Abstract
Marinating with calcium chloride had been employed to activate the endogenous proteolitic enzymatic system in muscle tissue. The activation of calpains leads to the disruption of major proteins responsible for myofilament structure. Nonetheless, intrinsic differences regarding enzymatic activity between different species need to be established, which concerns protein degradation and ultrastructural changes. In this study, four species—horse, chicken, rabbit and beef—were marinated in 150 mM CaCl2 solution. Calpain activity was determined by low and high molecular weight protein degradations (SDS-PAGE) and light microscopy, which was compared to a non-treated control. All the studied species presented an increase in the number of low molecular weight bands, corresponding to the 30 kDa polypeptide that resulted from tropomiosin degradation. High molecular weight protein degradation corresponds to the formation of breakdowns and amorphous regions in tissue micrographs. These results support the evidence of meat quality improvement by calcium marination.
INTRODUCTION
Natural meat proteolysis seems to be one of the main factors that contribute to the tenderizing process during meat aging.[Citation1] Two enzymatic systems are involved in meat tenderness: calpains and cathepsins.[Citation2] However, there are some discrepancies in regard to the cooperative action of both enzymatic systems.[Citation3] These proteolytic systems are the same in all muscles, regardless of species.[Citation4] Cathepsins are lysosomal enzymes involved in muscle proteolysis; 8 are present in skeletal muscle: cathepsin A, B, C, D, H, J, and lysosomal carboxypeptidase B.[Citation2] There are two main kinds of calpains: calpain I or m-calpain, which requires 50 to 70mM calcium, and calpain II or μ-calpain, needing 1 to 5 μM calcium for maximum activity.[Citation5,Citation6] This activation could be done by the injection of meat pieces with a CaCl2 solution or immersion into the solution.
Both enzymatic systems had proteolytic activity during meat aging, but it has been suggested that calpains are more important in myofibril breakdown after rigor mortis.[Citation7] Postmortem proteolysis plays an important role in meat tenderization with two main changes during this process: Z-disk breakdown and desmin degradation.[Citation8] Both Etherington[Citation9] and Roncalés et al.[Citation2] reported that calpains degraded troponin-T, nebulin, titin, and desmin in muscles stored under refrigeration, whereas myosin, actin, and α-actinin are not degraded under similar conditions. These changes could be accelerated by calcium chloride marination,[Citation10–17] improving meat quality.[Citation18,Citation19] The objective of this work was to determine the effect of calcium chloride marination on the low and high molecular weight muscular proteins, related to calpains activity, as well as the ultrastructural changes in tissue structure.
MATERIALS AND METHODS
Four animal species were employed in order to determine differences among them. Beef and horse Longissimus dorsi samples were obtained form local abattoirs, within 48 hr of post-mortem. Chicken and rabbit were slaughtered under humanitarian conditions at the Meat Science Plant at the University facilities. The carcases were eviscerated and washed then stored 14-16 hr at 3-4°C, in order to allow rigor resolution. L. dorsi samples were removed and the left leg and employed as a control, and the right muscle was used for marinating. Beef and horse samples, the muscle (2-3 kg), was perpendicularly divided into two sections, randomly divided for control and marinating samples. The muscle was divided in ca. ∼100 g portions and marinated by immersion in a 150 mM calcium chloride solution at 4°C for 48 h, under conditions as previously reported.[Citation20]
Myofibrillar Proteins Extraction
