Abstract
The sweet potato genotypes in the CTCRI germplasm collection were screened for anthocyanin content in the young top leaves and the values varied from 20-200 mg/100g fresh weight of the leaf samples, the highest being recorded for the genotype S-406. Total anthocyanin content (TAcy) showed significant difference among genotypes, growth phases, and growing seasons. The TAcy in the leaves reached a peak at 20 days after planting, declined after 35 days, returned to 20 day levels or higher by 50-65 days and remained stable or exhibited a slight decrease by 105 days after planting, for most of the genotypes selected for growth phase studies. Approximately 50-60 % decrease was observed in the TAcy of the frozen/thawed leaf samples. The stability of anthocyanin pigments in a model sugar-citrate solution at ambient (30 ± 2 °C) and refrigerated (8 ± 2 °C) conditions was studied. The solutions stored at low temperature and in dark showed higher retention of pigments. Compared to anthocyanins extracted from grape pomace, the sweet potato anthocyanins were more stable as indicated by the higher values of half-life and pigment retention. The anthocyanins were more stable at the pH range of 3.2-3.4 and the colour shifted from red to pale yellow at higher levels.
INTRODUCTION
Anthocyanins are phenolic plant metabolites belonging to the flavanoid group. They are very widespread in the plant kingdom and responsible for the red, violet, purple and blue colours found in plant parts like fruits, vegetables, flowers, leaves, roots, tubers etc. All natural anthocyanins are glycosides; the corresponding aglycones are called anthocyanidins. Differences between the aglycon bodies are due to the number of hydroxyl groups and the degree of methylation of these groups.[Citation1] Anthocyanins and other flavanoids are receiving renewed attention for their potential health benefits as antioxidants and anti-inflammatory agents. [Citation 2 ,Citation 3 ,Citation 4 ] Existing evidences indicate that anthocyanins are non-toxic, non mutagenic and also have positive therapeutic properties and used in the treatment of circulatory disorders and inflammatory diseases.[Citation5]
Anthocyanin expression in plants is controlled by both genetic and environmental factors. The latter include temperature, water potential, diseases, nutrient levels, and other nutritional factors.[Citation6] Esteban et al[Citation7] studied the effect of irrigation on anthocyanin formation in the skins of cv Tempranillo grape berries during ripening. Several workers have considered solar radiation to be critical factor in color development in the berries of many grape varieties. [Citation 8 ,Citation 9 ] Freezing of berries prior to final processing for anthocyanin extraction has been reported. Bronnum–Hansen et al [Citation10] noticed that freezing of elderberries produces much less pomace weight without any significant reduction of anthocyanin distribution in the pomace.
Grape pomace has been the single most abundant source from which anthocyanins are commercially obtained. More recently, alternative sources, such as vegetable juice extracts and berries, have drawn the attention of food industry. Studies conducted at Central Tuber Crops Research Institute (CTCRI) have shown that some varieties of sweet potato (Ipomoea batatas, Lam (L.) and yams (Dioscorea alata) contain anthocyanins in their tubers, leaves, vines, and flowers. In Japan, a sweet potato variety known as ‘Ayamurasaki’ has been used for the commercial extraction of anthocyanin pigments.[Citation11] The stability of anthocyanins is affected by various factors such as pH, temperature, oxygen, light, and certain enzymes.[Citation12] However, compared to other commonly available sources, sweet potato anthocyanins are reported to be more stable.[Citation13] Preliminary studies at CTCRI have shown in that the purple-coloured leaves of sweet potato are rich and in unexploited sources of anthocyanins with good stability in food systems even after one-month storage.[Citation14]
Since basic information on the anthocyanin concentration in the coloured leaves of sweet potato, changes during growth phases, and the stability of these pigments in various foods are scanty, a study was undertaken to screen the sweet potato genotypes for anthocyanin pigments and to understand the changes in anthocyanin concentration in the leaves during the life plant's cycle. The stability of sweet potato anthocyanins in a model sugar-citrate solution under long-term storage and the effect of pH on the colour and stability were studied.
