Abstract
We aimed to examine the growth suppressive effects of quercetin on acute promyelocytic and lymphoblastic leukemia and chronic myeloid leukemia, and to find out whether the growth suppression is related to the blocking of telomerase enzyme activity. Cytotoxic effects of quercetin were shown by trypan blue analyses. Apoptotic effects of quercetin were examined by acridine orange and ethidium bromide staining by fluorescence microscopy. The effects of quercetin on telomerase enzyme activity were shown by hTERT Quantification Kit. Our results demonstrated that quercetin has antiproliferative and apoptotic effects on T-cell acute lymphoblastic leukemia (ALL), acute promyelocytic leukemia, and chronic myeloid leukemia (CML) cells. We also showed for the first time by this study that quercetin suppresses the activity of telomerase in ALL and CML cells. The results of this study show the importance of quercetin for its therapeutic potential in treatment of leukemias.
Introduction
Flavonoids are naturally occurring polyphenolic compounds found widely in roots, stems and flowers of grains, fruits, and vegetables. Although initially they were not thought to have functions besides pigmentation, increasingly studies have revealed that flavonoids are involved in numerous physiological processes in plants. Flavoring of food plants,Citation1 antifungal and bactericidal protection,Citation2 protection from ultraviolet radiation,Citation3 and regulation of plant growthCitation4 are among the processes in which flavonoids have importance. Antioxidative protection is one of the most important features of flavonoids.Citation5 Additionally, flavonoids exert clinically relevant anti-atherosclerotic,Citation6 anti-inflammatory,Citation7 anti-thrombogenic,Citation8 and anti-viralCitation9 effects.
Quercetin is a member of the flavones subgroup of the flavonoids, and it is prevalently found in black and green tea, red onion, broccoli, and tomato.Citation10 This compound has antioxidant properties and it is especially known for its iron-chelating characteristics.Citation11 Quercetin has been shown to have beneficial effects on health such as reduction of the risks of coronary artery disease,Citation12 lung cancer,Citation13 asthma,Citation14 and disruption of pulmonary functions.Citation15 It is also known to have anti-inflammatory effects through inhibition of cytokines, such as TNF-alpha and IL8.Citation16,Citation17 This compound is also potent for inhibiting melanoma growthCitation18 and angiogenesis.Citation19 Because of those properties, quercetin deserves further research to delineate its possible usage as an antineoplastic agent in various cancers.
Leukemias are cancers of blood-forming tissues. As in other types of cancer, blood forming progenitor cells undergo uncontrolled cell proliferation in leukemias and interfere with the normal functioning of the circulatory system. Three leukemic cell lines used in this study represent three different types of leukemia. The first of these is chronic myeloid leukemia (CML) which is the first leukemia where its progression is documented to be caused by a chromosomal translocation.Citation20 Reciprocal translocation between chromosomes 9 and 22 producing the Philadelphia chromosome is the main driving force of the malignant transformation since it causes constitutive expression of an aberrant tyrosine kinase, BCR–ABL fusion protein.Citation21 The second leukemia type involved in the current study is acute promyelocytic leukemia (APL) which is a subtype of acute myelogenous leukemia. In the majority of the APL cases, retinoic acid receptor-alpha gene on the chromosome 17 undergoes a reciprocal translocation with promyelocytic leukemia gene on the chromosome 15.Citation22 The third and the last type of the leukemia within the scope of this study is acute lymphoblastic leukemia (ALL). While the exact causes of ALL are not known, some genetic and chromosomal aberrations and epigenetic changes are found in the leukemic cells that might disrupt the cellular signaling pathways and transcription factors.Citation23–Citation26 Chemotherapy and radiotherapy for the increased burden of the leukemic cells are the current options for the ALL therapy.Citation27
Telomeres are the repeat sequences found at the ends of chromosomes which protect against the replicative loss of the genetic information occurring each time the cell replicates its DNA. This loss is an inevitable outcome of the DNA replication machinery which cannot synthesize the daughter DNA strand fully at the farthermost ends. Integrity of the telomeres was shown to be affected by the oxidative stress in addition to the end-replication problem.Citation28 Deterioration of the telomeres is thought to be responsible for the limited capacity of replication of the cell under in vitro conditions.Citation29 However, cancer cells maintain the ability of limitless replication potential by re-activating the telomerase enzyme which prevents shortening of the ends of chromosomes by adding more repetitive sequences.Citation30 Telomerase composed of a reverse transcriptase (hTERT in humans) and an RNA component which would be used as the template for elongation of the telomeric DNA. Activation of this enzyme is one of the hallmarks of cancerCitation31,Citation32 and suppression of this ability might provide effective means to fight with cancer.
