Abstract
Experimental studies were carried out to determine thermal conductivity (k), thermal diffusivity (α), specific heat at constant pressure (cp), and density (ρ) of cooked ham as functions of both sample moisture content (M) and temperature (T). Thermal conductivity was measured using the heat-line-source probe, thermal diffusivity by Dickerson method, specific heat by differential scanning calorimeter, and density by pycnometer assembly. Temperature ranged from 3.08 °C to 74.08 °C, corresponding to the cooking process, and moisture ranged from 40.0 to 73.0% (w.b.). Equations are provided for α as a function of M, cp as a function of T, and ρ as a function of both M and T. Results for thermal conductivity are compatible with those published in the literature.
INTRODUCTION
Cooked ham is produced in large scale and consumed worldwide. Its main solid constituents are pork thighbone and fat. The processing of ham involves several thermal steps; among them cooking is crucial to provide the peculiar color, taste, and texture associated with ham. Cooking is also responsible for the thermal inactivation of vegetative pathogens such as Salmonella and Staphylococcus. Some substances, such as sodium nitrite, are added to the meat to complement the microorganism activity reduction. However, some additives might react with amino acids, free or bound to the meat, during either cooking or digestion, forming potentially cancerous substances.[Citation1] Thus, to reduce the quantities of additive and to produce a better organoleptic quality of meat, the internal temperature-time relationship should be known.
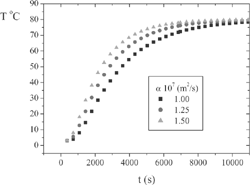
A simulation was performed to illustrate the importance of the thermal properties to predict the temperature field during the cooking process. shows the simulated center temperature for a hypothetical cylindrical-shape-ham of 3” diameter and infinitely long. The classical series solution of the heat conduction equation[Citation2] was used, assuming an initial sample temperature (Ti) of 3.0°C and a constant wall temperature (Tw) of 80.0°C, both usual temperatures in the manufacturing of ham. From this simulation, the estimated times required for Tc to reach 68.0°C are 196, 156, and 130 min for thermal diffusivities of 1.00×10−7, 1.25×10−7 and 1.50×10−7 m2/s, respectively. These time differences might be high enough to either overcook the meat or make deficient thermal inactivation of the microorganism. Only two works are available in the current literature concerning the physical properties of ham. Dickerson and Read[Citation3] tested smoked ham and measured thermal diffusivity (α) and density (ρ) in the temperature range from 43 to 66°C. Sweat[Citation4] determined thermal conductivity (k) and density of country ham at 23°C and specific heat at constant pressure (cp) from 1 to 40°C. Both papers did not present complete compositions of the samples. In this work, α, ρ, and cp were experimentally determined in wide ranges of temperature and moisture content. Thermal conductivity was measured for raw sample at 25.0°C to confirm the other measurements accuracy and to allow comparisons between the presented results with those of Dickerson and Read[Citation3] and Sweat.[Citation4]
MATERIALS AND METHOD
All tests were undertaken in triplicate to provide statistical confidence to the results. Every reference to ham composition is given at wet basis.
Cooked Ham Samples
Tested samples were bought from local retailers, and they were produced by a well known Brazilian industry, which has a good history of quality control. In all measurements, raw samples at 72.4% moisture content (M) were used. For determining α, M was reduced to 40.0 and 56.0%, and for ρ measurements to 15.0 and 44.0%. Raw samples were cut down into small pieces and dried in a convective oven at 50.0°C. Dried samples were wrapped in PVC plastic films and packed in a hermetically sealed container, which was kept refrigerated for a week in order to make uniform the moisture content within the samples. Fat content was determined through the Bligh and Dyer[Citation5] method. Protein, moisture, and ash were determined by AOAC[Citation6] standard procedures.