Miofibrillar proteins were extracted form the control and marinated samples according to the methodology reported by Samejima and Wolfe (Citation21), with some modifications. All steps were carried out at 4-5°C. One gram of meat was added with 10 mL of an extraction solution (0.6M NaCl, 2mM MgCl, 1mM EDTA, 0.5mM ditiothreitol, 10mM NaPO4, pH 7.0), homogenized for 1 min and centrifuged at 7,800 × g at 4°C for 20 min. The precipitate was resuspended (1:6 w/v) in another buffered solution (0.6M NaCl, 5mM MgCl, 5mM sodium pyrophosphate, 50mM NaPO4, pH 6.0). The suspension was stirred overnight at 4°C and then centrifuged at 9,000 × g for 20 min at 4°C, and the supernatant was diluted with deionised water at 4°C until ionic strength circa 0.1M was obtained. It was then stored overnight at 4°C to allow myofibrillar proteins precipitation. The precipitate was carefully removed and centrifuged at 7,800 × g for 25 min at 4°C. The precipitate was resuspended and stirred overnight at 4°C in a buffered solution (1:3 w/v)(0.6M NaCl, 50mM NaPO4, pH 6.0). The suspension was then centrifuged at 14,000 × g for 25 min, and the pellet was resuspended in the last solution, containing myofibrillar proteins. Protein content was adjusted and determined by the bicinchoninic acid protein assay described by Redinbaugh and Turley.[Citation22]
Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Muscular proteins degradation was determined by polyacrylamide gel electrophoresis, as described by Laemmli.[Citation23] Myofibrillar protein samples from marinated and control samples were prepared adding 4:1 v/v of sample buffer (4% sodium dodecyl sulfate, 10% mercaptoethanol, 0.002% bromophenol blue) solution and heated in boiling water for 4 min. Ten µL of the prepared sample and 10 µL of high molecular weight markers (500 to 10 kDa) (P-8906 for 584 to 97.4 kDa, Sigma, St. Louis, Missouri) were injected to polyacrylamide gels (3.5% w/v) in a Mini-Protean II slab cell (Bio-Rad, Richmond, California) and run at 200V. For low molecular weight, separation electrophoresis gels were prepared with higher poliacrylamide concentration (12% w/v) and 4% (w/v) in stacking gels with low molecular weight markers SDS-7 markers (66 to 14.2 kDa, Sigma Chemical Co., St. Louis, Missouri) at the same running conditions. Staining was carried out using 0.02% (w/v) Coomasie blue solution (10% acetic acid, 40% methanol) and destained by immersion into 10% acetic acid and 40% methanol solution for 30 min. Densitometry of the gels were determined in a Ultrascan XL densitometer (Pharmacia LKB, Bromma, Sweden), calculating the relative areas (peak area/total area) of band.
Light Microscopy
To determine tissue ultrastructural degradation, light microscopy of the non-treated and marinated samples was performed. Meat samples (1-2 g) were prepared by immersion in Bouin solution (75% picric acid solution, 5% acetic acid, 25% formol, 5:30 proportion w/v) and washed by consecutive immersions in xylene (3 min), absolute ethanol (2 min), and 95% ethanol (3 min). Samples were then washed with distilled water for 1 min and stained with hematoxilin for another min and then immersed in HCl: Etanol solution (1:99 v/v, ethanol 95%) for 10 sec, washed with distilled water, and dehydrated by immersion in absolute ethanol for 10 to 20 sec and xylene for 15 to 20 sec two during three consecutive cycles. Finally, samples were imbedded in paraffin at 65°C. Changes in the striated muscle due to marinating treatment were observed with an Olympus microscope with fluorescence (Olympus, model BH-2-RFCA, Tokyo, Japan).
RESULTS AND DISCUSSION
Protein Degradation
The densitometry patterns of low molecular weight myofibrillar proteins between 14 and 66 kDa, isolated from the four animal species studied, are shown in to , (a) and (b), for the Ca+2 treated and non-treated or control samples, respectively. Three main zones can be observed. The first one includes a peak for proteins with a molecular weight around 66 kDa. This peak was more intense in horse samples ( and ) and rabbit samples ( and ) than for chicken samples ( and ). It was not present in beef samples ( and ). The intensity of this band was higher in non-treated samples ( and ) than the Ca+2-treated samples ( and ), even in chicken samples ( and ). This could be an indication that calpains had a proteolytic activity only on high molecular weight proteins, such as nebulin, titin, or desmin.[Citation2,Citation9]
Figure 1 Densitometry of gel electrophoresis for chicken meat samples. LMW proteins for (a) Ca+2-treated and (b) un-treated samples. HMW proteins for (a) Ca+2-treated and (b) un-treated samples.