In the current study, we aimed to investigate quercetin-dependent apoptotic induction and inhibition of leukemic cell proliferation, and the effects of quercetin on telomerase activity.
Materials and Methods
Leukemic cell lines and chemical reagents
CCRF-CEM human T-cell acute lymphoblastic leukemia, HL-60 human acute promyelocytic leukemia, and K-562 human chronic myeloid leukemia cells were used as model systems in this study which was obtained from ATCC (Manassas, VA, USA). Quercetin was obtained from Sigma Chemical Co. (St Louis, MO, USA). The chemicals were diluted in 0·5% dimethylsulphoxide. Cell proliferation assay (XTT) was supplied from Roche Diagnostics (Munchen, Germany). LightCycler Telo TAGGG hTERT Quantification Kit was obtained from Roche Applied Science (Mannheim, Germany) for the quantification of hTERT mRNA. All other tissue culture supplies were obtained from Corning Incorporated (Corning, NY, USA) unless specified otherwise.
Assessment of cytotoxicity
All leukemia cells were incubated at the density of 5×105 cells/ml in RPMI-1640 medium containing 2 mM L-glutamine supplemented with 10% inactivated fetal bovine serum and 1% penicillin/streptomycin in a standard cell culture incubator at 37°C, under humidified 95% air, and 5% CO2 atmosphere.Citation33 Before each experiment, cells were split at 5×105 cells/ml in the RPMI 1640 medium and cell suspensions was aliquoted into flasks for subsequent treatments. Quercetin diluted in RPMI-1640 was used in treatments of 12·5, 25, 50, 75, and 100 μM. Cytotoxicity effects of quercetin on leukemia cells were determined by using trypan blue dye exclusion as indicated in manufacturers’ instructions.
Morphological evaluation of apoptosis using acridine orange/ethidium bromide staining
Apoptosis was determined morphologically after staining with acridine orange and ethidium bromide by fluorescence microscopy. Cells were washed with cold phosphate-buffered saline and adjusted to the cell density of 1×106 cells/ml in phosphate-buffered saline. Acridine orange and ethidium bromide (1∶1) (v/v) were added to the cell suspension in final concentrations of 100 μg/ml and then cells were incubated for 30 min. The cellular morphology was evaluated by fluorescent microscopy (Olympus, Tokyo, Japan). Apoptotic cells were essentially characterized by nuclear condensation of chromatin and/or nuclear fragmentation. Three hundred cells were evaluated for apoptosis and/or necrosis for each sample. When more than 50% of the pre-apoptotic plus apoptotic to total cell ratios were positive, the result was accepted positive for apoptosis.
Determination of hTERT activity
Total RNA was isolated from the leukemia cells treated with increasing concentrations of quercetin for 48 and 72 hours and, hTERT mRNA quantification was performed with commercially available LightCycler TeloTAGGGG hTERT Quantification Kit (Roche Applied Science) using the LightCycler instrument for real-time PCR. All subsequent quantification steps were carried out according to manufacturer’s instructions.
