Abstract
Glaucoma is the leading cause of irreversible blindness in industrialized countries and comprises a group of diseases characterized by progressive optic nerve degeneration. Glaucoma is commonly associated with elevated intraocular pressure due to impaired outflow of aqueous humor resulting from abnormalities within the drainage system of the anterior chamber angle (open-angle glaucoma) or impaired access of aqueous humor to the drainage system (angle-closure glaucoma). Oxidative injury and altered antioxidant defense mechanisms in glaucoma appear to play a role in the pathophysiology of glaucomatous neurodegeneration that is characterized by death of retinal ganglion cells. Oxidative protein modifications occurring in glaucoma serve as immunostimulatory signals and alter neurosupportive and immunoregulatory functions of glial cells. Initiation of the apoptotic cascade observed in glaucomatous retinopathy can involve oxidant mechanisms and different agents have been shown to be neuroprotective. This review focuses on the molecular mechanisms of oxidant injury and summarizes studies that have investigated novel free radical scavengers in the treatment of glaucomatous neurodegeneration.
Introduction
Glaucoma is the second most common cause of blindness among the elderly in developed countriesCitation1 and is an age-related heterogenous group of diseases affecting 67 million people worldwide.Citation2 It is commonly associated with elevated intraocular pressure (IOP) due to impairment of the outflow pathway of the trabecular meshwork, and charactarized by pathological changes in optic nerve head and lamina cribrosa leading to visual field defects and irreversible blindness due to apoptosis of retinal ganglion cells (RGCs).Citation3
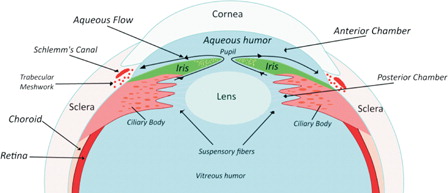
Elevated IOP is the most important risk factor for the disease.Citation4,Citation5 The mechanism of elevated IOP in glaucoma is impaired outflow of aqueous humor resulting from abnormalities within the drainage system of the anterior chamber angle (open-angle glaucoma) or impaired access of aqueous humor to the drainage system (angle-closure glaucoma).Citation6 Aqueous humor produced by the ciliary body enters the posterior chamber, passes through the pupil into the anterior chamber and then to the trabecular meshwork in the anterior chamber angle. Trabecular meshwork drains the aqueous fluid into the canal of Schlemm ().Citation2
Animal models of glaucoma are designed to produce either optic nerve injury or elevated IOP. However, these models may still not reflect the human disease in all aspects because they lack the heterogeneity present in the human pathological condition.Citation7 Although mechanical compression theory of glaucoma considers elevated IOP as the most important risk factor for the diseaseCitation8 and supports the essential signs of glaucomatous optic neuropathy, such as increased cuppingCitation9 and neuroretinal rim thinning,Citation10 it does not explain the existence of normal tension glaucoma.Citation11 Most experimental models of glaucoma also use young healthy animals, whereas glaucoma is a progressive optic neuropathy which often occurs in the elderly who are more likely to have macro- and micro-angiopathies that may influence neuronal responses to injury.Citation12 In this context, involvement of oxidative stress associated with glaucoma may not perfectly be mirrored in animal models of the disease.
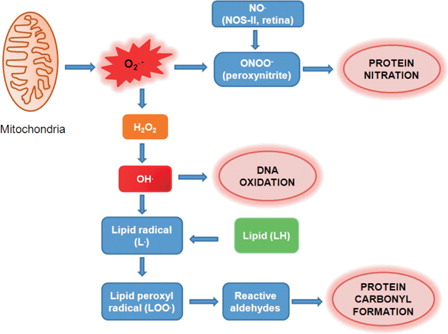
Oxidative stress has been implicated to cause trabecular meshwork degeneration and thus may contribute to alterations in the aqueous outflow pathway.Citation13 Altered antioxidant defense mechanisms and increased markers of oxidative stress such as DNA oxidation, protein carbonyl formation and lipid peroxidation has been documented in glaucoma patients and rat models of elevated IOP. Oxidative stress is also linked to retinal cell apoptosis and immunostimutalory signaling in glaucoma.Citation14 This review discusses the role of retinal oxidative injury in the pathogenesis of glaucomatous neurodegeneration and examines the relevance of free radical scavengers in altering and/or inhibiting neuronal degeneration associated with the disease.
Oxidative injury in glaucoma
DNA oxidation
8-Hydroxy-2′-deoxyguanosine (8-OH-dG) is a marker of oxidative DNA damage and was determined in human trabecular meshwork specimens collected from patients with primary open-angle glaucoma.Citation15 The relationship between DNA oxidation, disease duration, IOP, and visual field damage were also evaluated in the same study. 32P-postlabeling method was used to measure 8-OH-dG in extracted DNA samples.Citation15 A statistically significant correlation was found between oxidative DNA levels, IOP and visual field damage. It was suggested that oxidative DNA damage may induce human trabecular meshwork degeneration which could lead to increased IOP.
Glutathione S-transferases are enzymes which are important in cellular detoxification. These enzymes conjugate toxicants to glutathione and thereby neutralize electrophilic sites and increase the toxicants water solubility.Citation16 Polymorphism in the glutathione S-transferase M1 gene was examined in human trabecular meshwork specimens obtained from 45 primary open-angle glaucoma patients which had trabeculectomy and unaffected controls.Citation17 A genetic predisposition of defective Mu-class glutathione S-transferase gene was associated with primary open-angle glaucoma. A 2.2-fold higher 8-OH-dG was measured in primary open-angle glaucoma patients who had Mu-class glutathione S-transferase null allele deletion compared to Mu-class glutathione S-transferase null allele-positive subjects. Moreover, Mu-class glutathione S-transferase null allele deletion was significantly more frequent in primary open-angle glaucoma patients than in controls. Obtained results from this study showed that glutathione S-transferase genes are expressed in human trabecular meshwork under physiological conditions and that increased 8-OH-dG is associated with primary open-angle glaucoma.
Protein carbonyl formation
A significant increase was reported in serum protein carbonyl levels measured in 50 patients with pseudoexfoliation glaucoma compared to 55 healthy controls.Citation18 Increased protein carbonyl formation was also documented in a chronic pressure induced rat model of glaucoma.Citation19 Glyceraldehyde-3-phosphate dehydrogenase, heat shock protein 72, and glutamine synthetase showed a significant increase in the relative percentage of carbonyl immunoreactivity in chronic pressure induced rat models of glaucoma as compared with controls.Citation19
The function of heat shock protein 72 can be altered by posttranslational oxidative modification. The main function of heat shock protein 72 is to operate as an intracellular molecular chaperone of immature, abnormally folded or mutated proteins.Citation20 In fact, tolerance to hypoxic and excitotoxic injury has been shown to increase in cultured rat RGCs which have elevated levels of heat shock protein 72.Citation21 Likewise, RGC protection was shown in a rat model of glaucoma treated with a heat shock protein inducer, geranylgeranylacetone.Citation22
The conversion of glutamate to glutamine is catalyzed by glutamine synthetase which is an important enzyme involved in the metabolism of the excitatory neurotransmitter, glutamate.Citation23 Glutamine synthetase expression was shown in retinal ganglion Müller cells,Citation24 where it plays a significant protective role against neuronal excitotoxicity. The effect of post-translational carbonyl formation on the function of glutamine synthetase is unknown but may have potential consequences on RGC death associated with glaucoma.