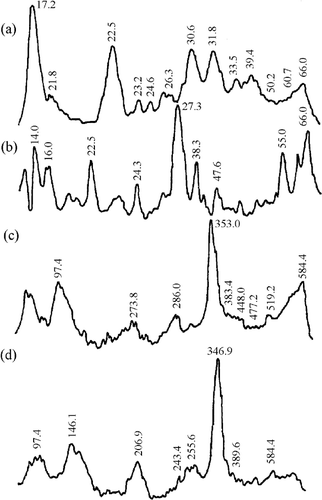
Figure 2 Densitometry of gel electrophoresis for beef samples. LMW proteins for (a) Ca+2-treated and (b) un-treated samples. HMW proteins for (a) Ca+2-treated and (b) un-treated samples.
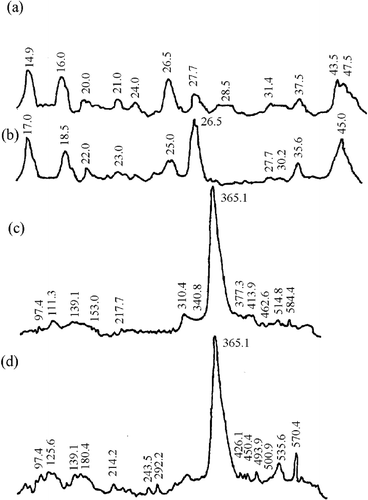
Figure 3 Densitometry of gel electrophoresis for rabbit samples. LMW proteins for (a) Ca+2-treated and (b) un-treated samples. HMW proteins for (a) Ca+2-treated and (b) un-treated samples.
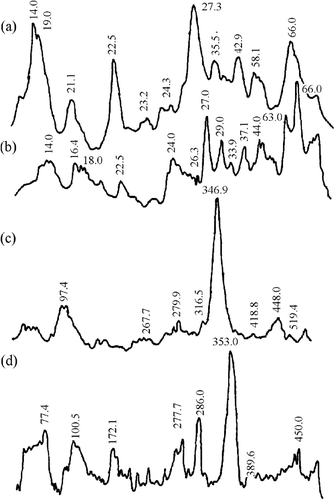
Figure 4 Densitometry of gel electrophoresis for horse samples. LMW proteins for (a) Ca+2-treated and (b) un-treated samples. HMW proteins for (a) Ca+2-treated and (b) un-treated samples.
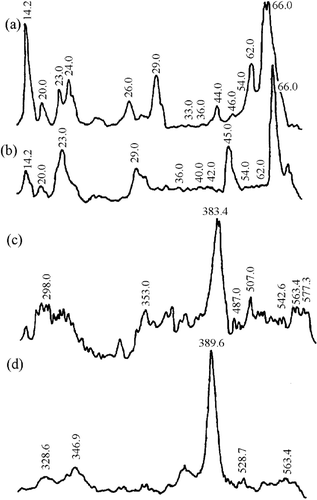
The second zone was for proteins with a molecular weight around 30 kDa, a polypeptide that had been reported as a result of myofibrillar protein degradation during maturation.[Citation4,Citation24] In horses, the Ca+2-treated samples () showed an increase in the intensity of this band (∼29.0 kDa) as compared to non-treated samples (). The same result was detected for chicken samples: the Ca+2-treated (, ∼27.3 kDa) had a higher intensity than the non-treated samples (; ∼30.6 kDa). The more remarkable change was observed in rabbit, where the Ca+2-treated samples (; ∼27.3 kDa) presented a notable increase in band intensity as compared with the non-treated samples (; ∼27.0 and 29.0 kDa). In beef samples ( and ), the changes were not as remarkable as in the other species. The presence of this band seems to be characteristic of chicken, bovine, and porcine muscles during marination.[Citation25] Other authors have reported the presence of the 30 kDa band as a consequence of troponin-T degradation.[Citation26] According to Morimoto and Ohtsuki (Citation27), troponin-T is the troponin subunit to most sensitive enzymatic attack; whereas Jaarsveld et al.[Citation3] reported that calpain-treated myofibrils showed a troponin-T degradation with the concomitant increase in the concentration of 27, 29, 30, 31, 32, and 33 kDa polypeptides, which agreed with the peaks present in our results. The appearance of a 27 kDa fraction was observed in rabbit muscle.[Citation28]
The third zone was for proteins with molecular weights around 23 kDa and lower. The number of bands in this region was more abundant for horse, chicken, and beef Ca+2-treated samples (, , and , respectively) than the non-treated samples (, , and , respectively). Rabbit samples showed the same two main bands (∼14.0 and 22.5 kDa) for Ca+2-treated () and non-treated () samples. The increase in protein-depleted fractions was probably due to calcium chloride marination. It has been reported that the mechanism responsible for meat tenderness is the same for all animal species, although some qualitative differences have been found between species.[Citation3]