MATERIALS AND METHODS
Screening of Germplasm
The anthocyanin content in the samples was determined according to the method described by Fuleki and Francis.[Citation15] The purple coloured leaf samples were collected from 3 to 4 month old sweet potato plants. The leaf samples were cut into small pieces and 5g of the pooled samples was homogenized in 50 ml of the extraction solvent (95% ethanol/1.5 N HCl, 85:15). The homogenate was allowed to stand in a refrigerator in the dark at 4°C overnight. The ethanolic extract was then filtered and more solvent was added to the residue; this procedure was repeated as many times as required until all colour had been extracted from the leaf tissues. Finally, the extracts were combined, filtered and made up to 100ml. The chlorophyll present in the extract was removed by treatment with chloroform and the upper layer containing anthocyanins was collected. An aliquot from the above extract was diluted with the solvent and stored in the dark for 2 h to equilibrate the colour. The OD was measured in a Unicam spectrophotometer at 535 nm using the solvent blank. The extraction was done in duplicate for each genotype. The total anthocyanin content was calculated with the aid of appropriate weight, volume, dilution factors, and an extinction coefficient value of 98.2. The absorption maxima of the anthocyanin extracts (λmax) were also recorded.
Growth Phase Studies
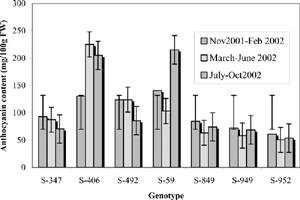
Seven genotypes of sweet potato selected from the germplasm were used for the growth phase studies. The quantitative changes that occurred in the total anthocyanin content of purple-coloured sweet potato leaves over a period from the date of planting to harvest were analysed and compared during three growing seasons. The young purple-coloured leaves were sampled at fortnightly intervals throughout the crop growth period until harvest. Samples from three seasons—, November 2001-February 2002, March-June 2002 and July- October 2002—were assayed individually in duplicate.
A portion of the freshly-plucked leaf samples was frozen and stored at –5°C. Frozen material was thawed by placing at room temperature for 2-3 h. Total anthocyanins from fresh and frozen/thawed sweet potato leaves were extracted and determined by the method described earlier. Optical Density (OD) of the extracts was measured directly at two wavelengths, 420 and 535 nm, corresponding to the yellow and red components in the pigment, respectively. The colour intensity of the extract was the sum (OD-420 + OD-535) and hue was the ratio (OD-420/OD-535) of the two-absorbance values.[Citation7]
Stability of Anthocyanins in a Model System
The fresh leaf samples were cut into small pieces, macerated with 1% HCl and kept in a refrigerator overnight. Then the samples were filtered through a Buckner funnel under vacuum. The residue was washed repeatedly with the solvent until the entire colour was extracted. The crude extract was purified by ion-exchange chromatography using HP-20 Diaion resin and concentrated in a vacuum flash evaporator at 50°C.
A sugar-citrate solution (13 ° Brix) was prepared by dissolving 130 g of sucrose in one litre of 0.1 M citric acid solution, and the pH was adjusted to 3.5. To this solution, colour concentrate was added at a concentration of 1% (1 ml for 100 ml solution); 10 ml samples were taken in glass tubes and stored under the following conditions: T1 at 8 ± 2 °C (refrigerator), T2 at 30 ± 2 °C (room temperature) in darkness, and T3 at 30 ± 2 °C in day light. The above conditions were selected, because soft drinks and beverages are normally stored under one of these conditions.
