub. lactis-bacteriocin had primary metabolite production kinetics at pH 6.0 to 6.5. Vignolo et al.Citation[14] found lactocin peak production by L. casei CRL705 in MRS broth at pH 6.5 to 7.5. Coventry et al.Citation[15] reported maximum production of brevicin 286 by L. brevis VB286 during the exponential phase at 20°C. It is assumed that bacteriocin-producing strains isolated from native food products are naturally selected, therefore they are more efficient against contaminant microflora than strains isolated from other food materials.
The objective of this study was to study the inhibition spectrum of a bacteriocin produced by L. buchneri, isolated from a Mexican sausage, against L. monocytogenes and several lactic acid bacteria. The effect of extrinsic fermentation parameters (culture media, gas atmosphere, surfactant addition, pH, and temperature) in in vitro bacteriocin and biomass production was also studied.
Materials and Methods
Bacteriocin Producing Strain
Lactobacillus buchneri, a bacteriocin-producing strainCitation[16] was isolated from Mexican “chorizo” (uncooked spice-added pork sausage). Although it was initially reported as Lactobacillus sake MXVK133 by Kuri et al.Citation[17] identification by molecular techniques (Midilabs, Newark, Delaware) confirmed it as L. buchneri. It was grown in MRS broth, pH 7.0 at 37°C for 24 h and stored in glycerol at −80°C until used.
Target Strains
Target strains were lactic acid bacteria (LAB) from culture collections (Table ) and Listeria (Table ). LAB were inoculated in MRS broth, pH 7.0, incubated at 37°C for 24 h, placed in glycerol and stored at −80°C until used.
Table 1 Sensitivity of LAB to a bacteriocin produced by L. buchneri
Table 2 Sensitivity of Listeria sp. strains to a L. buchneri-bacteriocin
A loop full of the stock Listeria culture was inoculated in TSB medium (Tryptic soy broth, Difco, Detroit, Michigan) and incubated at 35°C pH 7.0. After incubation the strains were inoculated on Palcam Listeria selective agar base (Difco, Detroit, Michigan), 5.0 mg polymixine-B-sulfate + 10 mg celfacide + 2.5 mg acriflavine were added as inhibitors. The plates were incubated at 35°C for 24 h; dark green colonies grown on this medium were again inoculated in TSB agarCitation[18] and incubated at 35°C, pH 7.0. Selected colonies were purified by inoculation in TSB medium and incubated at 35°C, pH 7.0 for 24 h. The colonies obtained were placed in glycerol and stored at −80°C until used.
Production and Partial Purification of L. Buchneri Bacteriocin
Lactobacillus buchneri, previously grown in 10 mL MRS broth (37°C, 24 h) under a N2/CO2 (50:50) atmosphere,Citation[19] was inoculated to MRS broth + 1% Tween 80. The medium was incubated at 37°C for 24 h and centrifuged at 10,000 g for 10 min at 4°C in a Beckman centrifuge, model J2-MI (Beckman, Fullerton, California). Ammonium sulfate (20, 40, 60, and 80%) was added with continuous agitationCitation[20] and left 1 h standing. Finally, samples were centrifuged at 11,000 g for 30 min at 4°C.Citation[7] The precipitate and supernatant were dialyzed against 3 mM phosphate buffer, pH 6.0 for 12 h to remove the ammonium sulfate. Protein concentration in the precipitate and supernatant was analyzed by the method reported by Lowry et al.Citation[21] and bacteriocin activity by the agar diffusion technique.Citation[3]
Microbial Inhibition of Target LAB and L. monocytogenes Strains by Bacteriocin Extract
It was also carried out by the agar diffusion technique.Citation[3] The strains (Table ) were grown in 10 mL MRS broth, pH 7.0, and incubated at 37°C for 24 h. Cultures were then diluted with peptone buffer, pH 7.0 (Scharlau Microbiology, Murcia, Spain). Two hundred microliters of 10−4 dilutions were inoculated to 0.8% agar + 1.0% Tween 80-added 20 mL MRS broth, pH 6.0 at 40°C and poured on top of 1.5% agar-added MRS medium, pH 6.0 in Petri dishes. Fifty microliters concentrated L. buchneri bacteriocin (450 AU/mL) was placed in holes drilled in the plates. Halo formation was considered positive. Similar technique was used to study the inhibitory effect of L. buchneri bacteriocin on Listeria sp. The strains were grown in 10 mL TSB broth, pH 7.0 and incubated at 35°C for 24 h.Citation[18] The culture was then diluted with peptone buffer, pH 6.0 (Scharlau Microbiology). Two hundred microliters 10−4 dilution was inoculated to 0.8% agar + 1.0% Tween 80 added 20 mL TSB broth, pH 6.0 at 40°C. It was poured on top of 1.5% agar-added TSB medium, pH 6.0 in Petri dishes. Fifty microliters of concentrated L. buchneri bacteriocin (450 AU/mL) was placed in holes drilled in the plates. Halo formation was considered positive.