Results
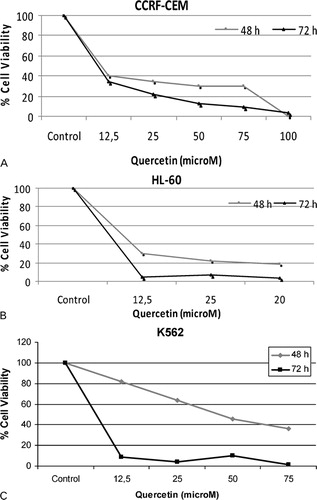
Quercetin decreases cell viability of leukemic cell lines in a time- and dose-dependent manner
Leukemic cell death upon treatment with quercetin was assessed by the trypan blue staining. Time- and dose-dependent decrease patterns were found in the viability of all the three leukemic cell lines (). K562 cells showed a more gradual survival curve than the CCRF-CEM and HL60 cells at 48 hours, but at 72 hours, K562 cells had the steepest decrease in the survival. Increasing concentrations of quercetin is linked to the increased cell death in leukemic cells.
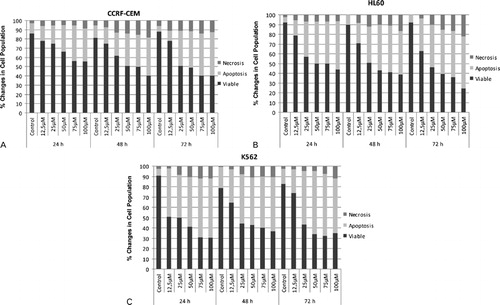
Evaluation of apoptotic induction
Treatment of the leukemic cells with quercetin decreased the viability and increased the apoptotic and necrotic cell death in a dose-dependent manner () in each cell line. This observation was valid for all of the tested time periods which are 24, 48, and 72 hours. While increasing concentration of quercetin decreased the ratio of viable cells in all three of the leukemic cell lines, treatment duration of quercetin did not appear to have a significant contribution to the apoptosis and necrosis. When the results are examined at the constant concentration of quercetin over the time periods, it appears that quercetin had higher potential of time-dependent suppression on CCRF-CEM and HL60 cells compared to the K562 cells, since K562 cells did not undergo apoptosis and necrosis in the increasing amounts over the tested time periods at the constant concentration of quercetin. In each of the three leukemic cell lines, ratio of the necrotic cells was found to be less than the ratio of the apoptotic cells. Ratio of the necrotic cells reached at most 22%, whereas the ratio of apoptotic cells was around 60% at maximum values.
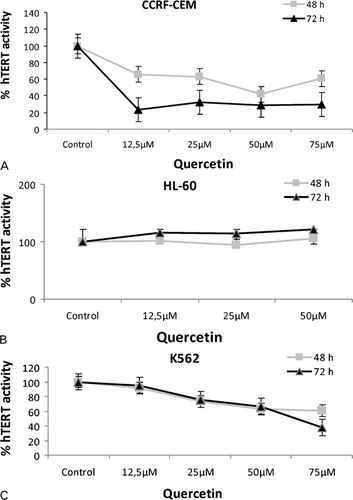
Determination of hTERT activity
Telomerase activity in the CCRF-CEM and K562 cell lines demonstrated decreasing patterns with the increasing concentrations of quercetin (). However, telomerase activity of HL60 cells remained relatively constant with insignificant amounts of increases in the presence of the increasing amounts of quercetin. Time-dependent suppression of hTERT activity is more apparent in CCRF-CEM cell line compared to the others.
Discussion
The effects of quercetin on leukemic cell death were investigated in the current study. When examined generally, results indicate that extent of cell death is proportional to the quercetin concentration in three leukemia cell lines. Cytotoxicity is assessed by the trypan blue exclusion assay in which living cells actively pump the dye out, while the dead cells remained stained. Counting the number of those cells gave an approximation about the cytotoxicity of the quercetin. Experimentations in 48 and 72 hours demonstrated slightly different survival plots in CCRF-CEM and HL60 cells. When results at the end of 48 hours are examined, it is apparent that K562 cells are less sensitive to the quercetin compared to the other cells. But all of the three cell lines have similar plots in 72 hours. Survival of HL60 and K562 cells are suppressed by more than 90% in 12·5 μM of quercetin, whereas CCRF-CEM cells seem to be less sensitive to the same concentration since 65% of the cells are killed in 72 hours. Remaining data points of CCRF-CEM cells showed higher number of surviving cells as well compared to the HL60 and K562 cells, indicating that CCRF-CEM cells might be less sensitive to quercetin.