Concerning high molecular weight fractions (molecular weight between 97 and 584 kDa), Ca+2-treated horse, chicken, and rabbit samples (c, c and c, respectively) showed an increase in the number of bands as non-treated samples (d, d, and d, respectively). In contrast, Ca+2-treated beef samples presented smaller bands (c) than the non-treated (d). The highest relative band area in the four studied species corresponded to 310-385 kDa proteins, indicating a possible degradation of intermediate filaments proteins, such as conectin, titin, and nebulin, as result of protease action.[Citation29] Nebulin is a thin filament regulatory protein in the skeletal muscle. Rong-Ghi et al.[Citation25] and Ho et al.[Citation26] found that calcium chloride marination increases the degree of postmortem degradation of titin and nebulin, producing subunits with a considerably lower molecular weight. However, in agreement with Jaarseveld et al,[Citation3] we might argue that high molecular weight protein identification had difficulties due to the presence of degradation products. A relative peak area of less than 97 kDa was observed in rabbit Ca+2-treated (c), probably as a consequence of the presence of α-actinin, the most abundant protein in Z-line. Koohmaraie et al.[Citation30] reported that the concentration of this protein decreased, and a 98 kDa band appeared as a result of Z-line degradation in meat marinated during 24 h. in same way. Rong-Ghi et al.[Citation25] also reported that fast α-actinin degradation can be related to Z-line breakdown during tenderisation.
Structural Changes
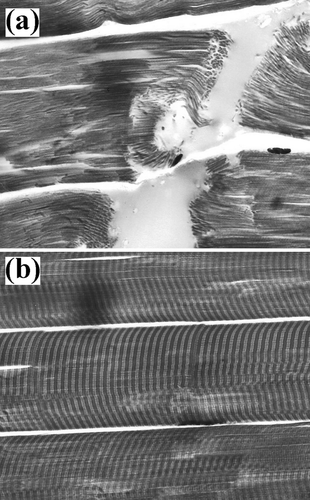
Light microscopy showed that marinated samples of the four animal species studied presented fractures, as well as dark zones, due to myofibrillar protein degradation during the experimental conditions employed ( to ). Beef tissue micrographs () presented more fractures than the other three species. All samples showed Z-line and M-band degradation, in agreement with the reported by Wu et al.[Citation31] Nonetheless, Shimada and Takahashi[Citation32] reported that the damage in Z-line is due to the liberation of phospholipids in pork and beef calcium-marinated samples, reducing the importance of calpains role in meat tenderisation. On the other hand, Ho et al.[Citation33] reported that changes in myofibrillar structure were mainly due to I-band degradation as reported by who described that myofibril structures in postmortem muscle are replaced by an amorphous, non-uniform bands adjacent to I-band ruptures, increasing intermyofibril gaps, as compared to premortem tissue. On the other hand, Taylor and Goll[Citation34] described myofibril fractures in I-bands adjacent to Z-lines, but not within this structure. Similarly, Boyer-Berri and Greaser[Citation35] reported titin depletion by calpains activity. Uytterhaegen et al.[Citation36] also found that desmin, titin, and nebulin were depleted by calpains. Tenderisation by calpain activation followed similar patters in the four species under this study, that is, disruption of I-bands adjacent to the Z-disks with the presence of fractures and amorphous zones. However, in parts of the myofibril structure, some areas of the tissue structure remained intact or were only partly disintegrated. Partial disintegration was observed both in treated and untreated samples of the four species, probably due to naturally occurring postmortem proteolysis. Intact tissue was the result of calpain inactivation probably due to the presence of calpastatin, a natural intracellular calpain inhibitor.[Citation37]
Figure 5 Myofibrillar tissue micrographs for horse samples (320x): (a) Ca+2-treated and (b) un-treated.