Bacteriocin Activity Analysis
Activity units were also analyzed by the agar diffusion method, in 10−1 to 10−8 bacteriocin extract dilution series, according to the technique indicated above. Fifty microliter bacteriocin samples were placed in holes drilled in the agar, allowing the bacteriocin to diffuse for 24 h at 4°C. The plates were then incubated at 30°C for 24 h. Bacteriocin activity was defined as the inverse of the highest dilution showing an inhibition halo. Specific activity was obtained by dividing activity into biomass dry weight. Lactobacillus hilgardi NRRL B-1139 was used as target strain.
Effect of Extrinsic Parameters on Bacteriocin Production by L. buchneri
Culture media
Air was evacuated by flushing N2/CO2 (50–50%) at 15 mL/min from 50 mL serological flasks containing 18 mL MRS broth (deMan, Rogosa y Sharpe, Difco, Detroit, Michigan) or APT (All Purpose Tween, Becton Dickinson) pH 7.0. In order to prevent target strain growth reduction by hydrogen peroxide production, both culture media were previously bubbled with the same gas mixture to assure oxygen-free conditions. Working under an anaerobic hood filled with the same gas mixture, 2 mL L. buchneri cell suspension (OD = 1.0) was inoculated to each flask, incubated for 24 h at 37°C and centrifuged for 10 min at 10,000 g, 4°C. The liquid fraction was adjusted to pH 6.0 with 0.1 N NaOH and filtered through 0.2 µm cellulose acetate membranes. Biomass production was analyzed by turbidimetry every 4 h using a Beckman DU spectrophotometer, model 650 (Fullerton, California) at λ = 640 nm. The medium was diluted in order to have readings below 0.3.Citation[22] Dry weight was analyzed by interpolating sample dry weight (105°C for 24 h) vs. optical density.
Gas atmosphere
Due that information about gas atmosphere effect on this microorganisms was scatter in literature, three gas atmospheres were studied (0:100, 50:50, and 0:100 N2/CO2).
Temperature
It was studied by inoculating the bacteriocin-producing strain in APT medium under a N2/CO2 (50:50) gas atmosphere. Samples were then incubated at 20, 25, 30, 35, and 40°C for 24 h.
Surfactant
The same conditions for bacteriocin production were also studied in APT broth added with 0.5, 1.0, or 1.5% Tween 80 (Baker).
Bacteriocin and biomass production was studied for each source of variation (gas atmosphere, temperature, and concentration of added surfactant) according to the techniques described for the effect of culture media.
Bacteriocin production in a bioreactor
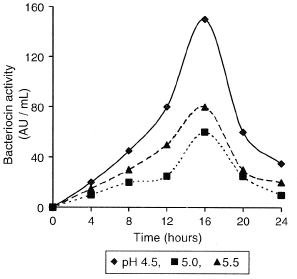
A 2 L FA-5000 bioreactor fitted with pH, temperature, and agitation digital controls was used (Sistemas y Equipos de Vidrio, Puebla, Mexico). Air was evacuated by flushing 15 mL/min N2 stream. Production conditions were: 900 mL APT broth, 100% N2 atmosphere, 1.0% Tween 80, 30°C, 150 rpm agitation, and 10% inoculum (L. buchneri grown in 100 mL APT broth at 30°C, pH 7.0, for 18 h). Bacteriocin production was studied at pH 4.5, 5.0, and 5.5. Bacteriocin activity and biomass production were analyzed as previously described.
Statistical analysis
The data were subjected to analysis of variance and Duncan's multiple range test using a SAS package.Citation[23] All analyses were carried out in triplicate.
Results and Discussion
Bacteriocin Inhibition Spectrum
Lactic acid bacteria sensitive to L. buchneri bacteriocin is listed in Table . Five out of ten sensitive strains tested belong to genus Lactobacillus, in agreement with other authors reporting that bacteriocins inhibit phylogenetically related microorganisms.Citation[2] Citation[24] The most sensitive strains were Staphylococcus xylosus DD-34 5000 509 and L. hilgardii NRRL B-1130.