Thermal Diffusivity
Thermal diffusivity was determined using the method of the infinity cylinder with constant temperature elevation at the tube wall, as first used by Dickerson[Citation7] for foodstuffs. The sample was packed into a brass tube, 35.9 mm diameter and 360.0 mm long, with two nylon caps at the endings. The tip of a sheathed 3mm diameter thermocouple was positioned at the tube center, and another thermocouple was soldered onto the outer tube wall. The measuring cell was submerged in a thermostatic water bath, which provided a constant heating rate (A) of 1.0°C/min. The thermocouples were connected to a computerized data acquisition system. The initial temperature was 3.0°C, and the final center temperature (Tc) was 68.0°C, as customarily used in industry for ham processing. The sample was ground before packing into the tube, and the quantity of meat was enough to reproduce the original ham density. Two techniques were used to pack the cell: without much care for the void spaces within the sample; and using a wood bar, with a central hole with the same thermocouple shaft diameter to eliminate voids. Calculation of α was done through the equation:
where R is the tube radius. Equation (1) is valid after the time (δ) when Tc starts to rise at a constant rate. To compute the value of A, linear regressions of Tw and Tc over the time t were performed and the slopes were averaged. The temperature difference Tw – Tc is the average of all differences after δ. The method was calibrated using water-carragenan gel at 1% weight, and the result agreed favorably with those from the literature for pure water.[Citation8]
Density
Density was measured as a function of both test temperature and sample moisture content. The measurements were made in a pycnometer of 50ml volume with a thermocouple inserted in the recipient cap. Soybean oil was used as pycnometric fluid since it has a high surface tension and does not penetrate the sample. The sample was cut down into cubes of 2g, and about 20g of ham was introduced in the recipient. An analytical balance, 10−4g precision, was used for the experiments. A thermostatic water bath was used for constant temperatures at 2.0°C, 25.0°C, and 74.0°C.
Specific Heat at Constant Pressure
A differential scanning calorimeter TA2010 (TA Instruments) was used to measure cp. Sapphire was employed as a reference material, after a baseline was obtained with empty aluminum pans. Temperature calibration was made with Indium. Ham samples were cut down to closely 15 mg and placed in the pans. The heating rate was 10.0°C/min, and the scanning was made from 5.0 to 90.0°C. The transient periods of start-up and shutdown reduced the valid temperature range from 30.0 to 75.0°C.
Thermal Conductivity
The well known line-heat-source probe[Citation9,Citation10] was used to measure k. The probe was made up of a hypodermic tube, 2.1mm diameter (D) and 50.0mm long (L), which houses both a constantan heating wire, 0.05mm diameter and 0.5Ω/m electrical resistance, and a copper-constantan thermocouple, 0.05 mm diameter for each wire. All elements inside the tube were electrically insulated. The ratio L/D = 23.8 is close to the value recommended by Blackwell[Citation9] in order to minimize the probe length effects. The heating wire was connected to a CC power supply, and the thermocouple connected to a computerized data acquisition system. To calculate k the following equation was used:
where q´ is the heating rate per unit of length; T1 is a reference temperature corresponding to a reference time t1; and to is the start-up time. The sample was cut down to fit a 50ml glass recipient, and the recipient was inserted in to a thermostatic water bath kept at 25.0°C. This method was also calibrated with the water-carragenan gel at 25.0°C and presented very good accuracy.
RESULTS AND DISCUSSION
Composition
shows the average ham composition. The moisture content is similar to that of the country ham tested by Sweat,[Citation4] and the fat content is about half of that of the smoked ham tested by Dickerson and Read.[Citation3]
Table 1 Centesimal ham composition.
Thermal Diffusivity
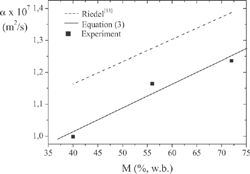
The tube packing technique presented a significant effect only for raw samples. Values of α from packs with voids were about 18% lower than those without voids, although the density was kept the same in both tests. For samples with moisture contents other than 72.45%, the packing technique did not affect α. A statistical student-t test was applied to compare the average values of alpha obtained at different moisture content levels. For this purpose, it was assumed normal distribution for the data population and equal data variances for the averages. Comparing the average values of alpha measured at two consecutive levels of moisture content, it was not observed any statistical difference between these averages at 95% siginificance level, although an increasing trend of alpha with M might be observed, as can be seen in Fig. 2. This small variation of α with M might be related to the presence of polyphosphates used as ham additive. These types of compounds strongly bind water to the meat, resulting in less free water available that would increase the value of α when M increases. This hypothesis is based in the results obtained by Oliveira[Citation12] on pork thighbone where the polyphosphates were not present and α increased significantly with M.