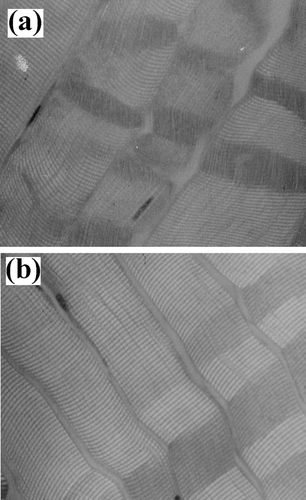
Figure 6 Myofibrillar tissue micrographs for chicken samples (200x): (a) Ca+2-treated and (b) un-treated.
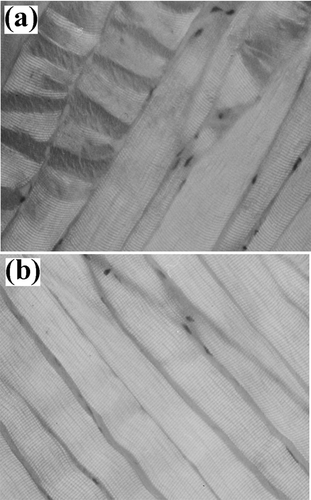
Figure 7 Myofibrillar tissue micrographs for rabbit samples (312x): (a) Ca+2-treated and (b) un-treated.
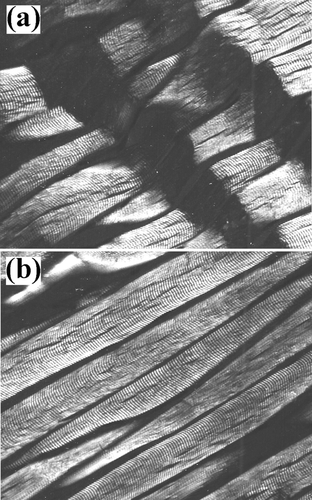
Figure 8 Myofibrillar tissue micrographs for beef samples (800x): (a) Ca+2-treated and (b) un-treated.
Although calcium chloride treatment, or marination, has been explored as a means to increase meat tenderness by calpain activation and, in turn, myofibrillar protein degradation, not all structural proteins are affected. Calpains have a proteolytic effect on cytoskeletal proteins as troponin-T, troponin-I, tropomyosin, α-actinin, titin, nebulin, and desmin,[Citation38,Citation39,Citation40] but not on myosin or actin.[Citation41,Citation42] In this way, any conditions that enhance the postmortem activity of calpains, as calcium chloride marination, would result in more degradation of these cytoskeletal proteins. Degradation of troponin-T may weaken the myofibrillar structure where the thin filaments are released form the Z-disk.[Citation33,Citation36,Citation43]
Protein gel electrophoresis showed that protein degradation was more extended in horse (44), chicken, and beef Ca+2-treated samples than non-treated samples. For rabbit, a similar number of bands were detected. Low molecular weight (LMW) proteins gel electrophoresis of myofibrillar extracts obtained from the four studied species showed that protein degradation was more extended in horse, chicken, and beef-marinated tissue, whereas treated and untreated rabbit samples had the similar number of bands. For rabbit samples, with the exception of a 30 kDa band in marinated samples, HMW and LMW proteins seemed to be not affected by calcium chloride treatment. High molecular weight (HMW) proteins had more extensive breakdowns in horse and chicken samples than in beef and rabbit. Ultrastructural changes (i.e., Z-line degradation, fractures, and formation of amorphous zones due to myofibrillar destruction) were observed in all marinated samples, probably due to degradation of intermediate proteins (titin, desmin, and nebulin).