In our study, four out of five Listeria sp. strains were sensitive to L. buchneri bacteriocin (Table ). Conversely, L. monocytogenes LM82 was not sensitive; this strain was previously reported as resistant to streptomycin.Citation[18] Citation[26] Citation[27] Inhibition resistance could be due to the presence of tolerant or/and structurally modified individuals, as reported by several authors.Citation28–31
Effect of Extrinsic Parameters in Bacteriocin Production
The maximum bacteriocin production, expressed as activity, was obtained in MRS broth although no significant difference was observed with respect to bacteriocin production in APT broth (Table ). Use of synthetic culture media resulted in high bacteriocin production, however it contains high molecular weight peptides (3–6 kDa) making bacteriocin purification difficultCitation[32] Citation[33] (results not shown). Temperature had a significant effect (p ≥ 0.001) on bacteriocin activity; the maximum was at 30°C (250 AU/mL). At this point biomass production also reached a peak suggesting that it is related to microbial growth. According to other authorsCitation34–38 most bacteriocin production by LAB follows a primary metabolite reaction rate.
Table 3 Effect of culture medium, gas atmosphere, temperature, and surfactant concentration on bacteriocin activity and biomass production
Gas atmosphere composition had a significant effect (p > 0.001) on bacteriocin activity, the maximum was observed in 100% N2 (180 AU/mL) (Table ) although no significant difference was observed between 100% N2 and N2-CO2 (50:50) (150 AU/mL). These results were in agreement with those reported by De Vuyst and VandammeCitation[34] who found that N2 could promote bacteriocin production, whereas Venema et al.Citation[39] reported that N2 atmospheres promotes anaerobic conditions increasing bacteriocin stability due to the reducing environment. The lowest bacteriocin and biomass production were in 100% CO2 atmosphere (Table ), in agreement with García et al.Citation[40] who explained this fact as microbial growth inhibition, although this effect depends on the specific strain.
Tween 80 concentration significantly affected (p > 0.016) bacteriocin production, although no significant difference was observed between 1% (20.0 AU/mL) and 1.5% (250 AU/mL) (Table ). It was probably due to formation of stable bacteriocin-Tween 80 micelles. Surfactants have been also reported to improve bacteriocin transport through the cell membrane.Citation[33]
Bacteriocin Production in a Bioreactor
Bioreactor-produced bacteriocin showed a maximum activity at pH 4.5 (150 AU/mL) (Fig. ) in agreement with Yang and Ray,Citation[32] who reported that as pH increases bacteriocins are adsorbed by the producing cells. In preliminary, small volume tests microbial growth increased acidity; under these conditions pH control was difficult, whereas it was automatic in a bioreactor. For this reason, studies on pH effect were carried out directly in the bioreactor.
The maximum specific activity (39.938 AU/g) was observed at 16 h although there was no significant difference between 100% N2 and N2/CO2 (5:50) atmospheres. It was also observed that the activity was significantly affected by CO2; samples incubated in this atmosphere showed the lowest bacteriocin activity with respect to incubation under 100% N2 or 50–50 CO2/N2 (Table ).
Bacteriocin production was related to L. buchneri exponential growth rate; the maximum specific activity occurred at 16 h (39.93 × 103 AU/g), the end of the exponential and beginning of the stationary phase (Table ). According to several authorsCitation[35] Citation[37] Citation[38] it is related to the presence of proteases and to bacteriocin adsorption by producing cells as pH increases. Zamfir et al.Citation[38] also observed an increase in bacteriocin activity at the end of the exponential phase; these authors reported that growth stops due to glucose exhaustion. Other authors related this phenomenon to the presence of proteases or to bacteriocin adsorption at pH 6.0 by the producing cellsCitation[25] when adsorption is around 90%, whereas at pH 2.0 adsorption is 1%.Citation[32] Carolissen-MacKay et al.Citation[33] also reported possible biochemical changes as the reason for activity reduction. These authors related activity loss to changes in the catalytic site promoted by a cofactor loss or covalent modifications.
Table 4 Specific activity and biomass production in a bioreactor
Conclusions
Antibacterial activity was due to bacteriocin production, not to other antimicrobials such as organic acids, hydrogen peroxide or diacetyl, as L. buchneri grown under anaerobic conditions (N2/CO2, 50:50). In addition, air in the culture medium was previously eliminated by bubbling N2/CO2 (50:50), therefore residual oxygen was eliminated therefore possible diacetyl formation did not act as antibacterial as it is a mild antimicrobial agent.Citation[40] Under our experimental conditions the highest bacteriocin production by L. buchneri, isolated from a Mexican uncooked sausage, occurred at the end of the exponential growth phase in 100% N2 atmosphere on MRS or APT medium at 30°C adding 1.0% surfactant. The studied bacteriocin also inhibited selected Listeria sp. strains.