The anthocyanin contents of the extracts and the sugar-citrate solutions were measured by pH differential method [Citation15] using the following two buffers: a) 0.2 N KCl-0.2 N HCl (25:67), pH 1.0; and b) 1 N sodium acetate-1 N HCl-water (100:60:90), pH 4.5. The mixtures of buffer and sample were equilibrated in darkness for 2 h, and the absorbance was measured at 510 nm. The anthocyanin content was calculated using an extinction coefficient value of 77.5. The pigment retention in the above sugar-citrate solutions was determined at 15-day intervals. The determinations were carried out in triplicates. Anthocyanins from grapes were also extracted, added to sugar-citrate solutions, and stored under the same conditions. The reaction rate constants were calculated by the formula of averages.[Citation16]
The half-life, T1/2 = 0.693 /k for each treatment was also calculated.
Effect of pH on Stability
The effect of pH on the colour and stability of anthocyanins was studied. The pH of the sugar-citrate solutions containing sweet potato anthocyanins was adjusted to different levels ranging from 2.6 to 10.0 and stored in daylight at room temperature for one week. The absorption maximum (λmax) and OD, which correspond to the anthocyanin content in the solutions, were determined on alternate days.
The experiments were conducted according to factorial completely randomized design with three replications. Analysis of variance was conducted to study the main effects of each factors and their interaction effect. The comparisons of mean values were made using least significance difference (LSD) at 5% levels of significance. The computations were carried out using GENSTAT 6.[Citation17]
RESULTS AND DISCUSSION
Screening of Sweet Potato Germplasm
Many accessions in the sweet potato germplasm collection at CTCRI had purple coloured tender leaves. Only such genotypes were selected for anthocyanin estimation. Wide variability was found to exist in the anthocyanin content of sweet potato leaves with values ranging from 20-220 mg/100g fresh weight (FW) of the samples. Depending on the anthocyanin content in the young leaves, sweet potato genotypes were grouped into four categories: those which contain less than 50, 50-100, 101-150 and above 150 mg of total anthocyanins/100g FW of the samples ().
Table 1. Distribution of total anthocyanins in the leaves of sweet potato genotypes.
Among the 30 genotypes screened, two were in the first group with low content of anthocyanins in their top leaves, and 21 belonged to the second group. Four genotypes possessed anthocyanin content in the range of 101-150 mg, while three were in the group with more than 150 mg of anthocyanins in their tender leaves. The genotype S-59, S-402, and S-406 was found to be particularly rich in anthocyanin content with 182.22, 215.85, and 218.43 mg/100g FW of the leaves, respectively. The quantity of purple coloured leaves produced by these varieties was also observed to be higher.
Generally, anthocyanin pigmentation in tender leaves is temporary and disappears rapidly as the leaves mature. The cause of anthocyanin formation at this stage in leaf development is probably due to the sugar accumulation in the tissues in excess of the immediate requirements for growth. Anthocyanin synthesis in leaves seems to be rather closely related to carbohydrate metabolism.[Citation18] In the present study, all the seven genotypes of sweet potato accumulated anthocyanins in only young top leaves.
Growth Phase Studies
Analysis of variance showed a significant difference in the TAcy of the tender leaves during different growth phases and genotypes of sweet potato (P< 0.001) (). Over all the growth phases, the genotype S- 406 was found to have significantly higher anthocyanin content in the leaves with a mean value of 182.70 mg/100g FW, followed by S-59 (102.85 mg), and S-492 (98.98 mg) (). The quantity of purple colored leaves produced by S- 406 was also higher compared to other genotypes. Significantly higher values for TAcy in the leaves was observed at 65 DAP with an average value of 102.14 mg, which was on par with the anthocyanin content at 20 and 95 DAP (102.04 and 101.72 mg/100g FW, respectively). The genotype S- 406 recorded the highest average anthocyanin concentration of 227.53 g at 20 DAP and 225.23mg/100g FW at 95 DAP.
Table 2. Analysis of variance of Total anthocyanin content (TAcy) in sweet potato leaves.
Table 3. Effect of growth phases and freezing/thawing on the anthocyanin concentration (expressed as mg/100g fresh wt.) of the leaves of sweet potato.