Lipid peroxidation
Conjugated dienes are end products of lipid peroxidation and were measured in lipid extracts from aqueous humor, trabecular tissues and lenses obtained from 49 patients with primary open-angle glaucoma. Significantly higher levels of lipid peroxidation products were found in the aqueous humor and trabecular tissue of glaucoma patients compared with control subjects.Citation25 Likewise, malondialdehyde levels showed a 2.5-fold increase in lens capsule samples of patients with pseudoexfoliation syndrome compared with age-matched control subjects.Citation26 Increased retina and vitreous malondialdehyde levels were also measured in rats with elevated IOP.Citation27
Formation of 4-hydroxynonenal occurs via peroxidation of polyunsaturated fatty acids. 4-Hydroxynonenal is able to induce apoptosis in neuronal cells.Citation28 The dose and time-dependent effects of 4-hydroxynonenal were examined on primary cultures of human optic nerve head astrocytes, generated from normal and glaucomatous human eyes.Citation29 Treatment with 4-hydroxynonenal at concentrations of 50 mM and higher led to a greater than 50% reduction in cell viability of normal optic nerve head astrocytes over 6 hours.Citation29
Altered antioxidant defense mechanisms in glaucoma
As presented in this review, chronic oxidative stress is implicated in the pathogenesis of glaucoma, particularly, its age-dependent clinical onset. Antioxidants play a key role in protecting against oxidative damage and are present in both ocular fluids and tissues. Superoxide (O2•−) is removed by superoxide dismutases, which dismutate superoxide (O2•−) to yield hydrogen peroxide (H2O2) and oxygen (O2).Citation30 Hydrogen peroxide formed in the anterior segment tissues of the eye is removed by catalase or by glutathione peroxidase.Citation31 Catalase and superoxide dismutase can be measured in both normal fresh human cadaver trabecular meshworkCitation32 and in the iris and corneal endothelium of rabbits.Citation33 Normal cadaver human trabecular meshwork demonstrate an age-dependent decline in the specific activity of superoxide dismutase, but not catalase. However, age-dependent decline of catalase activity is observed in the iris and corneal endothelium of rabbits. Patients with pseudoexfoliation syndrome also show a decrease in serum superoxide dismutase and catalase activity compared with age-matched controls.Citation34
Low-molecular-weight antioxidants such as glutathione and ascorbic acid (vitamin C) can be detected in the aqueous humor of humans.Citation35 Pseudoexfoliation lens epithelial cells display decreased glutathione and oxidized glutathione levels compared with non-pseudoexfoliation controls.Citation26 In addition, mean ascorbic acid concentration in the aqueous humor of patients with pseudoexfoliation syndrome is significantly lower than that found in control patients.Citation36 Ascorbic acid, which is known to recycle the vitamin E radical, can prevent rapid loss of vitamin E during lipid peroxidation.Citation37 Blood levels of ascorbic acid measured in 38 patients with chronic open-angle glaucoma did not show a significant difference when compared to 12 controls.Citation38 Conversely, plasma glutathione levels measured in 21 patients with newly diagnosed primary open-angle glaucoma and 34 age- and gender-matched control subjects showed that glaucoma patients had significantly lower levels of reduced and total glutathione than did control subjects.Citation39
Glutathione is the most abundant non-protein thiol in the cell and a major cellular antioxidant important in the inactivation of 4-hydroxynonenal.Citation40 Glutathione levels were significantly depleted in normal astrocytes after exposure to 4-hydroxynonenal for over an hour.Citation28 Primary cultures of human optic nerve head astrocytes from glaucomatous eyes were reported to have basal glutathione levels below the level of detection compared with primary cultures from normal astrocytes.Citation29 This finding suggests the presence of an altered antioxidant defense mechanism in glaucoma. A significant increase in glutathione levels were measured in both normal and glaucomatous astrocytes 24 hours after 4-hydroxynonenal removal.Citation29
Glaucoma induced in rats, via 6-week injection of hyaluronic acid into the anterior chamber, was accompanied by increased lipid peroxidation and altered antioxidant enzyme activities.Citation41 A time- and hypertension-dependent increase was observed in retina lipid peroxidation, measured as thiobarbituric acid-reacting substances. The observed increase in thiobarbituric acid-reacting substances was accompanied by a decrease in glutathione, superoxide dismutase, and catalase enzyme activities suggestive of an altered antioxidant defense mechanism.Citation41
Apoptosis in glaucoma
The major mechanism of visual loss in glaucoma is RGC apoptosis, leading to thinning of the inner nuclear and nerve fiber layers of the retina and axonal loss in the optic nerve.Citation4 Atrophy occuring in neurons located at magno- and parvocellular layers in the lateral geniculate nucleus of the thalamus is also reported in experimental glaucoma.Citation42 The lateral geniculate nucleus is the primary relay center for visual information received from the retina of the eye.Citation43 It is reported that neurons in parvocellular layers undergo significantly more shrinkage than neurons in magnocellular layers.Citation42
The external environment can generate reactive oxygen species (ROS) from many sources including light.Citation44 Indeed, cell viability was assessed in RGC cultures exposed to visible light and it was observed that light reduced cell viability, increased the number of terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)-positive cells and enhanced labeling for ROS.Citation45 It was suggested that light entering the eye interacted with RGC axon mitochondria to generate ROS which may jeopardize cellular survival.
RGCs have been shown to die by apoptosis in models of experimental glaucomaCitation46 and in human glaucoma.Citation47 The molecular basis of RGC death in glaucoma has been discussed recently in a comprehensive review.Citation48 The emerging mechanisms are complex and points to a variety of molecular signals which can also act synergistically to induce RGC death. These include axonal transport failure, neurotrophic factor deprivation, toxic pro-neurotrophins, activation of intrinsic and extrinsic apoptotic signals, mitochondrial dysfunction, excitotoxic damage, oxidative stress, misbehaving reactive glia, and loss of synaptic connectivity.
Oxidative stress is implicated in neuronal cell death but whether this is the reason for a neuron dying or a symptom of what takes place when death occurs is of significant importance. Optic nerve transection caused formation of O2•− which was visualized in RGCs 24 hours after transaction. Superoxide oxidized intravitreal-injected hydroethidine to its reaction product 2-hydroxyethidium which was detected by real-time imaging of fluorescent cells via confocal scanning laser ophthalmoscopy (CSLO). RGC O2•− increased within 24 hours after axotomy, peaking at 4 days, and was not observed in contralateral untransected eyes. The O2•− signal preceded phosphatidylserine externalization and binding of annexin V which was used to visualize apoptotic cells by CSLO. Intravitreal pegylated superoxide dismutase blocked O2•− generation after axotomy and delayed RGC death. These results indicated that superoxide generation was an upstream signal for RGC apoptosis after optic nerve injury.Citation49
A recent study performed on primary cortical neurons also revealed that there were multiple phases of neuronal cell death modalities under sustained O2•− exposure. Cortical neurons initially responded to O2•− by non-apoptotic death as indicated by inactive caspases and depolarized mitochondria. A combination of autophagic cell death and programmed necrosis were observed at 4 hours. At later times the specific siRNAs that initially suppressed death become ineffective. Ultimately most neurons became overwhelmed by the consequences of severe oxidative stress and died.Citation50
Peroxidation of polyunsaturated fatty acids result in the formation of 4-hydroxynonenal which is also capable of inducing apoptosis in neuronal cells by inhibiting ion-motive ATPases.Citation28,Citation51 Taken together, these results implicate that oxidative stress is the cause for a neurone dying rather than a reflection of what occurs when death is induced.
