Abstract
Objectives
Neoplastic niche is a specific microenvironment for growth and proliferation of malignant cells. Here we review the leukemic niche and its constituent stem cells, signaling pathways and essential chemokines.
Methods
Relevant literature was identified by a PubMed search (2000–2013) of English-language literature using the terms neoplastic niche, chemokines, and leukemia.
Discussion
Leukemia is caused by malignant hematopoietic stem cells and precursors. Important molecules and signals are involved in interactions between leukemic cells and their microenvironment. MicroRNAs (miRNAs) play an important role in expression regulation of oncogenes, transcription factors, signaling molecules and in eventual fate of the cell. It seems necessary to evaluate the relationship between aberrant miRNA expression and malignant transformation of bone marrow niche.
Conclusions
Characterizing malignant leukemic cells, activated signaling pathways, and molecules involved in disease progression will result in understanding the causes of drug resistance, relapse factors, and effective treatments.
Introduction
Bone marrow (BM) niche is a specific physiological microenvironment for hematopoietic and non-hematopoietic stem cells (HSCs) such as mesenchymal stem cells (MSCs). Maintaining such stem cell characteristics as pluripotency, self-renewal, control of stem cell number, proliferation, and determination of the fate of these cells is among the duties of this niche.Citation1,Citation2
BM microenvironment supporting the maintenance of HSCs is composed of two separate niches: osteoblastic (endosteal) and vascular.Citation3 In the osteoblastic niche, there are molecules such as bone morphogenetic protein (BMP), osteopontin, angiopoietin-1 and Notch, which appear to play important regulatory roles.Citation4 This niche also provides a microenvironment for long-term HSCs involved in hematopoiesis. Vascular niche composed of sinusoidal endothelial cells promotes the differentiation and proliferation of short-term HSCs.Citation3,Citation4
MSCs are another type of stem cells in BM niche in the vicinity of endosteum, and together with osteoblasts are involved in maintenance of HSCs and regulation of osteoblastic differentiation by secretion and synthesis of important factors. Metalloproteinase-3, agrin, and BMP6 are a number of factors involved in normal bone formation, supplying survival signals to hematopoietic progenitor cells and resulting in increased osteoblastic differentiation in BM niche, respectively.Citation2,Citation5,Citation6
Myeloid cells are of particular importance in HSC niche. In this regard, macrophages are positive regulators of MSCs and osteoblasts in BM niche by secreting such proteins as IL-1 and IGF-1, and play important roles for retention of HSCs.Citation5,Citation6
In a normal niche, BM microenvironment is formed by stromal cells such as osteoblasts and non-stromal cells like osteoclasts. Osteoclasts and osteoblasts play critical roles in remodeling and structure of niche. Osteoblasts regulate maturation and proliferation of BM cells through expression and secretion of a number of molecules and factors.Citation2 Typically, a normal niche maintains the balance between stasis expansion of stem cells.Citation7 Signals such as wnt/β-catenin growth inducing signal and BMP anti-growth signal are also involved in regulating the balance between stasis and expansion of niche.Citation2 If the stable balance of niche is egregiously influenced by activation of proliferative signals, loss of anti-growth signals, or infiltration of malignant cells such as myeloma cells, cancer stem cells (CSCs) are generated and reside in both osteoblastic and vascular niches, and this condition converts the normal niche to neoplastic one.Citation8–Citation10 Leukemia is the result of malignancy of HSCs and precursors, and changes in BM niche are considered as the basis for leukemia.Citation2 Studies have indicated that concomitant with leukemogenic events in hematopoietic system of the niche, secretory signals of niche promote the growth and proliferation of leukemic cells.Citation3
In this situation, leukemic microenvironment is created, in which leukemic cells disrupt the normal niche of hematopoietic precursor cells of BM, and create a cancerous microenvironment that can be called leukemic niche. According to investigations, leukemic cells in this niche receive antiapoptotic signals such as survivin and anti-apoptotic BCL-2 family members not only from osteoblasts but also from vascular endothelium.Citation3,Citation11,Citation12