In general, the TAcy in the leaves reached a peak at 20 days after planting, declined after 35 days, and returned to 20 day level or higher by 50–65 days and remained stable or exhibited a slight decrease by 105 days after planting. Maximum foliage was observed at 35 DAP. The distribution of anthocyanins among the larger number of leaves during this period may be responsible for the observed decrease in TAcy. Statistical analysis of the data of anthocyanin content in the leaves during different growing seasons showed that the TAcy was significantly higher in the genotype S- 406 with an average value of 187mg/100g FW,followed by S-59 and S- 492 with 153.2 mg and 111.3 mg /100g FW, respectively. The genotypes did not perform uniformly across the seasons and there were significant effect of seasons (p = 0.037) and accessions (p<0.001) on the anthocyanin content in the leaves. The genotype S- 406 recorded significantly higher TAcy (225.3 g) during March–June 2002, while S-59 showed higher TAcy during July–October 2002 (215 mg). For S-492, the TAcy values were almost same during November 2001–February 2002 and March–June 2002. The other genotypes showed higher TAcy in leaves during November 2001–February 2002 ().
Figure 1. Changes in the total anthocyanin concentration (expressed as mg/100g fresh wt.) of the sweet potato leaves during different growing seasons.
The TAcy pattern over different growth periods was almost same for the leaf samples frozen/thawed. However, a significant decrease of about 50% was observed in the TAcy of the freeze/thawed leaf samples (). Here also, highest TAcy was observed in S-406 with an average value of 102.91 mg/100g FW. Bronnum-Hansen et al.[Citation10] studied the influence of freezing the freshly-harvested elderberry fruits on colour extract production. They observed that freezing of berries prior to pressing for anthocyanin extraction resulted in a small change only in the distribution of anthocyanin between pomace and juice. Apparently, freezing step gives less pomace for extraction without significantly reducing the anthocyanins available in the pomace. However, a slight decrease in % TAcy in pomace was observed.
Lewis et al. [Citation6] studied the effect of cold storage on anthocyanin concentration in the skin of coloured potato tubers. They observed that during storage at 4°C, the concentration of anthocyanins, other flavanoids, and phenolic acids increased in the tubers. Cold storage of tubers caused a gradual but significant increase in anthocyanin concentration over a period of five months. Cold storage of tubers is known to cause the production of sugars from starch (Cold sweetening).[Citation19] Because sugars are anthocyanin precursors, this increase in sugars may play a part in anthocyanin synthesis during cold storage. However, in the case of sweet potato leaves, during storage in a freezer at –5°C and thawing at room temperature, a significant reduction was noticed in anthocyanins extracted. In the case of leaf samples, the possibility of rise in anthocyanins is negligible. Garcia-Viguera et al., [Citation20] observed that when jam was made from frozen red raspberries, the total anthocyanin concentration was lower compared to fresh fruits. Thawing of the fruits after freezing at –20°C for 24 h resulted in jam with smaller anthocyanin content (9-24% lower). During freezing and thawing, the cell structures are disrupted and the plastidic oxidative enzymes (polyphenol oxidase) and the vacuolar substrates (phenolics) interact, and during thawing, phenolic pigments are lost due to this enzymatic process. The loss of anthocyanin pigment in the frozen/thawed sweet potato leaves could be attributed to similar enzymatic process, which brought about destruction of phenolic pigments.