Quercetin was shown to induce apoptosis and necrosis in CML, APL, and ALL cell lines by this study. However, apoptotic induction appears to be more important than the necrosis in the cytotoxicity of quercetin since the amount of necrotic cells showed minor changes unlike the amount of apoptotic cells. Increasing concentrations of quercetin is in accordance with the decreasing amounts of cellular viability in each cell line; but this explicit trend is not observable when the results of the experiments involving similar concentrations of quercetin are examined over different time periods, especially for the K562 cells. This may show that quercetin might be reaching full functionality as soon as the 48 hours of treatment, and therefore, the results at the 48 and 72 hours might not be varying much.
Assessment of hTERT activity in three leukemic cell lines showed varying results. Telomerase activity remains unaffected by the presence of quercetin in HL60 cells, though there was a slight dose-dependent increase which is seemingly not very significant. On the other hand, CCRF-CEM and K562 cells showed time- and dose-dependent decreases in telomerase activity. This difference might be indicative of the distinct nature of malignancy of HL60 cells (APL cells) compared to the CCRF-CEM and K562 cells (ALL and CML cells, respectively). Despite the constant hTERT activity, quercetin is still cytotoxic to the HL60 cells as it is to the other two cell lines. This fact might suggest that, for at least APL cells, quercetin exerts its tumor suppressive effects through a telomerase-independent pathway.
The results of this study might be useful for attributing new roles to quercetin for the treatment of different types of leukemias. Quercetin was previously shown to suppress tumor growth in various experimental set-ups.Citation18,Citation19,Citation34 The current study provides evidence of its potential for use in the treatment of leukemia. Despite advances in therapeutic outcomes in leukemia cases, development of resistance to known chemotherapeutic agents and, therefore, the lack of a definitive cure, pushes scientists forward to look for new therapeutic options and chemotherapy agents. Quercetin may prove to be an important compound in this research since it has cytotoxic effects on the leukemic cell lines. However, the findings of this study must be tested further in animal systems to reveal the actual physiological feasibility of its use in leukemia therapeutics. Along with further evidence supporting the findings of the current study, quercetin might be incorporated in the future therapeutic options for leukemia.
References
- Harborne JB. Nature, distribution and function of plant flavonoids. Prog Clin Biol Res 1986;213:15–24.
- Salvador MJ, Pereira PS, França SC, Candido RC, Ito IY, Dias DA. Bioactive chemical constituents and comparative antimicrobial activity of callus culture and adult plant extracts from Alternanthera tenella. Z Naturforsch C 2009;64:373–81.
- Li J, Ou-Lee TM, Raba R, Amundson RG, Last RL. Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 1993;5:171–9.
- Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, et al.. Flavonoids act as negative regulators of auxin transport in vivo in arabidopsis. Plant Physiol 2001;126:524–35.
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 1996;20:933–56.
- Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, et al.. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med 1995;155:381–6.
- Ferrandiz ML, Alcaraz MJ. Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids. Agents Actions 1991;32:283–8.
- Osman HE, Maalej N, Shanmuganayagam D, Folts JD. Grape juice but not orange or grapefruit juice inhibits platelet activity in dogs and monkeys. J Nutr 1998;128:2307–12.
- Wang HK, Xia Y, Yang ZY, Natschke SL, Lee KH. Recent advances in the discovery and development of flavonoids and their analogues as antitumor and anti-HIV agents. Adv Exp Med Biol 1998;439:191–225.
- Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr 2002;22:19–34.
- Ferrali M, Signorini C, Caciotti B, Sugherini L, Ciccoli L, Giachetti D, et al.. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett 1997;416:123–9.
- Hertog MG, Feskens EJ, Kromhout D. Antioxidant flavonols and coronary heart disease risk. Lancet 1997;349:699.