Induction of nitric oxide synthases-2 expression, increased protein nitration, and apoptosis are observed in retinal cells of animals with elevated IOPCitation52 signifying that nitrative stress aggravates disease progression in clinical conditions accompanied by ocular hypertension. Studies also show that nitric oxide synthases-2 is present in glaucomatous optic nerve head with consistent staining of nitrotyrosine, indicating that reactive nitrogen species may contribute to RGC death associated with elevated IOP.Citation53,Citation54 In fact, pharmacological studies have shown that inhibition of nitric oxide synthases-2 by aminoguanidine provides neuroprotection to RGCs in a rat model of chronic glaucoma.Citation55
The capability of nitric oxide (NO•) to induce apoptosis and nitric oxide-mediated cytotoxicity have been documented in macrophages,Citation56 astrocytes,Citation57 and neuronal cells.Citation58 Apoptosis, which can be regulated by reactive oxygen and nitrogen species, can be induced by two major pathways. The extrinsic pathway involves binding of tumor necrosis factor-α (TNF-α) and Fas ligand to membrane receptors leading to caspase-8 activation, while the intrinsic pathway participates in stress-induced mitochondrial cytochrome c release. Released cytochrome c makes a complex with apoptotic protease activating factor-1 and procaspase-9, which activates caspase-9. Both pathways converge on caspase-3 activation, resulting in cellular morphological changes such as blebbing and nuclear degradation ().Citation59
Figure 2. Intrinsic and extrinsic pathways for apoptosis. The extrinsic pathway can be induced by members of the TNF family receptors such as tumor necrosis factor receptor (TNFR) and FAS. The intrinsic pathway can be activated by release of cytochrome c from mitochondria. In the cytosol, cytochrome c binds and activates Apaf-1, allowing it to bind and activate caspase-9. Caspase-9 and -8 activate caspase-3. Apaf-1, apoptotic protease-activating factor-1; Cyt c, cytochrome c; JNK, c-Jun N-terminal kinase; p38 MAPK, p38 mitogen-activated protein kinase; FASL, FAS Ligand.
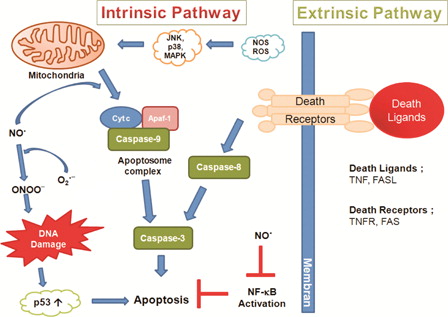
There is strong evidence indicating that the extrinsic pathway is involved in RGC apoptosis in glaucoma. The cell death in the retina after chronic elevation of IOP was studied in an experimental rat model of human glaucomatous disease. Activation of several cell death programs represented by Fas ligand, FADD (Fas-associated death domain/Mort1) and the caspase cascade (caspase-8 and -3) were examined using immunohistochemistry and western blotting. Following injury, two major events occurred simultaneously in the retina: activation of programmed cell death pathways and activation of survival mechanisms to maintain the cellular homeostasis of the retina. At the later stage of injury, markers of an activated cell death program appeared to be concentrated in the RGCs. The data suggest that FasL and Fas associated death domain increase upon elevated IOP.Citation60
The activation of c-Jun N-terminal kinase (JNK; also referred to as stress activated protein kinase or SAPK) and p38 mitogen-activated protein (MAP) kinase by nitrative and oxidative stress induces apoptotic cell death by leading to caspase-3 activation.Citation61,Citation62 Nitric oxide can also participate in tyrosine nitration of cytochrome c which induces cytochrome c release from the mitochondria.Citation63 Excessive amounts of nitric oxide (NO•) and peroxynitrite (ONOO−) are reported to cause DNA damage and lead to p53-mediated growth arrest and apoptosis.Citation64
Active, cleaved caspases-3 has been detected in experimental models of glaucomaCitation65 and, as discussed, its formation can be induced by both nitrative and oxidative stress. Although caspase-3 is implicated in the primary and secondary waves of RGC apoptosis, it is active for a longer period of time and with greater intensity during the primary wave of RGC loss.Citation66 This may explain why ganglion cells die at different time points during oxidative stress.
The involvement of caspase-3 and members of the MAP kinase pathway were evaluated in experimental rat models of glaucoma. Protein levels of caspase-3 were elevated from days 15 day 30. All investigated members of the MAP kinase pathway were significantly activated. P-SAPK/JNK activation began on day 2, reaching a 6-fold elevation by day 30. The p-P38 level was elevated on days 2 and 8, followed by a decrease to baseline on day 15. The level of p-ATF-2, the substrate of P38, was significantly elevated at all time points tested, up to day 30. Reported data suggest that RGC death in glaucoma involves activation of the MAP kinase pathway and the caspase family at different time points.Citation67
Nuclear factor-κB up-regulates genes encoding anti-apoptotic proteins and therefore plays a protective role against apoptosis.Citation68 Nitric oxide inhibits nuclear factor-κB activation, by inducing the expression of the nuclear factor-κB inhibitor, and stabilization of the nuclear factor-κB/nuclear factor-κB inhibitor complex.Citation69 On the contrary, oxidative stress activates nuclear factor-κB inhibitor kinase, which leads to the phosphorylation of nuclear factor-κB inhibitor and activation of nuclear factor-κB. The activation of nuclear factor-κB inhibitor kinase and phosphorylation of nuclear factor-κB inhibitor can be blocked by antioxidants and nitric oxide (NO•).Citation68 The induction of apoptosis requires fine biochemical interplay between oxygen and nitrogen species. The main signaling components of nitric oxide (NO•)-initiated apoptotic cell death are illustrated in .