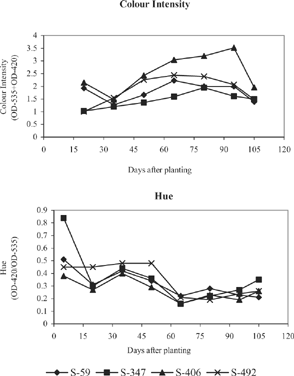
shows the colour intensity and hue of the leaf anthocyanin extracts from the genotypes S-59, S- 406, S-347 and S-492. The extracts from S-59 and S-492 recorded higher colour intensity of 2.23 and 2.44 respectively, at 65 days after planting. The maximum colour intensity of 3.52 was observed at 95 days for S- 406. For the genotype S-347, the colour intensity showed a maximum of 1.94 at 80 days and thereafter a gradual decrease was observed. For S- 492, the colour intensity values increased up to 65 days and declined gradually over the remaining period. Anthocyanins were the components that made the highest contribution to colour intensity. Among the genotypes, S - 406 showed higher colour intensity of the extracts during the period of 65-95 DAP followed by S-492 and S-59. All the four genotypes recorded higher values for hue at 5 days and 35 days after planting. The genotypes S- 59, S-347 and S- 406 showed lowest hue values i.e., higher absorbance in the region of the red component of the pigment, at 65 days and S-492 at 80 days of planting.
Stability of Anthocyanins in a Model System
illustrates the effect of two storage parameters, temperature and light, on the stability of anthocyanins from sweet potato leaves in a model sugar-citrate solution. Analysis of variance showed that there was a gradual decrease in % pigment retention values on storage of the sugar-citrate solutions in all the three storage conditions studied.
Table 4. Anthocyanin pigment retention in the model sugar-citrate solutions under different storage conditions.
The treatment T1 showed significantly higher pigment retention (96.73%), followed by T2 and T3 with mean values of 90.53 and 86.20 %, respectively. In the samples stored in a refrigerator, there was no significant difference in anthocyanin content up to 75 days. After 135 days, the pigment retention was 93.19%.There was no significant loss in pigment content in the samples stored at room temperature in darkness until 30 days of storage. After this period, significant difference in the % pigment retention was observed. The sugar-citrate solutions stored at room temperature in light showed lower pigment retention with an average value of 70.35 %, remaining after 135 days of storage. At high storage temperature (30 ± 2°C), the pigment degradation was greater in the solutions, especially when stored in exposure to light.
The stability of sweet potato and grape anthocyanins in sugar-citrate solutions on storage was studied and the data is presented in . Although pigment content decreased significantly with increasing storage time in both cases, comparatively higher pigment retention was observed for the systems containing sweet potato anthocyanins. The pigment retention decreased to 79.34% for the solutions containing sweet potato anthocyanins after135 days of storage; however, only 56.14 % pigment retention was observed in the sample containing grape anthocyanins.
Table 5. Pigment retention in the sugar–citrate solutions containing grape and sweet potato anthocyanins and stored at ambient temperature.
The studies of Bassa and Francis [Citation21] showed that anthocyanins from sweet potatoes are an effective colourant for beverages. The sweet potato samples were much more stable than a commercial sample of enocyanin in a model beverage. Our results also agree with the observations of Palamidis and Markakis [Citation16] who studied the effect of temperature and light on the stability of grape anthocyanins added to a carbonated beverage. Increase in the storage temperature accelerated the pigment destruction in the beverage. In darkness, at 38°C, only 23% of the original amount of the pigment was left in the beverage after 135 days, while at 3.5 °C, under the same conditions, 92% of the pigment was retained. Exposure to light accelerated the degradation of anthocyanins. The degradation of the pigments in sugar-citrate solution followed first order reaction kinetics. The reaction rate constants (k) and half-lives (T1/2) are listed in . The pigment extracted from sweet potato leaves was found to be more stable than that from grape pomace.
Table 6. Reaction rate constants (k days –1) and half-life time (T1/2) of anthocyanins in the stored sugar-citrate solutions.
Effect of pH
In a model system of sugar-citrate solution, a decrease in pH from 5 to 2.6 enhanced the pigment retention (). Since the λmax of the solutions shifted from 530 nm at higher pH levels, only those values below pH 5.0 are presented in . A gradual decrease in OD values was observed with storage time at all pH levels from 2.6 to 5.0. However, in the pH range of 3.2- 3.4, this change was not significant. At pH levels below 3.2 and above 3.4, there was significant reduction in OD values on storage. When the pH of the solutions increased from 6.0 to 10.0, the λmax values increased to the range of 540-590 nm. The colour of the systems also changed from red to light yellow.