- Hirvonen T, Virtamo J, Korhonen P, Albanes D, Pietinen P. Flavonol and flavone intake and the risk of cancer in male smokers (Finland). Cancer Causes Control 2001;12:789–96.
- Knekt P, Kumpulainen J, Järvinen R, Rissanen H, Heliövaara M, Reunanen A, et al.. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr 2002;76:560–8.
- Tabak C, Arts IC, Smit HA, Heederik D, Kromhout D. Chronic obstructive pulmonary disease and intake of catechins, flavonols, and flavones: the MORGEN Study. Am J Respir Crit Care Med 2001;164:61–4.
- Manjeet KR, Ghosh B. Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-alpha production in murine macrophages. Int J Immunopharmacol 1999;21:435–43.
- Geraets L, Moonen HJ, Brauers K, Wouters EF, Bast A, Hageman GJ. Dietary flavones and flavonoles are inhibitors of poly(ADP-ribose)polymerase-1 in pulmonary epithelial cells. J Nutr 2007;137:2190–5.
- Caltagirone S, Rossi C, Poggi A, Ranelletti FO, Natali PG, Brunetti M, et al.. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int J Cancer 2000;87:595–600.
- Chen Y, Li XX, Xing NZ, Cao XG. Quercetin inhibits choroidal and retinal angiogenesis in vitro. Graefes Arch Clin Exp Ophthalmol 2008;246:373–8.
- Tough IM, Court Brown WM, Baikie AG, Buckton KE, Harnden DG, Jacobs PA, et al.. Cytogenetic studies in chronic myeloid leukaemia and acute leukaemia associated with monogolism. Lancet 1961;1:411–7.
- Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood 2000;96:3343–56.
- Kakizuka A, Miller WH, Umesono K, Warrell RP, Frankel SR, Murty VV, et al.. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell 1991;66:663–74.
- Roman-Gomez J, Cordeu L, Agirre X, Jiménez-Velasco A, San José-Eneriz E, Garate L, et al.. Epigenetic regulation of Wnt-signaling pathway in acute lymphoblastic leukemia. Blood 2007;109:3462–9.
- Omura-Minamisawa M, Diccianni MB, Batova A, Chang RC, Bridgeman LJ, Yu J, et al.. Universal inactivation of both p16 and p15 but not downstream components is an essential event in the pathogenesis of T-cell acute lymphoblastic leukemia. Clin Cancer Res 2000;6:1219–28.
- Roman-Gomez J, Castillejo JA, Jimenez A, Gonzalez MG, Moreno F, Rodriguez Mdel C, et al.. 5′ CpG island hypermethylation is associated with transcriptional silencing of the p21(CIP1/WAF1/SDI1) gene and confers poor prognosis in acute lymphoblastic leukemia. Blood 2002;99:2291–6.
- Mi S, Lu J, Sun M, Li Z, Zhang H, Neilly MB, et al.. MicroRNA expression signatures accurately discriminate acute lymphoblastic leukemia from acute myeloid leukemia. Proc Natl Acad Sci USA 2007;104:19971–6.
- Hoelzer D, Gokbuget N, Ottmann O, Pui CH, Relling MV, Appelbaum FR, et al.. Acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program 2002;2002:162–92.
- Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci 2004;117:2417–26.
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 1965;37:614–36.
- Blackburn EH. Telomerases. Annu Rev Biochem 1992;61:113–29.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70.
- Meyerson M. Role of telomerase in normal and cancer cells. J Clin Oncol 2000;18:2626–34.
- Piskin O, Ozcan MA, Ozcan HG, Ates H, Demirkan F, Alacacioglu I, et al.. Synergistic effect of imatinib mesylate and fludarabine combination on Philadelphia chromosome-positive chronic myeloid leukemia cell lines. Turk J Hemat 2007;24:23–7.
- Knekt P, Järvinen R, Seppänen R, Hellövaara M, Teppo L, Pukkala E, et al.. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am J Epidemiol 1997;146:223–30.