Metastatic niche, the growth-supportive environment for extravasated cancer cells, is created in two stages of metastasis initiation and metastatic growth, and certain factors and molecules are involved in each stage.Citation13,Citation14 Metastasis initiation is triggered by cytokines and enzymes secreted by primary induced tumor. These include matrix protein fibronectin, matrix cross-linking enzyme lysil oxidase (LOX) and matrix metalloproteinase (MMP2 and MMP9).Citation14 Wnt and Notch signaling pathways, TGFβ along with proteolytic enzymes MMPs and cathepsin have been recognized as prominent components in metastatic outgrowth induction.Citation13,Citation14
Hypoxic conditions, growth factors, signaling molecules and secreted cytokines are different in normal as compared with malignant niche. Stem cell factor secreted by cancer stem cells leads to malignant transformation of resident cells in the niche. In addition, CD44 is involved in leukemic niche through homing and engraftment of these cells by binding selectins and hyaluronic acid.Citation15–Citation17 In addition, the expression level of IL-6 as an important growth factor in BM environment is higher in malignant niche in comparison with normal BM stromal cells, which will lead to resistance to apoptosis. Excessive proliferation of cells in leukemic endosteal niche will result in expansion of hypoxic conditions in the niche.Citation15,Citation18
ATP-binding cassette (ABC) transporters are conserved transmembrane proteins highly expressed in HSCs, protecting stem cells from genetic damage due to xenobiotics.Citation19 In parallel with normal stem cells, malignant cells specifically express ABC transporters, which is responsible for multidrug resistance of malignant cells. ABCG2, ABCB1, and ABCC1 are three important ABC transporters known as multidrug resistance genes in malignant cells.Citation20 Therefore, ABC transporters play important roles in both normal and abnormal physiology. However, further studies are required in relation to the interaction among ABC transporters and drugs in neoplastic niche.Citation19,Citation20
Neoplastic niche microenvironment provides for circumstances leading to homing of normal and malignant cells. Higher expression of a number of signaling molecules like Notch is likely to result in chemoprotection and resistance to apoptosis in CSCs.Citation15,Citation21
According to the above, as HSCs niche in normal and neoplastic state of the leukemia involves complex interactions between multiple cell types and molecules, and as chemokines, cytokines, and growth factors are also secreted in niche, better understanding the nature of stem cells, leukemia types, cytokines, and chemokines secreted in normal and neoplastic niches will lead to improved treatment methods for some types of cancers and malignant tumors. In this regard, this review article will assess the factors affecting the normal and neoplastic niches in some acute and chronic leukemias such as acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), chronic lymphoblastic leukemia (CLL), and multiple myeloma (MM).
Cancer stem cells in leukemic niche
CSC is a biologically distinct cell in a neoplastic clone capable of initiating tumor growth and self-renewal of cancer cells, leading to creation of heterogeneous lineages of cancer cells. In addition to progenitors and other differentiated cells, normal stem cells are the most likely targets for mutants causing CSCs due to their active self-renewal routes.Citation22,Citation23 Leukemic stem cells are a type of cancer stem cell identified in leukemias with similar self-renewal capacity with normal HSCs but with some significant differences (). Leukemic niche or microenvironment of leukemic stem cells provides a site for homing of malignant leukemic cells, and plays an essential role in growth and progression of leukemia. Interactions between CSCs and their microenvironment require a number of molecules and factors. For example, CD44 is a key factor in homing and engraftment of CSCs to their leukemic niche in AML and CML.Citation1,Citation24
Table 1. Similarities and differences between normal stem cells and cancer stem cells in leukemia
In addition to CD44, other molecules are involved in homing of HSCs in BM niche. Adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1), E and P-endothelial selectins and integrins like very late activation antigen-4 (VLA-4) and α4-integrin are among other molecules involved in stem cell homing. In addition to the above molecules, soluble factors such as granulocyte colony-stimulating factor (G-CSF), granulocyte /macrophage colony stimulating factor (GM-CSF), and growth factors such as vascular endothelial growth factor, angiopoietin-1, and chemokines such as IL-8, stromal cell-derived factor 1 (SDF-1) as well as other factors play a role in homing of stem cells. Among these factors, α4-integrin/VCAM-1 play prominent roles in BM homing.Citation25–Citation28
Signaling in leukemic niche