Oxidative stress and immune response regulation in glaucoma
Oxidative stress can regulate the immune response in many different ways in glaucoma.Citation14 One of these events is oxidative protein modifications as detected by proteomic analysis of the retina in experimental glaucoma.Citation19 Oxidation may change the antigenic features of these proteins, thereby serving as an immunostimulatory signal during glaucomatous neurodegeneration.Citation14 In addition to increasing antigenity, oxidative modifications may also affect the neurosupportive and immunoregulatory functions of glial cells.Citation14 Oxidized proteins, lipids, and DNA become para-inflammatory stimuli and signal to resident immune cells, mainly including microglia, to initiate an innate immune response.Citation70 With enhanced scavenger functions, microglial cells are able to remove oxidation products by phagocytosis and they may release growth factors and cytokines to promote tissue healing.Citation71,Citation72 This is in the same notion proposed for regulatory T cells.Citation73 However, if oxidative stress reaches to a certain level, the physiological homeostasis may be impaired, thereby evolving into an injury process. In this case, initial glial response expands and leads to increased production of proinflammatory cytokines.Citation14
Complement activation constitutes another important component of the innate immune activities detected in glaucomatous neurodegeneration. The regulation of complement activation was studied in oxidative stress-mediated glaucoma. Human retinal protein samples obtained from donor eyes with or without glaucoma were analyzed by a quantitative proteomic approach using mass spectrometry. Cellular localization of protein expression for different complement components and regulators were also determined by immunohistochemical analysis of an additional group of human donor eyes with glaucoma compared with age-matched control eyes without glaucoma. In addition, to determine the regulation of complement factor H by oxidative stress, in vitro experiments were performed using rat retinal cell cultures incubated in the presence and absence of an oxidant treatment. Proteomic and immunohistochemical analysis identified an increase in complement components C1q and C3b and the membrane attack complex C5b-9. In addition, several complement regulatory proteins were detected in the human retinal proteome, and glaucomatous samples exhibited a trend toward down-regulation of complement factor H expression. In vitro experiments revealed that oxidative stress, which was also prominently detectable in the glaucomatous human retinas, down-regulated complement factor H expression in retinal cells. These findings expand the current knowledge of complement activation by presenting new evidence in human glaucoma. A potential deficiency in intrinsic regulation of complement activation, as is evident in the presence of oxidative stress, may lead to uncontrolled complement attack with neurodestructive consequences.Citation74
Other consequences of oxidative stress facilitating an aberrant immune activity in glaucoma include the augmented generation of advanced glycation end products through oxidative stress-dependent processes.Citation75 Advanced glycation end products may act as persistent antigenic stimulus and also be immunostimulatory through a specific receptor for advanced glycation end products-mediated signaling that leads to pro-inflammatory cytokine production.Citation76 Oxidative stress provides a common trigger for many downstream pathways compromising the perivascular barrier functionCitation77 may similarly affect blood vessels in human glaucoma.Citation78
The regulation of immune response through glial toll-like receptor signaling was studied in glaoucumatous oxidative stress.Citation79 Retinal protein samples obtained from human donor eyes were analyzed by a quantitative proteomic approach involving mass spectrometry. Cellular localization of toll-like receptor-2, -3, and -4 was also determined by immunohistochemical analysis of an additional group of human donor eyes with glaucoma and control eyes. In addition, in vitro experiments were performed in rat retinal microglia and astrocytes to determine glial toll-like receptor expression and immunoregulatory function after exposure to hydrogen peroxide (H2O2)-induced oxidative stress. Proteomic analyses of the human retina detected expression and differential regulation of different toll-like receptors in glaucomatous samples. Immunohistochemical analysis supported up-regulated expression of toll-like receptors on both microglia and astrocytes in the glaucomatous retina. In vitro experiments provided additional evidence that oxidative stress up-regulate glial toll-like receptor and MHC class II expression and cytokine production through toll-like receptor signaling and stimulate proliferation and cytokine secretion of co-cultured T cells during antigen presentation. This study supports the up-regulation of toll-like receptor signaling in human glaucoma, which may be associated with innate and adaptive immune responses. In vitro findings showed that oxidative stress mediated glaucomatous tissue stress and may initiate the immunostimulatory signaling through glial toll-like receptors.Citation79
Potential applications of free radical scavengers in glaucoma
Lutein and zeaxanthin are oxygenated carotenoids that form the macular pigment. Of the 10 carotenoids that have been reported in the human serum, only two, zeaxanthin and lutein are found in the human retina.Citation64 Although it is suggested that zeaxanthin and lutein are concentrated in the retina because of their ability to cross the blood brain barrier of the retinal pigment epithelium and scavenge free radicals,Citation80 no strong association was found between dietary intake of lutein and zeaxanthin and the risk for glaucoma.Citation81 Conversely, a recent study showed that treatment with astaxanthin, a naturally occurring carotenoid pigment and a powerful biological antioxidant, reduced oxidant-induced protein oxidation, lipid peroxidation, and apoptotic cell death in experimental rat models of elevated IOP.Citation82
Polyphenolic flavonoids (present in tea, coffee, wine, dark chocolate, and Ginkgo bilboa), alpha lipoic acid, coenzyme Q10, and melatonin are natural substances with antioxidant activity in glaucomatous neurodegeneration.Citation83 Melatonin is not only an antioxidant but also acts by decreasing the IOP.Citation84 Eriodictyol, a flavonoid found in citrus fruits, is reported to be a potent compound which protects human retinal pigment epithelial cells from oxidative stress-induced cell death.Citation85
The effects of resveratrol, which is a naturally occurring polyphenol found in berries, nuts, and red wine was studied in primary porcine trabecular meshwork cells subjected to chronic oxidative stress.Citation86 Primary porcine trabecular meshwork cells were submitted to chronic treatment with resveratrol or vehicle every 3 days for 15 days. Cells under resveratrol or vehicle treatment were incubated under oxidative stress conditions (40% oxygen) and control cultures were treated with vehicle and incubated at physiological oxygen concentration (5%). The level of endogenous reactive oxygen species was significantly decreased by resveratrol treatment (4 fold) compared with cells treated with vehicle. The amount of intracellular reactive oxygen species in resveratrol-treated cells under oxidative stress was similar to that of non-stressed control cells. The induction of mRNA expression of the inflammatory markers interleukin-1α, -6, -8, and endothelial leukocyte adhesion molecule after chronic oxidative stress was significantly inhibited by chronic treatment with resveratrol. The accumulation of carbonylated proteins induced by oxidative stress was significantly lower in resveratrol-treated samples compared with samples treated with vehicle under oxidative stress. Resveratrol-treated cells showed protection against apoptosis after acute oxidative stress (200, 400, and 800 µM of hydrogen peroxide [H2O2]), when compared with cells treated with vehicle. Vehicle-treated cells showed a linear correlation between hydrogen peroxide (H2O2) concentration and apoptosis; cells treated with resveratrol exhibited protection against apoptosis in all hydrogen peroxide (H2O2) concentrations. Resveratrol treatment did not result in significant changes in proliferation and in the amount of DNA damage when compared with cells treated with vehicle. The data suggest that resveratrol could potentially have a role in preventing the trabecular meshwork tissue abnormalities observed in primary open-angle glaucoma.Citation86