A correlation for α as a function of M was obtained by applying a linear regression to the data, as shown in and . also presents the equation of Riedel[Citation13] for meats, and although the same slope is observed for both curves, there is a shift between the curves, probably due to the presence of the polyphosphates in the ham. By using Eq. (3) to estimate α for 68% moisture content, the same water content as the smoked ham used by Dickerson and Read,[Citation3] the resulting value 1.22x 10−7 m2/s is about 13% lower than the value measured by those authors. This difference may be attributed to the ham type and composition, to the different temperature ranges, and mainly to the voids likely to be present in the sample tested by Dickerson and Read.
Table 2 Correlations for thermal diffusivity, density, and specific heat, and average experimental value for thermal conductivity.
Density
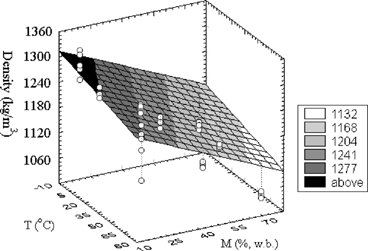
There was no statistical difference between average densities at 2.0°C and 25.0°C by a Student-t test, but they were different for 74.0°C and for the different moisture contents. The significant difference at 74.0°C might be due to the structural thermal modification of the sample. According to Baker,[Citation14] at temperatures above 40.0°C both fat melting and destruction of the supporting fat structure occur. Perez and Calvelo[Citation15] pointed out that for temperatures around 70.0°C protein denaturation, water loss, and air void formation occur. These effects reduce the sample mass but keep constant the volume, decreasing ρ. The increase of ρ with the decrease of M was attributed to the water loss, since water has a lower molecular weight than that of the protein, the second major compound. Sweat[Citation4] and Perez and Calvelo[Citation15] observed the same behavior for fresh meat of nonspecified animals. When ρ was represented separately as a function of moisture content and temperature, a linear trend of ρ with both variables was verified. Thus, a multiple regression was performed, resulting in the Eq. (4), presented in , where T is the temperature in degree Celsius. shows the surface response for the proposed model and all experimental data. Eq. (4) was used to compute ρ for the same experimental condition and the same moisture content as used by Dickerson and Read[Citation3] and Sweat,[Citation4] and the calculated densities agreed very favorably with the experimental results of those authors (Dickerson and Read,[Citation3] ρ = 1080 kg/m3; Sweat,[Citation4] ρ = 1030 kg/m3).
Specific Heat at Constant Pressure
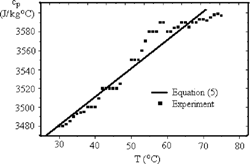
presents cp as a function of the temperature. As previously mentioned, results for cp are limited to a range from 30.0°C to 75.0°C due to the time-lag. A linear regression of these data resulted in the Eq. (5), shown in , and , where a statistical residual bias between the experimental and the calculated values can be seen. Moreover, the Student-t test for two consecutive temperatures was always nonsignificant, even though cp always increased from one temperature to another. To eliminate this residual bias, more repetitions are needed in the same range at the same temperatures, increasing data population and turning out the data distribution closer to a normal distribution. If Eq. (5) is extrapolated to 20.0°C, temperature at which Sweat[Citation4] presented his results, the difference between Sweat's results and the present estimation is less than 0.5%.
Thermal Conductivity
The average value for k was 0.46W/m°C, very similar to the one obtained by Sweat,[Citation4] 0.48 W/m°C. Computing k from the thermal diffusivity definition (), and using Eq. (3), (4), and (5) applied to a moisture content of 72.45% and temperature of 25.0°C, the estimated value is 0.47 W/m°C, an excellent agreement with the experimental result that validates the proposed equations.