Interactions between HSCs and their microenvironment are of particular importance to the extent that they affect the function of these cells. Therefore, regulating the activity of hematopoietic cells is a complicated process requiring moderating signals provided by the microenvironment around them.Citation24,Citation33 For example, the interaction between anti-growth signal BMP and Wnt is responsible for regulating homeostatic balance of HSCs, disruption of which will lead to tumor formation.Citation7 Wnt/beta-catenin signaling pathway is a major signaling pathway in regulating HSC functions such as differentiation and apoptosis.Citation34 Moreover, SDF-1-mediated CXCR4 signaling, Notch1 activation, and B lymphoma Mo-MLV insertion region 1 homolog (BMI-1) are respectively involved in implantation and mobilization, increased self-renewal capacity and maintenance of HSCs.Citation35 Notch and Gs signaling pathways are among other signaling pathways playing important roles in maintenance of HSCs and osteoblastic differentiation, respectively.Citation36,Citation37Concomitant with growth of leukemic stem cells, signaling mechanisms between HSCs, and stem cell niche are hijacked by these malignant cells.Citation7 Aberrant activation or dysregulation of these signaling pathways and molecules involved in them can lead to a variety of malignant disorders. Disruption of Wnt signaling pathway has been observed in AML cell lines, during which decreased intracellular level of beta-catenin in vitro can reduce proliferation in the cell line without affecting viability of the cells.Citation34 Interference with the above signaling pathway has also been reported in MM, such that downregulation of beta-catenin leads to G1/G2 phase increase of cells and reduced S phase cells.Citation34,Citation38 In MM, increased expression of DKK1 as Wnt inhibitor by BM stem cells, especially MSCs, has been reported followed by inhibition of osteoblastic differentiation of MSCs.Citation39 Dysregulation or activation of Wnt signaling pathway through abnormal promoter methylation of Wnt inhibitors like DKK3 and WIF1 has also been reported in ALL patients, and is involved in pathogenesis of lymphoid leukemia. Dysregulation of this pathway in CML patients causes progression of disease from chronic phase to advanced phase.Citation40,Citation41 Continuous Gs signaling leads to accumulation of osteoblast cells, resulting in reduced expression of key factors influencing the maintenance of HSCs in the BM.Citation37 Transcription factor genes and signaling molecules are among the most common mutated or dysregulated genes observed in leukemias ().
Table 2. Some of important transcription factors/signaling molecules involved in leukemic niche
CXCR4/SDF-1 axis as a key chemokine/chemokine receptor interaction in leukemic niche
In addition to their role in leukocyte migration and hematopoiesis, chemokines play important roles in pathological processes such as hematological neoplasias.Citation57 As shown in , chemokine (C-X-C motif) receptor type 4 (CXCR4) plays an important role in many types of cancers indicating leukemia.Citation58 Most leukemic cells express CXCR4, but the expression level of this receptor is different in various types of leukemia.Citation59,Citation60 Interaction between chemokines and their receptors (known as cluster) plays important roles during leukemogenesis. For example, CXCL12 (also known as SDF1) as a member of CXC chemokine family along with CXCR4 forms CXCL12/CXCR4 cluster, and during leukemogenesis in AML patients regulates migration of AML cells.Citation61 SDF1/CXCR4 signaling together with oncogenic proteins involved in various types of leukemia is important in prognosis of the disease, so that ITD-Flt3 signaling in AML and BCR/ABL signaling in CML along with SDF1/CXCR4 is respectively involved in development of leukemia and migration of malignant cells.Citation60 This chemokine receptor is essential for homing of ALL cells in bone microenvironment, the expression of which is regulated by a wide range of cytokines.Citation59,Citation60 In addition to cytokines, a wide range of transcription factors affect transactivation of this chemokine receptor. FOXA2, FOXC2, and FOXH1 are among the transcription factors enhancing transactivation of this chemokine receptor, and Ying Yang 1 (YY1) is among those that reduce its transactivation.Citation62,Citation63
Figure 1. Schema of the relationship between changing level of miRNAs in stem cells and malignant neoplastic cells in neoplastic niche (51 and 54). (A) In normal niche respectively from top to bottom: osteoblasts, hematopoietic stem cells, among which there is a macrophage and an osteoclast is shown at the end. (B) In neoplastic niche, a variety of hematologic malignancies and miRNAs changes during the disease process has been shown, in which the upregulated miRNAs are shown in red and those downregulated in green. CXCR4/SDF1 axis has been shown as the important chemokine/chemokine receptor expressed in a variety of malignancies in neoplastic niche.