CONCLUSIONS
Calcium chloride promoted calpain activation, affecting myofibrillar proteins in the four species studied, with different degrees of action due to inherent animal differences. Beef and horse seem to be most affected by calcium marination. The appearance of low molecular weight components could be a reflection of high molecular weight proteins, specifically troponin-T, resulting in ∼30 kDa components, depending on species. Calpain activity seems to be higher in large animal species (horse and beef) than in small––rapid rigor resolution––species (chicken and rabbit). These results support previous reports on the improvement of meat quality by calcium chloride marination, where marinated samples of these species resulted in more tenderness, increasing juiciness.[Citation15]
REFERENCES
- Koohmaraie , M. 1992 . Effect of pH, temperature and inhibitors on autolysis and catalytic activity of bovine skeletal muscle m-calpain . J. Anim. Sci. , 70 : 3071 – 3080 . [PUBMED] [INFOTRIEVE]
- Roncalés , P. , Geesink , G.H. , vanLaack , R.L.J.M. , Jaime , I. , Beltran , J.A. , Barnier , V.M.H. and Smulders , F.J.M. 1995 . “ Meat tenderization: enzymatic mechanism ” . In Expression of Tissue Proteinases of Protein Degradation as Related to Meat Quality , Edited by: Ouali , A. , Demeyer , D.I. and Smulder , F.J.M. Utrecht, The Netherlands : ECCEAMST .
- Jaarsveld , F.P. , Naudé , R.J. and Oelofsen , W. 1997 . The effects of Ca ions, EGTA and storage time on myofibrillar protein degradation, levels of Ca dependent proteases and cathepsins B, H, L and D of ostrich skeletal muscle . Meat Science , 45 : 517 – 529 . [CROSSREF]
- Ouali , A. 1992 . Proteolytic and physicochemical mechanism involved in meat texture . Biochemie , 74 : 251 – 256 . [CROSSREF]
- Thompson , B.C. , Dobbie , P.M. , Singh , K. and Speck , P.A. 1996 . Post-mortem kinetics of meat tenderness and the component of the calpain system in bull skeletal muscle . Meat Sci. , 44 ( 1 ) : 151 – 157 . [CROSSREF]
- Koohmaraie , M. , Seideman , S.C. , Scholmeyer , J.E. , Dutson , T.R. and Babiker , A.S. 1988 . Acceleration of postmortem tenderization in ovine carcasses through activation of Ca dependent proteases . J. Food Sci. , 53 : 1638 – 1643 .
- Etherington , D.J. , Taylor , M.A.J. and Dransfield , E. 1987 . Conditioning of meat from different species. Relationship between tenderising and the levels of cathepsin B,cathepsin L,calpain I, calpain II and beta-glucoronidase . Meat Sci. , 20 ( 1 ) : 1 – 14 . [CROSSREF]
- Bandman , E. 1992 . AMI Rec. Meat Conf. Proc. , 45 : 47 – 50 .
- Etherington , D.J. 1984 . The contribution of proteolytic enzymes to postmortem changes in muscle . J. Animal Sci. , 59 : 1644 – 1649 .
- Slinde , E. and Kryvi , H. 1986 . Z-disc digestion of isolated bovine myofibrils by an endogenous calcium activated neutral proteinase . J. Animal Sci. , 16 ( 1 ) : 45 – 51 .
- Alarcón Rojo , A.D. and Dransfield , E. Acceleration of proteolysis in beef by addition of calcium ions . Proc. 36th International Congress on Meat Science and Technology . August 27–September 1 , La Habana, Cuba. pp. 178 – 185 .
- Morgan , J.B. , Miller , R.K. , Mendez , F.M. , Hale , D.S. and Savell , J.W. 1991 . Using calcium chloride injection to improve tenderness of beef from mature cows . J. Animal Sci. , 69 : 4469 – 4476 .
- Geesink , G.H. , Ouali , A. , Smulders , F.J.M. , Talmant , A. , Tassy , C. , Guignot , F. and vanLaack , H.L.J.M. 1992 . The role of ultimate pH in proteolysis and calpain/calpastatin activity in bovine muscle . Biochimie , 74 : 283 – 289 . [PUBMED] [INFOTRIEVE] [CROSSREF]
- Got , F. , Rousset-Akrim , S. , Bayle , M.C. and Culioli , J. Effects of injection of calcium as a lactate salt on tenderness and flavour of beef . Proc. 42th. International Congress on Meat Science and Technology . September 1–6 , Lillehammer, Norway. pp. 394 – 395 .