Acknowledgments
Author H. Minor thanks CONACYT (National Council of Science and Technology, Mexico) for a graduate scholarship. The authors also thank Drs. Victor Kuri (University of Plymouth, UK), Margarita Salazar (Universidad Autónoma Metropolitana, Mexico), and Fulgencio Marín Iniesta (Universidad de Murcia, Spain) for their comments and supplying some of the studied strains.
References
- Stiles , M.E. 1996 . Biopreservation by lactic acid bacteria . Antonie van Leeuwenhoek , 70 : 235 – 249 .
- Klaenhammer , T.R. 1988 . Bacteriocins of lactic acid bacteria . Biochimie. , 70 : 337 – 349 .
- Schillinger , U. and Lucke , F.K. 1989 . Antibacterial activity of Lactobacillus sake isolated from meat . Appl. Environ. Microb. , 55 : 1901 – 1906 .
- O'Sullivan , L. , Ross , R.P. and Hill , C.C. 2002 . Potential of bacteriocin-producing lactic acid bacteria for improvements in food safety and quality . Biochimie. , 84 : 593 – 604 .
- Cutter , C.N. and Siragusa , G.R. 1994 . Decontamination of beef carcass tissue with nisin using a pilot scale model carcass washer . Food Microb. , 11 : 481 – 489 .
- Schillinger , U. , Kaya , M. and Lücke , F.K. 1991 . Behaviour of Listeria monocytogenes in meat and its control by a bacteriocin-production strain of Lactobacillus sake . J. Appl. Bacteriol. , 70 : 473 – 478 .
- Hugas , M. , Garriga , M. , Aymerich , M.T. and Monfort , J.M. 1995 . Inhibition of Listeria monocytogenes in dry fermented sausages by the bacteriocinogenic Lactobacillus sake CTC494 . J. Appl. Bacteriol. , 79 : 322 – 330 .
- Foegeding , P.M. , Thomas , A.B. , Pilkington , D.H. and Klaenhammer , T.R. 1992 . Enhanced control of Listeria monocytogenes by in situ produced pediocin during dry fermented sausage production. Appl . Environ. Microb. , 58 : 884 – 890 .
- Luchansky , J.B. 1999 . Overview on applications for bacteriocin-producing lactic acid bacteria and their bacteriocins . Antonie van Leeuwenhoek , 76 : 335
- Blasubramanyam , B.V. and Varadaraj , M.C. 1998 . Cultural conditions for the production of bacteriocin by a native isolate of Lactobacillus delbruecki ssp. bulgaricus CFR 2028 in milk medium . J. Appl. Bacteriol. , 84 : 97 – 102 .
- Kaiser , A.L. and Montville , T.J. 1993 . The influence of pH and growth rate on production of the bacteriocin, bavaricum MN, in batch and continuous fermentations . J. Appl. Bacteriol. , 75 : 536 – 540 .
- Parente , E. and Hill , C. A . 1992 . comparison of factors affecting the production of two bacteriocins from lactic acid bacteria . J. Appl. Bacteriol. , 73 : 290 – 298 .
- Parente , E. and Ricciardi , A. 1994 . Influence of pH on the production of enterocin 1146 during batch fermentation . Lett. Appl. Microb. , 19 : 12 – 15 .
- Vignolo , G.M. , Kairuz , M.N. , Ruiz , H.A. and Oliver , G. 1994 . Influence of growth conditions on the production of lactocin 705, a bacteriocin produced by Lactobacillus casei CRL705 . J. Appl. Bacteriol. , 80 : 90 – 93 .
- Coventry , M.J. , Wan , J. , Gordon , J.B. , Mawson , R.F. and Hickey , M.W. 1996 . Production of brevecin 286 by Lactobacillus brevis VB286 and partial characterization . J. Appl. Bacteriol. , 80 : 91 – 98 .
- Kuri , H.V. 1998 . “ Lactic Acid Bacteria and Salmonella from Mexican Pork Products: Characterization and Antagonism ” . In Ph.D. thesis Belfast, , Northern Ireland : The Queen's University of Belfast .
- Kuri , V. , Madden , R.H. and Collins , M.A. 1996 . Hygienic quality of raw pork and chorizo (raw pork sausage) on retail sale in Mexico City . J. Food Protec. , 59 ( 2 ) : 141 – 145 .
- Marin-Iniesta , F. 2002 . Personal Communication Spain : Universidad de Murcia .
- Brink , B. , Huisin’t Veld , J.H.J. and Minekus , M. 1990 . Antimicrobial activity of Lactobacillus M46; optimization of production and partial characterization . FEMS Microb. Rev. , 87 : 91 – 95 .