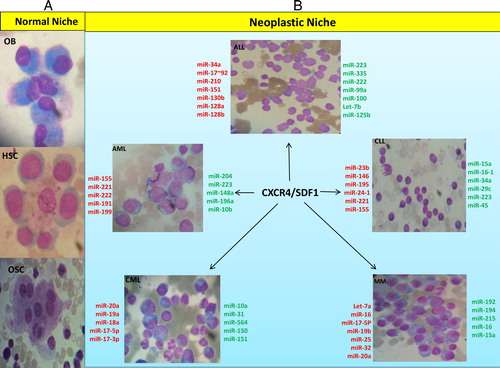
Interaction of leukemic stem cells with BM vasculature is strongly dependent upon CXCR4 expression and binding to SDF-1 expressed by vessel endothelium. Small peptide inhibitors of CXCR4 have been shown to be capable of overcoming BM stroma-mediated resistance to drug-induced apoptosis in AML and CLL. Therefore, CXCR4/SDF-1 can be considered as an autocrine mechanism with an indirect sanctuary role in drug resistance. In addition to the important role of this cytokine, autocrine IL-6 generation in myeloma cell clones can be a resistance mechanism against drug-induced apoptosis.Citation7,Citation15
MiRNAs, cancer stem cells, and therapeutic approach in neoplastic niche
MiRNAs as small RNA molecules affect many biological processes such as proliferation and apoptosis of the cells.Citation64,Citation65 In addition, during the tumorigenesis process, miRNAs regulate important features of cancer stem cells such as migration and invasion causing increased metastatic capacity of these malignant cells. Studies conducted in this regard indicate the oncogenic role of miR-15/miR-16 cluster in CLL, such as targeting the apoptotic inhibitor Bcl-2.Citation65 In addition to the role of miRNA in regulating adhesion molecules, such as the role of miR-10a and miR-126 in controlling the expression of VCAM-1, the activity of adhesion-related miRNAs can be changed during development and progression of cancer metastasis. MiR-10b, miR-31 and miR-200 are among the adhesion-related miRNAs participating in metastatic progression in tumor models.Citation66
As shown in , miRNAs are involved in a number of leukemias such as AML, ALL, CML, and CLL as well as MM. Considering the expression of MiRNAs in leukemic cells and specificity of some types of them for a number of leukemias like down regulation of miR-92 in acute leukemia, this group of small noncoding RNAs can be used as biomarkers to develop appropriate therapeutic approaches for some leukemia types.Citation67 Taking advantage of the tumor suppressor property of miRNAs is also possible in some types of leukemia-like tumor suppressor miR-451 in T-ALL.Citation64,Citation68
Using monoclonal antibodies conjugated with cytotoxic antibiotics against cell surface markers in leukemic cells in a variety of hematologic malignancies is also one of the important practical therapeutic strategies to eliminate leukemic stem cells. Considering the expression of CXCR4 in a variety of leukemic cell types, using the CXCR4 antagonists can be a therapeutic prospect in treatment of some leukemias.Citation29
Discussion
Recent studies on neoplastic niche have indicated that Polo like kinases (PLK) as serine/threonine protein kinase family members act as important regulators of cell cycle progression and cytokinesis. However, overexpressed PLK1 results in oncoprotein neoplastic niche. Recent studies consider conventional anti-leukemic chemotherapy combined with PLK inhibitor as a promising progress in treatment of AML. In addition, development of selective histone methyltransferase inhibitors such as EZH2 has recently been considered in treatment of hematologic malignancies like AML.Citation69
Normal and neoplastic niches have their specific molecules and microenvironment. Each of these niches with their own stem cells including normal and cancer stem cells along with signaling pathways, microenvironment specific chemokines and chemokine receptors are respectively involved in the growth and survival of normal stem cells and cancer progression, as observed in various types of leukemia. Accordingly, research aiming to identify molecules and factors involved in neoplastic niche is useful for therapeutic purposes.
Authors' contributions
N.S, S.B, and F.R conceived the manuscript and revised it; N.S, Sh.A, and M.Sh wrote the manuscript. N.S prepared the figure.
Acknowledgments
We wish to thank all our colleagues in Shafa Hospital and Allied Health Sciences School, Ahvaz Jundishapur University of Medical Sciences.
References
- Borovski T, De Sousa EMF, Vermeulen L, Medema JP. Cancer stem cell niche: the place to be. Cancer Res. [Research Support, Non-U.S. Gov't Review] 2011;71(3):634–9.
- Saki N, Abroun S, Hagh MF, Asgharei F. Neoplastic bone marrow niche: hematopoietic and mesenchymal stem cells. Cell 2011;13(3):131–6.
- Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. J Clin Oncol. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Review] 2011;29(5):591–9.
- Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] 2008;322(5909):1861–5.
- Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. [Research Support, Non-U.S. Gov't Review] 2011;208(3):421–8.
- Shen Y, Nilsson SK. Bone, microenvironment and hematopoiesis. Curr Opin Hematol. [Review] 2012;19(4):250–5.
- Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. [Comparative Study Review] 2006;66(9):4553–7.
- Haramis AP, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus GJ, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 2004;303(5664):1684–6.
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. [Research Support, Non-U.S. Gov't] 2004;36(10):1117–21.
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] 2004;351(7):657–67.
- Paydas S, Tanriverdi K, Yavuz S, Disel U, Sahin B, Burgut R. Survivin and aven: two distinct antiapoptotic signals in acute leukemias. Ann Oncol. [Comparative Study] 2003;14(7):1045–50.