- Pérez-Chabela , M.L. , Escalona , H. and Guerrero , I. 1998 . Effect of calcium chloride marination on calpain and quality characteristics of meat from chicken,horse, cattle and rabbit . Meat Sci. , 48 ( 1/2 ) : 125 – 134 .
- García-Barrientos , R. Efecto de las enzimas endógenas sobre las propiedades fisicoquímicas y estructurales de carne de bovino durante la maduración . 2001 . Master Sc. Dissertation. Universidad Autónoma Metropolitana, Mexico
- González-Tenorio , R. Propiedades fisicoquímicas y de textura del músculo Braqueocephalicus de bovino marinado con cloruro de calcio. . 2003 . Master Sc. Dissertation. Universidad Autónoma del Estado de Hidalgo, Mexico
- Lawrence , T.E. , Dikeman , M.E. , Stephens , J.W. , Obuz , E. and Davis , J.R. 2004 . In situ investigation of the calcium-induced proteolytic and salting-in mechanisms causing tenderization in calcium-enhanced muscle . Meat Sci. , 66 ( 1 ) : 69 – 75 . [CROSSREF]
- Lawrence , T.E. , Dikeman , M.E. , Hunt , M.C. , Kastner , C.L. and Johnson , D.E. 2003 . Effects of calcium salts on beef Longissimus quality . Meat Sci. , 64 ( 3 ) : 299 – 308 . [CROSSREF]
- Pérez-Chabela , M.L. , Escalona-Buendia , H and Guerrero , I. 2003 . Physicochemical and sensory characteristics of calcium chloride-treated horse meat . Int. J. Food Prop. , 6 : 73 – 85 . [CROSSREF]
- Samejima , K. and Wolfe , F.H. 1976 . Degradation of myofibrillar protein components during postmortem aging of chicken muscle . J. Food Sci. , 41 : 250 – 254 .
- Redinbaugh , M.G. and Turley , R.B. 1986 . Adaptation of the bicinchoninic acid protein assay for the use in microtiter plates and sucrose gradient fractions . Anal. Biochem. , 53 : 267 – 271 . [CROSSREF]
- Laemmli , U.K. 1970 . Cleavage of structure proteins during the assembly of the head of bacteriophage T4 . Nature , 227 : 680 – 685 . [PUBMED] [INFOTRIEVE] [CROSSREF]
- Olson , D.G. , Parrish , F.C. and Stromer , H.M. 1976 . Myofibrillar fragmentation and shear resistance of three bovine muscles during postmortem storage . J. Food Sci. , 41 : 1036 – 1041 .
- Rong-Ghi , R.C. , Lin , K.J. , Tseng , T.F. and Wu , C. 1995 . Post-mortem changes in goose (Anser anser) breast muscles as affects by calcium chloride marination . J. Sci. Food Agric. , 68 : 293 – 297 .
- Ho , C.-Y , Stromer , M.H. and Robson , R.M. 1994 . Identification of the 30 kDa polypeptide in postmortem skeletal muscle as a degradation product of troponin-T . Biochimie , 76 : 369 – 375 . [PUBMED] [INFOTRIEVE] [CROSSREF]
- Morimoto , S. and Ohtsuki , I. 1994 . Role of troponin C in determining the Ca+2 sensitivity and cooperativity of the tension development in rabbit skeletal and cardiac muscles . J. Biochem. , 115 : 114 – 146 .
- Ouali , A. and Talmant , A. 1990 . Calpains and calpastatin distribution in bovine, porcine and skeletal muscles . J. Meat Sci. , 28 : 331 – 336 . [CROSSREF]
- Kawamura , Y. , Kume , H. , Itoh , Y. , Ohtsuka , S. , Kimura , S. and Maruyama , K. 1995 . Localization of three fragments of connectin in chicken breast muscle sarcomeres . J. Biochem. , 117 : 201 – 207 . [PUBMED] [INFOTRIEVE]
- Koohmaraie , M. , Kennick , W.H. , Elgasim , E.A. and Anglemier , A.F. 1984 . Effects of prerigor pressurization of the activity of calcium-activated-factor . J. Food Sci. , 49 : 680 – 684 .