The antioxidative properties of ginkgo are due to its direct radical scavenging activity. Ginkgo biloba prevents oxidative damage to mitochondria, exhibits neuroprotective properties, inhibits LDL oxidation, has a relaxing effect on vascular walls, and an antagonistic action on platelet activating factor.Citation83 Administration of ginkgo increases ocular blood flow velocity in patients,Citation87 and improves visual field in normal tension glaucoma patients.Citation88 The beneficial properties of G. biloba suggest it to be of major therapeutic value in the treatment of glaucoma.Citation89
The water- and fat-soluble vitamin alpha lipoic acid is found in foods such as red meat, liver, and yeast. Alpha lipoic acid is capable of regenerating several other antioxidants back to their active states, including vitamin C, vitamin E, glutathione and coenzyme Q10.Citation83 Coenzyme Q10 is a coenzyme for the inner mitochondrial enzyme complexes involved in energy production within the cell.Citation90 Coenzyme Q10 has been demonstrated to prevent lipid peroxidation and DNA damage induced by oxidative stress.Citation91 Oral administration of ubiquinone was shown to be useful in mitigating cardiovascular side-effects without affecting IOP in glaucoma patients.Citation92
Melatonin reduces the elevation of cGMP by suppressing nitric oxide synthase activity, indicating a neuroprotective role in the retina.Citation83,Citation93 Findings indicate that melatonin reduces nitric oxide (NO•)-induced retinal oxidative damage both in vitro and in vivo. Furthermore, several of the metabolites that are generated when melatonin inactivates toxic reactants are themselves free radical scavengers.Citation83 In addition, melatonin stimulates a number of antioxidative enzymes, which further promote antioxidative protection.Citation94
The role of dorzolamide, which is a topical carbonic anhydrase inhibitor that plays significant IOP-lowering activity and vasoactive effect, was studied on the oxidative/antioxidant status of aqueous humor in patients with primary open-angle glaucoma.Citation95 One hundred thirty patients were divided into three groups; patients with primary open-angle glaucoma without dorzolamide administration (n = 34); patients with primary open-angle glaucoma with dorzolamide administration (n = 36); and subjects with cataract (comparative group, n = 60). Oxidative activity was measured in the aqueous humor by malondialdehyde determination by thiobarbituric acid reacting substances assay. Antioxidant status was assessed in the aqueous humor samples by measuring the superoxide dismutase activity and the total antioxidant status. Oxidative activity was significantly higher in both glaucoma groups than in the cataract group and was significantly higher in subjects without dorzolamide administration. Superoxide dismutase activity was significantly higher in both glaucoma groups than in the cataract group, and was significantly higher in glaucoma without dorzolamide administration than in glaucoma with dorzolamide treatment. Total antioxidant status was significantly decreased in both glaucoma groups compared with the cataract group, and was more significantly decreased in glaucoma without dorzolamide administration than in glaucoma with dorzolamide administration.Citation95 The data suggest that topical administration of dorzolamide diminishes oxidative stress in patients with glaucoma.
The protective effects of prostaglandin analogues (bimatoprost, travoprost, and latanoprost) on oxidative stress-induced trabecular meshwork changes in primary open-angle glaucoma was studied in primary cell cultures of human trabecular meshwork and furthermore whether these protective effects of prostaglandin analogs could be blocked by pretreatment with prostaglandin F receptor antagonists was investigated.Citation96 The cells were exposed to hydrogen peroxide (H2O2) for 1 hour. The effects of prostaglandin analogs and benzalkonium chloride, which is the most widely used preservative in commercially available eye drops, on trabecular meshwork were investigated by preincubation of non-stressed or hydrogen peroxide (H2O2)-treated cells with 1:100 diluted commercial solutions of bimatoprost, travoprost, and latanoprost or their corresponding BAC concentrations. Pretreatment with BAC further increased the typical glaucomatous trabecular meshwork changes, which were characterized by cell loss, increased accumulation of extracellular matrix, and cellular senescence in vitro. These effects were reduced by pre-incubation with prostaglandin analogs in hydrogen peroxide (H2O2)-treated and non-stressed cells. There was no reduction in the presence of prostaglandin F receptor antagonists in hydrogen peroxide (H2O2)-treated cells. These data suggest that oxidative stress-induced trabecular meshwork changes can be minimized by the use of prostaglandin analogs and prevention of oxidative stress exposure to the trabecular meshwork may help to reduce the progression of primary open-angle glaucoma.
A refined monosodium luminal, Galavit, has antioxidant and anti-inflammatory effects in humanCitation97 and glaucomatous mice.Citation98 The immunohistochemical distribution of glutathione, glutamine synthetase, and glutamate was examined in normal C57BL/6 mice (negative control), glaucomatous DBA2J mice (positive control), and glaucomatous DBA/2J mice treated with Galavit.Citation99 Serial sections were immunogold stained for glutamate, glutamine synthetase, and total glutathione, followed by image analysis for staining patterns and density. Focal decreases in glutamate immunostaining were common in the inner nuclear layer of glaucomatous DBA/2J retinas, but not in C57BL/6 or Galavit-treated glaucomatous DBA/2J retinas. Decreases in glutathione and glutamine synthetase immunostaining were found in glaucomatous DBA/2J retinal regions where neuronal glutamate immunostaining was reduced. Retinas from Galavit-treated glaucomatous DBA/2J had no significant decreases in inner nuclear layer levels of glutamate, glutathione, or glutamine synthetase. The data suggest that the antioxidant Galavit may prove to be effective in delaying or preventing retinal dysfunction and damage in at least some types of glaucoma.
The effect of nitric oxide synthase (NOS) inhibition and therapeutic potential of a newly designed metalloporphyrin-based catalytic antioxidant Mn (III) meso-tetrakis (N-n-hexylpyridinium-2-yl) porphyrin (MnTnHex-2-PyP5+) was studied in a rat model of elevated intraocular pressure (EIOP). Rats were randomly divided into different experimental groups which received either intraperitoneal MnTnHex-2-PyP5+ (0.1 mg/kg/day), intragastric NOS inhibitor (S-methylthiourea; 5 mg/kg/day) or both agents for a period of 6 weeks. Ocular hypertension was induced by unilaterally cauterizing three episcleral vessels and the unoperated eye served as the control. Neuroprotective effects of given treatments were determined via electrophysiological measurements of visual-evoked potentials (VEP) while retina and vitreous levels of MnTnHex-2-PyP5+ were measured via liquid chromatography tandem mass spectrometry. Latencies of all VEP components (P(1), N(1), P(2), N(2), P(3)) were significantly prolonged in EIOP and returned to control levels following all three treatment protocols. Ocular hypertension significantly increased retinal protein nitration which returned to baseline levels in all treated groups. NOS-2 expression and nitrate/nitrite levels were significantly greater in non-treated rats with EIOP. Retinal TUNEL staining showed apoptosis in all ocular hypertensive rats. The data confirm the role of oxidative injury in EIOP and highlight the protective effect of MnTnHex-2-PyP5+ treatment and NOS inhibition in ocular hypertension.Citation100
Naturally occuring imidazole-containing peptidomimetic, N-acetylcarnosine, possesses strong and specific antioxidant properties, by preventing and reducing the accumulation of oxidized products derived from the lipid peroxidation of biological membranes. Carnosine has been shown to act as a competitive inhibitor of the non-enzymatic glycosylation of proteins.Citation101 The combination therapy with N-acetylcarnosine lubricant eye drops and oral formulation of non-hydrolysed carnosine has been developed and studied in primary open-angle glaucoma.Citation102 In the treatment of primary open-angle glaucoma this dual therapy was combined with the conventional antiglaucoma therapy with beta-blocking and/or adrenergic agonist medicines providing the significant IOP-lowering effect and significant increase in outflow facility.Citation102 Potential applications of free radical scavengers in glaucomatous neurodegeneration are shown in .