CONCLUSIONS
The physical properties density, thermal diffusivity, specific heat, and thermal conductivity of cooked ham were experimentally determined. The composition of the sample was obtained and protein, fat, moisture, and ash contents presented. The experimental methods were calibrated with standard materials, and they were considered very accurate. Predictive equations for α as a function of moisture content, of cp as a function of temperature, and for ρ as a function of both temperature and moisture, were proposed. Thermal conductivity was used to validate these equations, from the thermal diffusivity definition, obtaining excellent agreement. Except for α, all other properties presented values similar to the ones available in the literature for similar products. In the determination of α, the tube packing technique was crucial for reliable experimental results. The difference between the values of α presented in this work with those from the previous literature might be related to the existence of void spaces in the sample in the previous experiments and to the differences of both ham formulation and temperature range.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Paulo J.A. Sobral (USP) and Dr. Fernanda E.X. Murr (UNICAMP) for their valuable help during the experimental work; to Dr Róger D. Barbosa (UNESP) for his helpful cooperation with the text; and to CAPES for the financial support.
Notes
12. Oliveira, G.S. Experimental Study of the Thermal Properties of Cooked Ham. M.Sc. Thesis Paulista State University: São José do Rio Preto, 2002. (in Portuguese)
13. Riedel, L. Measurements of thermal diffusivity of foodstuffs rich in water. Cited by Dickerson and Read3
REFERENCES
- Varnan , A.H.E. and Sutherland , J.P. 1995 . Meat and Meat Products: Technology, Chemistry and Microbiology , London : Chapman and Hall .
- Carslaw , H.S. and Jaeger , J.C. 1959 . Conduction of Heat in Solids , Oxford : Oxford University Press .
- Dickerson , R.W. and Read , R.B. 1975 . Thermal diffusivity of meats . Transactions of the ASHRAE , 81 : 356 – 364 .
- Sweat , V.E. 1985 . Thermal properties of low and intermediate moisture contents food . Transactions of ASHRAE , 91 : 369 – 389 .
- Bligh , E.G. and Dyer , W.J. 1959 . A rapid method of total lipid extraction and purification . Canadian Journal of Biochemistry and Physiology , 37 : 911 – 917 . [PUBMED] [INFOTRIEVE]
- AOAC . 1984 . Official Methods of Analysis , 14 , Washington, DC : Association of Official Analytical Chemists .
- Dickerson , R.W. 1965 . An apparatus for measurement of thermal diffusivity of foods . Food Technology , 22 : 37 – 52 .
- Perry , R.H. , Green , D.W. and Maloney , J.O. 1997 . Perry's Chemical Engineers’ Handbook , 7th , New York : McGraw-Hill .
- Blackwell , J.H. 1956 . The axial flow error in the thermal conductivity probe . Canadian Journal of Physics , 34 : 412 – 417 .
- Elustondo , D. , Elustondo , M.P. and Urbicain , M.J. 2001 . New thermal conductivity probe design based on the analysis of error sources . Journal of Food Engineering , 48 : 325 – 333 . [CROSSREF]
- Levine , D.M. , Berenson , M.L. and Stephan , D. 2001 . Statistics for Managers Using Microsoft Excel , 3rd , Upper Saddle River : Prentice Hall .
- 12. Oliveira, G.S. Experimental Study of the Thermal Properties of Cooked Ham. M.Sc. Thesis Paulista State University: São José do Rio Preto, 2002. (in Portuguese)
- 13. Riedel, L. Measurements of thermal diffusivity of foodstuffs rich in water. Cited by Dickerson and Read3
- Baker , L.C. and Lawrie , R.A. 1998 . Lawrie's Meat Science , 6 th , Woodhead : Cambridge (UK) .
- Perez , M.G.R. and Calvelo , A. 1984 . Modeling the thermal conductivity of cooked meat . Journal of Food Science , 49 : 152 – 156 . 1984