- Wu , J.Y. , Mills , E.W. and Henning , W.R. 1995 . Postmortem delay time and heating rate affect tenderness and ultrastructure of prerigor cooked bovine muscle . J. Food Sci. , 60 ( 3 ) : 565 – 569 .
- Shimada , K. and Takahashi , K. 2003 . Relationship between fragmentation of myofibrils and liberation of phospholipids from Z-disks induced by calcium ions at 0.1mM: Mechanism of tenderization of pork and beef during postmortem aging . J. Food Sci. , 68 ( 9 ) : 2623 – 2629 .
- Ho , C-Y. , Stromer , M.H. and Robson , R.M. 1996 . Effect of electrical stimulation on postmortem titin,nebulin, desmin and troponin-T degradation and ultrastructural changes in bovine Longissimus muscle . J. Animal Sci. , 74 : 1563 – 1575 .
- Taylor , R.G. and Goll , D.E. 1995 . “ Enzyme localization during postmortem muscle tenderization ” . In Composition of Meat in Relation to Processing, Nutritional and Sensory Quality; from Farm to Fork , Edited by: Lundström , K. , Hansson , I and Wicklund , E. 347 – 358 . Utrecht, , The Netherlands : ECCEAMST .
- Boyer-Berri , C. and Greaser , M.L. 1998 . Effect of postmortem storage on the Z-line region of titin in bovine muscle . J. Animal Sci. , 76 : 1034 – 1044 .
- Uytterhaegen , L. , Claeys , E. and Demeyer , D. 1994 . Effects of exogenous protease effectors on beef tenderness development and myofibrillar degradation and solubility . J. Animal Sci. , 72 : 1209 – 1223 .
- Di Lisa , F. , Tullio , R.L. , Salamino , F. , Barbato , R. , Melloni , E. , Siliprandi , N. , Schiaffino , S. and Pontremoli , S. 1995 . Specific degradation of troponin T and I by m-calpain and its modulation by substrate phosphorylation . Biochem. J. , 308 : 57 – 61 . [PUBMED] [INFOTRIEVE]
- Zeece , M.G. , Woods , T.L. , Kee , M.A. and Reville , W.J. 1995 . Role of proteinases and inhibitors in postmortem muscle protein degradation. . AMI Rec. Meat Conf. Proc., , 45 : 51 – 61 .
- Robson , R.M. 1995. 1995 . “ Myofibrillar and cytoskeletal structures and proteins in mature skeletal muscle cells ” . In Expression of Tissue Proteinases of Protein Degradation as Related to Meat Quality , Edited by: Ouali , A. , Demeyer , D.I. and Smulder , F.J.M. Utrecht, , The Netherlands : ECCEAMST .
- Taylor , R.G. , Geesink , G.H. , Thompson , V.F. , Koohmaraie , M. and Goll , E. 1995 . J. Animal Sci. , 73 : 1351 – 1367 .
- Whipple , G. and Koohmaraie , M. 1991 . Degradation of myofibrillar proteins by extractable lysosomal enzymes and m-calpain, and the effects of zinc chloride . J. Anim. Sci. , 69 : 4449 – 4460 . [PUBMED] [INFOTRIEVE]
- Greaser , L.M. and Fritz , D.J. “ Post-mortem changes in myofibrillar proteins in relation to meat texture ” . In Composition of Meat in Relation to Processing, Nutritional and Sensory Quality; from Farm to Fork , Edited by: Lundström , K. , Hansson , I. and Wicklund , E. 293 – 303 . Utrecht, , The Netherlands : ECCEAMST .
- Olson , D.G. , Parrish Jr , F.C. , Dayton , W.R. and Goll , D.E. 1977 . Effect of postmortem storage and calcium activated factor on the myofibrillar proteins of bovine skeletal muscle . J. Food Sci. , 42 : 117 – 124 .
- Pérez-Chabela , M.L. , Escalona-Buendia , H.B. and Guerrero-Legarreta , I. 2003 . Physicochemical and sensory characteristics of calcium chloride-treated horse meat . Int. J. Food Prop. , 6 : 73 – 85 . [CROSSREF]