Table 1. Potential applications of free radical scavengers in glaucomatous neurodegeneration
Conclusion
Oxidative and nitrative processes have an important role in the pathogenesis of glaucomatous neurodegeneration (). In spite of all reported experimental data there is still incomplete knowledge to understand whether free radical generation is a primary or a secondary event in glaucomatous neurodegeneration. As discussed herein, oxidative stress has been implicated to cause trabecular meshwork degeneration and thus may contribute to alterations in the aqueous outflow pathway. An elevation in IOP can occur via disruption of the eye's outflow leading to clinical onset of glaucoma. In such conditions, oxidative stress can be considered as a secondary event in the pathogenesis of glaucoma. Retinal oxidative injury occurring in models of elevated IOP or in normal tension glaucoma could also directly damage the RGC layer, leading to glaucomatous optic neuropathy. Although the principle pharmacological approach for glaucoma treatment is to decrease IOP, continued trials of therapeutic interventions to reduce in vivo oxidative stress seem relevant in patients with the disease. Conceivably, an effective approach of protective treatment would be to start the therapeutic interventions at an early stage of the disease and target-specific sites of reactive species generation. Future studies and clinical trails can further improve our insight on mechanisms of neuronal degeneration in glaucoma and help in the design of more effective therapies.
Acknowledgments
This work was supported in part by a grant from Akdeniz University Research Foundation (no. 2007.01.0103.018) and in part by TUBITAK (The Scientific and Technological Research Council of Turkey; no. 111S419).
References
- Tuulonen A, Airaksinen PJ, Erola E, Forsman E, Friberg K, Kaila M, et al. The Finnish evidence-based guideline for open-angle glaucoma. Acta Ophthalmol Scand 2003;81:3–18.
- Prokofyeva E, Zrenner E. Epidemiology of major eye diseases leading to blindness in Europe: a literature review. Ophthalmic Res 2012;47:171–88.
- Aslan M, Cort A, Yucel I. Oxidative and nitrative stress markers in glaucoma. Free Radic Biol Med 2008;45:367–76.
- Fechtner RD, Weinreb RN. Mechanisms of optic nerve damage in primary open angle glaucoma. Surv Ophthalmol 1994;39:23–42.
- Yan DB, Coloma FM, Metheetrairut A, Trope GE, Heathcote JG, Ethier CR. Deformation of the lamina cribrosa by elevated intraocular pressure. Br J Ophthalmol 1994;78:643–8.
- Parisot TJ, Wood EM. A comparative study of the causative agent of a mycobacterial disease of salmonoid fishes. 2. A description of the histopathology of the disease in Chinook salmon (Oncorhynchus tshawytscha) and a comparison of the staining characteristics of the fish disease with leprosy and human tuberculosis. Am Rev Respir Dis 1960;82:212–22.
- Danesh-Meyer HV, Levin LA. Neuroprotection: extrapolating from neurologic diseases to the eye. Am J Ophthalmol 2009;148:186–91.
- Yan DB, Coloma FM, Metheetrairut A, Trope GE, Heathcote JG, Ethier CR. Deformation of the lamina cribrosa by elevated intraocular pressure. Br J Ophthalmol. 1994;78:643–8.
- Greenfield DS. Glaucomatous versus nonglaucomatous optic disc cupping: clinical differentiation. Semin Ophthalmol 1999;14:95–108.
- Jonas JB, Budde WM, Lang P. Neuroretinal rim width ratios in morphological glaucoma diagnosis. Br J Ophthalmol 1998;82:1366–71.
- Yamamoto T, Kitazawa Y. Vascular pathogenesis of normal-tension glaucoma: a possible pathogenetic factor, other than intraocular pressure, of glaucomatous optic neuropathy. Prog Retin Eye Res 1998;17:127–43.
- Flammer J. The vascular concept of glaucoma. Surv Ophthalmol 1994;38(Suppl.):S3–6.
- Sacca SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res 2007;84:389–99.
- Tezel G. The immune response in glaucoma: a perspective on the roles of oxidative stress. Exp Eye Res 2011;93:178–86.
- Sacca SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol 2005;123:458–63.
- Mannervik B, Alin P, Guthenberg C, Jensson H, Tahir MK, Warholm M, et al. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci USA 1985;82:7202–6.
- Izzotti A. DNA damage and alterations of gene expression in chronic-degenerative diseases. Acta Biochim Pol 2003;50:145–54.
- Yagci R, Gurel A, Ersoz I, Keskin UC, Hepsen IF, Duman S, et al. Oxidative stress and protein oxidation in pseudoexfoliation syndrome. Curr Eye Res 2006;31:1029–32.
- Tezel G, Yang X, Cai J. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Invest Ophthalmol Vis Sci 2005;46:3177–87.
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet 1988;22:631–77.
- Caprioli J, Kitano S, Morgan JE. Hyperthermia and hypoxia increase tolerance of retinal ganglion cells to anoxia and excitotoxicity. Invest Ophthalmol Vis Sci 1996;37:2376–81.
- Caprioli J, Ishii Y, Kwong JM. Retinal ganglion cell protection with geranylgeranylacetone, a heat shock protein inducer, in a rat glaucoma model. Trans Am Ophthalmol Soc 2003;101:39–50; discussion 50–1.
- Albrecht J, Sonnewald U, Waagepetersen HS, Schousboe A. Glutamine in the central nervous system: function and dysfunction. Front Biosci 2007;12:332–43.
- Linser PJ, Sorrentino M, Moscona AA. Cellular compartmentalization of carbonic anhydrase-C and glutamine synthetase in developing and mature mouse neural retina. Brain Res 1984;315:65–71.
- Babizhayev MA, Bunin A. Lipid peroxidation in open-angle glaucoma. Acta Ophthalmol 1989;67:371–7.
- Gartaganis SP, Patsoukis NE, Nikolopoulos DK, Georgiou CD. Evidence for oxidative stress in lens epithelial cells in pseudoexfoliation syndrome. Eye 2007;21:1406–11.
- Yucel I, Akar Y, Yucel G, Ciftcioglu MA, Keles N, Aslan M. Effect of hypercholesterolemia on inducible nitric oxide synthase expression in a rat model of elevated intraocular pressure. Vision Res 2005;45:1107–14.
- Kruman I, Bruce-Keller AJ, Bredesen D, Waeg G, Mattson MP. Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. J Neurosci 1997;17:5089–100.
- Malone PE, Hernandez MR. 4-Hydroxynonenal, a product of oxidative stress, leads to an antioxidant response in optic nerve head astrocytes. Exp Eye Res 2007;84:444–54.
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol 1986;58:61–97.
- Costarides AP, Riley MV, Green K. Roles of catalase and the glutathione redox cycle in the regulation of anterior-chamber hydrogen peroxide. Ophthalmic Res 1991;23:284–94.