- Scopes , R.K. 1994 . Protein Purification: Principles and Practice 38 – 39 . New York : Springer-Verlag . 71–101
- Lowry , O.H. , Rosebrough , N.J. , Farr , A.L. and Randall , R.J. 1951 . Protein measurement with the folin phenol reagent . J. Biol. Chem. , 193 : 265 – 275 .
- Dalgaard , P. , Ross , T. , Kamperman , L. , Neumeyer , K. and McMeekin , T. 1994 . Estimation of bacteria growth rates from turbidimetric and viable counts data . Int. J. Food Microb. , 31 : 391 – 404 .
- SAS, Institute. 1990 . SAS User's Guide Cary, North Caroline : SAS Institute .
- Kuri , V. , Collins , M.A. and Madden , R.H. 44th Proceedings of the International Congress of Meat Science and Technology . Sept 1–5 . Stability and Antibacterial Activity of Bacteriocin Preparations in Pork Model , Vol. 1 , pp. 342 – 343 . Barcelona, , Spain : ICOMST .
- Ennahar , S. , Sashihara , T. , Sonomoto , K. and Ishizaki , A. 2000 . Class IIa bacteriocins: biosynthesis, structure and activity . FEMS Microb. Rev. , 34 : 85 – 106 .
- Chen , Y. and Montville , T.J. 1995 . Efflux of ions and ATP depletion induced by pediocin PA-1 are concomitant with cell death in Listeria monocytogenes Scott A . J. Appl. Bacteriol. , 79 : 684 – 690 .
- Kaiser , A.L. and Montville , T.J. 1996 . Purification on the bacteriocin brevicin MN and characterization of its mode of action against Listeria monocytogenes Scott A cells and lipid vesicles . Appl. Environ. Microb. , 64 : 4529 – 4535 .
- Chen , Y. , Ludescher , R.D. and Montville , T.J. 1998 . Influence of lipid composition on pediocin PA-1 binding to phospholipid vesicles . Appl. Environ. Microb. , 64 : 3530 – 3532 .
- Hanlin , M.B. , Kalchayanad , N. , Ray , P. and Ray , B. 1993 . Bacteriocins of lactic acid bacteria in combination have greater antibacterial activity . J. Food Protect. , 56 : 252 – 255 .
- Noerlis , Y. and Ray , B. 1993 . Factors influencing immunity and resistance of Pediococcus acidilactici to the bacteriocin, pediocin AcH . Lett. Appl. Microb. , 18 : 138 – 143 .
- Ray , B. 1993 . Sublethal injury, bacteriocins and food microbiology . ASM News , 59 : 285 – 291 .
- Yang , R. and Ray , B. 1994 . Factors influencing production of bacteriocins by lactic acid bacteria . Food Microb. , 11 : 281 – 291 .
- Carolissen-MacKay , V. , Arendse , G. and Hastings , W.J. 1997 . Purification of bacteriocins of lactic acid bacteria: problems and pointers . Int. J. Food Microbiol. , 34 : 1 – 16 .
- De Vuyst , L. and Vandamme , E.J. 1994 . Bacteriocins of Lactic Acid Bacteria: Microbiology, Genetics and Applications London : Blackie Academic and Professional .
- De Vuyst , L. , Callewaert , R. and Crabbé , K. 1996 . Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions . Microbiol. , 142 : 817 – 827 .
- Lejeune , R. , Callewaert , R. , Crabbé , K. and De Vuyst , L. 1995 . Modeling the growth and bacteriocin production by DCE 471 in batch cultivation . J. Appl. Microb. , 84 : 159 – 168 .
- Parente , E. , Brienza , C. , Ricciardi , A. and Addario , G. 1997 . Growth and bacteriocin production by Enterococcus faecium DPC1146 in batch and continuous culture . J. Ind. Microb. Biotech. , 18 : 62 – 67 .
- Zamfir , M. , Callewaert , R. , Cornea , P.C. , Savu , L. , Vatafu , I. and De Vuyst , L. 2000 . Purification and characterization of a bacteriocin produced by Lactobacillus acidophilus IBB 801 . J. Appl. Microb. , 87 : 923 – 931 .
- Venema , K. , Venema , G. and Kok , J. 1995 . Lactococcal bacteriocins: mode of action and immunity . Trends Microb. , 3 : 299 – 304 .
- García , T. , Martín , R. , Sanz , B. and Hernández , P.E. 1995 . Extensión de la vida útil de la carne fresca. I: envasado en atmósferas modificadas y utilización de bacterias lácticas y bacteriocinas . Rev. Española Ciencia Tec. Alim. , 35 : 1 – 10 .