Table 7. Effect of pH on the colour and stability of sugar-citrate solutions containing sweet potato anthocyanins.
Anthocyanin pigments are more stable at low pH values [Citation22]. Temperature, time of processing, and storage were found to exert a significant influence on anthocyanin stability. [Citation23,Citation24,Citation25] Daravingas and Cain [Citation26] studied the thermal degradation of anthocyanin pigments of black raspberries as influenced by pH, oxygen, and sugar. The degradation of the total isolated pigments and the pigments in the natural berry juice were retarded as the pH decreased. Their studies, using freshly extracted black raspberry juice at various pH levels, indicated that lowering the pH from 4.15 to 2.15 increased the stability of anthocyanin pigment in the juice. In a model system of cyanidin – 3 - diglucoside, the major anthocyanin present in black raspberry, a decrease in pH from 4.25 to 0.95 enhanced the pigment retention. This phenomenon has been explained on the basis of the fact that the concentration of the more stable cation-form of the pigment decreased at higher pH values giving rise to the more labile non-ionic form.
CONCLUSION
The total anthocyanin content in the young purple coloured leaves of sweet potato genotypes showed significant variation among genotypes, growth phases, and growing seasons. The anthocyanin pigment extracted from sweet potato leaves was found to be more stable than grape anthocyanins in a model system of sugar-citrate solution, under different storage conditions. Presently, there is no economic value for sweet potato leaves and vines; they are either fed to cattle or use as a green manure. The results of our study indicate that the tender leaves can be used as a cheap source of anthocyanin pigments. Defoliation of young leaves will bring about the regeneration of fresh new leaves and, therefore, do not affect the yield of the vines for cattle feed. Studies on the characterization of anthocyanins in sweet potato leaves are in progress in our laboratory.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the help and encouragement given by Dr. S. Edison, Director, Central Tuber Crops Research Institute and Dr. G. Padmaja, Head, Division of Crop Utilization. Thanks are also due to Mr. J. Sreekumar, Scientist, CTCRI, for the statistical analysis of the data.
REFERENCES
- Mazza , G. and Miniati , E. 1993 . Anthocyanins in Fruits, Vegetables and Grains , London : CRC Press .
- Wang , H. , Cao , G. and Prior , R.L. 1997 . Oxygen radical absorbing capacity of anthocyanins . J.Agric. Food Chem. , 45 : 304 – 305 .
- Kalt , W. and Dufour , D. 1997 . Health functionality of blueberries . Hortic. Technol. , 7 : 216 – 221 .
- Wang , H. , Nair , M.G. , Strasburg , G.M. , Chang , Y.C. , Booren , A.M. , Gray , J.I. and Dewitt , D.L. 1999 . Antioxidant and anti-inflammatory activities of anthocyanins and their aglycone, cyanidin, from Tart cherries . J.Nat. Prod. , 62 : 294 – 296 . [CROSSREF]
- Saija , A. 1994 . Pharmacological effects of anthocyanins from blood orange juice . Essenze-Deriv. Agrum , 64 ( 2 ) : 229 – 233 .
- Lewis , C.E. , Walker , R.L and Lancaster , J.E. 1999 . Changes in anthocyanin, flavanoid and phenolic acid concentrations during development and storage of coloured potato (Solanum tuberosum L) tubers . J. Sci. Food Agric. , 79 : 311 – 316 . [CROSSREF]
- Esteban , M.A. , Villanueva , M.J. and Lissarrague , J.R. 2001 . Effect of irrigation on changes in the anthocyanin composition of the skin of cv Tempranillo (Vitis vinifera L) grape berries during ripening . J. Sci. Food Agric. , 81 : 409 – 420 . [CROSSREF]
- Kliewer , W.M. 1970 . Effect of day temperature and light intensity on colouration of Vitis vinifera L grapes . J.Am Soc Hort Sci. , 95 : 693 – 697 .