- De La Paz MA, Epstein DL. Effect of age on superoxide dismutase activity of human trabecular meshwork. Invest Ophthalmol Vis Sci 1996;37:1849–53.
- Riley MV. Physiologic neutralization mechanisms and the response of the corneal endothelium to hydrogen peroxide. CLAO J 1990;16:S16–21; discussion S21–2.
- Zoric L, Miric D, Milenkovic S, Jovanovic P, Trajkovic G. Pseudoexfoliation syndrome and its antioxidative protection deficiency as risk factors for age-related cataract. Eur J Ophthalmol 2006;16:268–73.
- Richer SP, Rose RC. Water soluble antioxidants in mammalian aqueous humor: interaction with UV B and hydrogen peroxide. Vision Res 1998;38:2881–8.
- Koliakos GG, Konstas AG, Schlotzer-Schrehardt U, Hollo G, Katsimbris IE, Georgiadis N, et al. 8-Isoprostaglandin F2a and ascorbic acid concentration in the aqueous humour of patients with exfoliation syndrome. Br J Ophthalmol 2003;87:353–6.
- May JM. Is ascorbic acid an antioxidant for the plasma membrane? FASEB J 1999;13:995–1006.
- Asregadoo ER. Blood levels of thiamine and ascorbic acid in chronic open-angle glaucoma. Ann Ophthalmol 1979;11:1095–100.
- Gherghel D, Griffiths HR, Hilton EJ, Cunliffe IA, Hosking SL. Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci 2005;46:877–83.
- Dickinson DA, Levonen AL, Moellering DR, Arnold EK, Zhang H, Darley-Usmar VM, et al. Human glutamate cysteine ligase gene regulation through the electrophile response element. Free Radic Biol Med 2004;37:1152–9.
- Moreno MC, Campanelli J, Sande P, Sanez DA, Keller Sarmiento MI, Rosenstein RE. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med 2004;37:803–12.
- Yucel YH, Zhang Q, Weinreb RN, Kaufman PL, Gupta N. Atrophy of relay neurons in magno- and parvocellular layers in the lateral geniculate nucleus in experimental glaucoma. Invest Ophthalmol Vis Sci 2001;42:3216–22.
- Xu X, Ichida JM, Allison JD, Boyd JD, Bonds AB, Casagrande VA. A comparison of koniocellular, magnocellular and parvocellular receptive field properties in the lateral geniculate nucleus of the owl monkey (Aotus trivirgatus). J Physiol 2001;531:203–18.
- Zhao B, Ranguelova K, Jiang J, Mason RP. Studies on the photosensitized reduction of resorufin and implications for the detection of oxidative stress with Amplex Red. Free Radic Biol Med 2011;51:153–9.
- Wood JP, Lascaratos G, Bron AJ, Osborne NN. The influence of visible light exposure on cultured RGC-5 cells. Mol Vis. 2007;14:334–44.
- Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci 1994;14:4368–74.
- Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol 1997;115(8):1031–5.
- Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res 2012;31:152–81.
- Kanamori A, Catrinescu MM, Kanamori N, Mears KA, Beaubien R, Levin LA. Superoxide is an associated signal for apoptosis in axonal injury. Brain 2010;133:2612–25.
- Higgins GC, Devenish RJ, Beart PM, Nagley P. Transitory phases of autophagic death and programmed necrosis during superoxide-induced neuronal cell death. Free Radic Biol Med 2012;53:1960–7.
- Springer JE, Azbill RD, Mark RJ, Begley JG, Waeg G, Mattson MP. 4-Hydroxynonenal, a lipid peroxidation product, rapidly accumulates following traumatic spinal cord injury and inhibits glutamate uptake. J Neurochem 1997;68:2469–76.
- Aslan M, Yucel I, Akar Y, Yucel G, Ciftcioglu MA, Sanlioglu S. Nitrotyrosine formation and apoptosis in rat models of ocular injury. Free Radic Res 2006;40:147–53.
- Liu B, Neufeld AH. Expression of nitric oxide synthase-2 (NOS-2) in reactive astrocytes of the human glaucomatous optic nerve head. Glia 2000;30:178–86.
- Shareef S, Sawada A, Neufeld AH. Isoforms of nitric oxide synthase in the optic nerves of rat eyes with chronic moderately elevated intraocular pressure. Invest Ophthalmol Vis Sci 1999;40:2884–91.
- Neufeld AH, Sawada A, Becker B. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma. Proc Natl Acad Sci USA 1999;96:9944–48.
- Sarih M, Souvannavong V, Adam A. Nitric oxide synthase induces macrophage death by apoptosis. Biochem Biophys Res Commun 1993;191:503–18.
- Hu J, Van Eldik LJ. S100 beta induces apoptotic cell death in cultured astrocytes via a nitric oxide-dependent pathway. Biochim Biophys Acta 1996;1313:239–45.
- Heneka MT, Loschmann PA, Gleichmann M, Weller M, Schulz JB, Wullner U, et al. Induction of nitric oxide synthase and nitric oxide-mediated apoptosis in neuronal PC12 cells after stimulation with tumor necrosis factor-alpha/lipopolysaccharide. J Neurochem 1998;71:88–94.
- Reed JC. Mechanisms of apoptosis. Am J Pathol 2000;157:1415–30.
- Kim HS, Park CK. Retinal ganglion cell death is delayed by activation of retinal intrinsic cell survival program. Brain Res 2005;1057:17–28.
- Saeki K, Kobayashi N, Inazawa Y, Zhang H, Nishitoh H, Ichijo H, et al. Oxidation-triggered c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein (MAP) kinase pathways for apoptosis in human leukaemic cells stimulated by epigallocatechin-3-gallate (EGCG): a distinct pathway from those of chemically induced and receptor-mediated apoptosis. Biochem J 2002;368:705–20.
- Jun CD, Oh CD, Kwak HJ, Pae HO, Yoo JC, Choi BM, et al. Overexpression of protein kinase C isoforms protects RAW 264.7 macrophages from nitric oxide-induced apoptosis: involvement of c-Jun N-terminal kinase/stress-activated protein kinase, p38 kinase, and CPP-32 protease pathways. J Immunol 1999;162:3395–401.
- Hortelano S, Alvarez AM, Bosca L. Nitric oxide induces tyrosine nitration and release of cytochrome c preceding an increase of mitochondrial transmembrane potential in macrophages. FASEB J 1999;13:2311–7.
- Kim YM, Bombeck CA, Billiar TR. Nitric oxide as a bifunctional regulator of apoptosis. Circ Res 1999;84:253–6.
- McKinnon SJ, Lehman DM, Kerrigan-Baumrind LA, Merges CA, Pease ME, Kerrigan DF, et al. Caspase activation and amyloid precursor protein cleavage in rat ocular hypertension. Invest Ophthalmol Vis Sci 2002;43:1077–87.
- Levkovitch-Verbin H, Dardik R, Vander S, Melamed S. Mechanism of retinal ganglion cells death in secondary degeneration of the optic nerve. Exp Eye Res 2010;91:127–34.
- Levkovitch-Verbin H, Harizman N, Dardik R, Nisgav Y, Vander S, Melamed S. Regulation of cell death and survival pathways in experimental glaucoma. Exp Eye Res 2007;85:250–8.
- Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-kappa B, a ubiquitous transcription factor in the initiation of diseases. Clin Chem 1999;45:7–17.
- Peng HB, Libby P, Liao JK. Induction and stabilization of I kappa B alpha by nitric oxide mediates inhibition of NF-kappa B. J Biol Chem 1995;270:14214–9.
- Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res 2009;28:348–68.
- Schwartz M. Macrophages and microglia in central nervous system injury: are they helpful or harmful? J Cereb Blood Flow Metab 2003;23:385–94.
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 2009;27:119–45.
- Schwartz M, Kipnis J. Autoimmunity on alert: naturally occurring regulatory CD4(+)CD25(+) T cells as part of the evolutionary compromise between a 'need' and a 'risk'. Trends Immunol 2002;23:530–4.
- Tezel G, Yang X, Luo C, Kain AD, Powell DW, Kuehn MH, et al. Oxidative stress and the regulation of complement activation in human glaucoma. Invest Ophthalmol Vis Sci 2010;51:5071–82.
- Tezel G, Luo C, Yang X. Accelerated aging in glaucoma: immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head. Invest Ophthalmol Vis Sci 2007;48:1201–11.
- Lin L. RAGE on the Toll Road? Cell Mol Immunol 2006;3:351–8.
- Pun PB, Lu J, Moochhala S. Involvement of ROS in BBB dysfunction. Free Radic Res 2009;43:348–64.
- Feilchenfeld Z, Yucel YH, Gupta N. Oxidative injury to blood vessels and glia of the pre-laminar optic nerve head in human glaucoma. Exp Eye Res 2008;87:409–14.
- Luo C, Yang X, Kain AD, Powell DW, Kuehn MH, Tezel G. Glaucomatous tissue stress and the regulation of immune response through glial Toll-like receptor signaling. Invest Ophthalmol Vis Sci 2010;51:5697–707.
- Roberts RL, Green J, Lewis B. Lutein and zeaxanthin in eye and skin health. Clin Dermatol 2009;27:195–201.
- Rhone M, Basu A. Phytochemicals and age-related eye diseases. Nutr Rev 2008;66:465–72.
- Cort A, Ozturk N, Akpinar D, Unal M, Yucel G, Aslan M, et al. Suppressive effect of astaxanthin on retinal injury induced by elevated intraocular pressure. Regul Toxicol Pharmacol 2010;58:121–30.
- Mozaffarieh M, Grieshaber MC, Orgul S, Flammer J. The potential value of natural antioxidative treatment in glaucoma. Surv Ophthalmol 2008;53:479–505.
- Agorastos A, Huber CG. The role of melatonin in glaucoma: implications concerning pathophysiological relevance and therapeutic potential. J Pineal Res. 2011;50:1–7.
- Johnson J, Maher P, Hanneken A. The flavonoid, eriodictyol, induces long-term protection in ARPE-19 cells through its effects on Nrf2 activation and phase 2 gene expression. Invest Ophthalmol Vis Sci. 2009;50:2398–406.
- Luna C, Li G, Liton PB, Qiu J, Epstein DL, Challa P, et al. Resveratrol prevents the expression of glaucoma markers induced by chronic oxidative stress in trabecular meshwork cells. Food Chem Toxicol 2009;47:198–204.
- Chung HS, Harris A, Kristinsson JK, Ciulla TA, Kagemann C, Ritch R. Ginkgo biloba extract increases ocular blood flow velocity. J Ocul Pharmacol Ther 1999;15:233–40.
- Quaranta L, Bettelli S, Uva MG, Semeraro F, Turano R, Gandolfo E. Effect of Ginkgo biloba extract on preexisting visual field damage in normal tension glaucoma. Ophthalmology 2003;110:359–62; discussion 62–4.
- Ritch R. Potential role for Ginkgo biloba extract in the treatment of glaucoma. Med Hypotheses 2000;54:221–35.
- Choi JH, Ryu YW, Seo JH. Biotechnological production and applications of coenzyme Q10. Appl Microbiol Biotechnol 2005;68:9–15.
- Tomasetti M, Alleva R, Borghi B, Collins AR. In vivo supplementation with coenzyme Q10 enhances the recovery of human lymphocytes from oxidative DNA damage. FASEB J 2001;15:1425–7.
- Takahashi N, Iwasaka T, Sugiura T, Onoyama H, Kurihara S, Inada M, et al. Effect of coenzyme Q10 on hemodynamic response to ocular timolol. J Cardiovasc Pharmacol 1989;14:462–8.
- Saenz DA, Turjanski AG, Sacca GB, Marti M, Doctorovich F, Sarmiento MI, et al. Physiological concentrations of melatonin inhibit the nitridergic pathway in the Syrian hamster retina. J Pineal Res 2002;33:31–6.
- Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 2004;36:1–9.
- Zanon-Moreno V, Garcia-Medina JJ, Gallego-Pinazo R, Vinuesa-Silva I, Moreno-Nadal MA, Pinazo-Duran MD. Antioxidant status modifications by topical administration of dorzolamide in primary open-angle glaucoma. Eur J Ophthalmol 2009;19:565–71.
- Yu AL, Fuchshofer R, Kampik A, Welge-Lussen U. Effects of oxidative stress in trabecular meshwork cells are reduced by prostaglandin analogues. Invest Ophthalmol Vis Sci 2008;49:4872–80.
- Butorov IV, Nikolenko IA, Butorov SI. [Efficacy of galavit in patients with duodenal ulcer]. Klin Med (Mosk) 2005;83:72–5.
- Jiang Y, Scofield VL, Yan M, Qiang W, Liu N, Reid AJ, et al. Retrovirus-induced oxidative stress with neuroimmunodegeneration is suppressed by antioxidant treatment with a refined monosodium alpha-luminol (Galavit). J Virol 2006;80:4557–69.
- Gionfriddo JR, Freeman KS, Groth A, Scofield VL, Alyahya K, Madl JE. alpha-Luminol prevents decreases in glutamate, glutathione, and glutamine synthetase in the retinas of glaucomatous DBA/2J mice. Vet Ophthalmol 2009;12:325–32.
- Dogan S, Unal M, Ozturk N, Yargicoglu P, Cort A, Aslan M, et al. Manganese porphyrin reduces retinal injury induced by ocular hypertension in rats. Exp Eye Res 2011;93:387–96.
- Babizhayev MA, Kasus-Jacobi A. State of the art clinical efficacy and safety evaluation of N-acetylcarnosine dipeptide ophthalmic prodrug. Principles for the delivery, self-bioactivation, molecular targets and interaction with a highly evolved histidyl-hydrazide structure in the treatment and therapeutic management of a group of sight-threatening eye diseases. Curr Clin Pharmacol 2009;4:4–37.
- Babizhayev MA. Biomarkers and special features of oxidative stress in the anterior segment of the eye linked to lens cataract and the trabecular meshwork injury in primary open-angle glaucoma: challenges of dual combination therapy with N-acetylcarnosine lubricant eye drops and oral formulation of nonhydrolyzed carnosine. Fundam Clin Pharmacol 2012;26:86–117.