- Crippen , D.D. Jr and Morrison , J.C. 1986 . The effects of sun exposure on the phenolic content of cabernet Sauvignon berries during development . Am J Enol Vitic , 37 : 275 – 280 .
- Bronnum-Hansen , K. , Jacobsen , F. and Flink , J.M. 1985 . Anthocyanin colourants from elderberry (Sambucus nigra L.). 1. Process considerations for production of the liquid extract . J. Food. Tecnol. , 20 : 703 – 711 .
- Yamakawa , M. , Okunu , S. , Yoshinaga , M. , Yamakawa , O. , Yamaguchi , M. and Yamada , J. 1999 . Antimutagenicity of sweet potato (Ipomoea batatas) roots. Biosci . Biotechnol.Biochem. , 63 : 537 – 541 . [CROSSREF]
- Markakis , P. 1974 . Anthocyanins and their stability in foods . CRC Critical Reviews in Food Technology , 4 : 437
- Philip , T. , Cited in Bassa , I.A. and Francis , F.J. 1987 . Stability of anthocyanins from sweet potatoes in a model beverage . J. Food. Sci. , 52 ( 6 ) : 1753 – 1754 .
- Jyothi , A.N. , Moorthy , S.N. and Eswariamma , C.S. Sweet potato leaves-A potential source of anthocyanins as natural food colours . Abstracts of Invited and Contributed Papers, International Conference on Vegetables . Nov11-14 2002 , Bangalore, India. Vol. X-6-P , pp. 380 Bangalore : Dr. PremNath Agricultural Science Foundation .
- Fuleki , T. and Francis , F.J. 1968 . Quantitative methods for anthocyanins . J. Food. Sci. , 33 : 72 – 83 .
- Palamidis , N. and Markakis , P. 1975 . Stability of grape anthocyanins in a carbonated beverage . J Food Sci. , 40 : 1047
- Payne , RW. , ed. 2000 . The Guide to Genstat Part 2 , Oxford, U.K. : VSN International Ltd .
- Harborne , J.B. 1965 . “ Flavanoids: Distribution and contribution ” . In Chemistry and Biochemistry of Plant Pigments , Edited by: Goodwin , TW. 247 – 277 . London : Academic Press .
- Isherwood , F.A. 1976 . Mechanism of starch-sugar interconversion in Solanum tuberosum . Phytochem , 15 : 33 – 41 . [CROSSREF]
- Garcia-Viguera , C. , Zafrilla , P. , Ates , F. , Romero , F. , Abellan , P. and Tomas-Barberan , F.A. 1998 . Colour and anthocyanin stability of red raspberry jam . J. Sci. Food Agric. , 78 : 565 – 573 . [CROSSREF]
- Bassa , I.A. and Francis , F. J. 1987 . Stability of anthocyanins from sweet potatoes in a model beverage . J Food Sci , 52 ( 6 ) : 1753 – 1754 .
- Meschter , E.E. 1953 . Effects of carbohydrates and other factors on strawberry products . J Agric Food Chem , : 574 – 579 . [CROSSREF]
- Decareu , R.V. , Livingaton , G.E. and Fellers , C.R. 1956 . Colour changes in strawberry jellies . Food Res , 10 : 125 – 128 .
- Markakis , P. 1982 . “ Stability of anthocyanins in food ” . In Anthocyanins as Food Colours , Edited by: Markakis , P. 163 – 180 . New York : Academic Press .
- Pilano , L.S. , Wrolstad , R.E. and Heatherbell , D.A. 1985 . Influence of fruit composition maturity and mold concentration on the colour and appearance of strawberry wine . J Food Sci. , 50 : 1121 – 1125 .
- Daravingas , G. and Cain , R.F. 1968 . Thermal degradation of black raspberry anthocyanin pigments in model systems . J.Food. Sci. , 33 : 138 – 142 .