- Tzifi F, Economopoulou C, Gourgiotis D, Ardavanis A, Papageorgiou S, Scorilas A. The role of BCL2 family of apoptosis regulator proteins in acute and chronic leukemias. Adv Hematol. 2012; doi: 10.1155/2012/524308.
- Irmisch A, Huelsken J. Metastasis: New insights into organ-specific extravasation and metastatic niches. Exp Cell Res. 2013;319(11):1604–10.
- Descot A, Oskarsson T. The molecular composition of the metastatic niche. Exp Cell Res. 2013;319(11):1679–86.
- Nwajei F, Konopleva M. The bone marrow microenvironment as niche retreats for hematopoietic and leukemic stem cells. Adv Hematol. 2013; doi: 10.1155/2013/953982.
- Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. [Research Support, N.I.H., Extramural] 2006;12(10):1175–80.
- Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. [Research Support, Non-U.S. Gov't] 2006;12(10):1167–74.
- Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. [Review] 2008;14(9):2519–26.
- Raaijmakers MH. ATP-binding-cassette transporters in hematopoietic stem cells and their utility as therapeutical targets in acute and chronic myeloid leukemia. Leukemia. [Review] 2007;21(10):2094–102.
- de Jonge-Peeters SD, Kuipers F, de Vries EG, Vellenga E. ABC transporter expression in hematopoietic stem cells and the role in AML drug resistance. Crit Rev Oncol Hematol. [Research Support, Non-U.S. Gov't Review] 2007;62(3):214–26.
- Colombo M, Mirandola L, Platonova N, Apicella L, Basile A, Figueroa AJ, et al. Notch-directed microenvironment reprogramming in myeloma: a single path to multiple outcomes. Leukemia 2013;27(5):1009–18.
- Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. [Review] 2005;15(9):494–501.
- Hu Y, Fu L. Targeting cancer stem cells: a new therapy to cure cancer patients. Am J Cancer Res. 2012;2(3):340–56.
- Kaplan RN, Psaila B, Lyden D. Niche-to-niche migration of bone-marrow-derived cells. Trends Mol Med. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review] 2007;13(2):72–81.
- Govindan R, Kumar V, Sisodia S, Mallick PJ. Molecular interactions in stem cell homing and bone marrow transplantation therapy. Int J Pharm. 2012;3(4):210–3.
- Bonig H, Priestley GV, Papayannopoulou T. Hierarchy of molecular-pathway usage in bone marrow homing and its shift by cytokines. Blood. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] 2006;107(1):79–86.
- Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood. 2001;98(8):2403–11.
- Guzman ML, Jordan CT. Considerations for targeting malignant stem cells in leukemia. Cancer Control. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review] 2004;11(2):97–104.
- Deng CH, Zhang QP. Leukemia stem cells in drug resistance and metastasis. Chin Med J (Engl). [Research Support, Non-U.S. Gov't Review] 2010;123(7):954–60.
- O'Hare T, Corbin AS, Druker BJ. Targeted CML therapy: controlling drug resistance, seeking cure. Curr Opin Genet Dev. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review] 2006;16(1):92–9.
- O'Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. [Research Support, Non-U.S. Gov't] 2009;16(5):401–12.
- Swerts K, De Moerloose B, Dhooge C, Laureys G, Benoit Y, Philippe J. Prognostic significance of multidrug resistance-related proteins in childhood acute lymphoblastic leukaemia. Eur J Cancer. [Research Support, Non-U.S. Gov't Review] 2006;42(3):295–309.
- Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. [Research Support, N.I.H., Extramural] 2008;2(3):274–83.
- Kim Y, Thanendrarajan S, Schmidt-Wolf IG. Wnt/ss-catenin: a new therapeutic approach to acute myeloid leukemia. Leuk Res Treatment. 2011; doi: 10.4061/2011/428960.
- Rizo A, Vellenga E, de Haan G, Schuringa JJ. Signaling pathways in self-renewing hematopoietic and leukemic stem cells: do all stem cells need a niche? Hum Mol Genet. [Research Support, Non-U.S. Gov't Review] 2006;15(2):R210–9.
- Weber JM, Calvi LM. Notch signaling and the bone marrow hematopoietic stem cell niche. Bone [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review] 2010;46(2):281–5.
- Schepers K, Hsiao EC, Garg T, Scott MJ, Passegue E. Activated Gs signaling in osteoblastic cells alters the hematopoietic stem cell niche in mice. Blood [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] 2012;120(17):3425–35.
- Abroun S, Saki N, Fakher R, Asghari F. Biology and bioinformatics of myeloma cell. Lab Hematol. 2012;18(4):30–41.
- Noll JE, Williams SA, Purton LE, Zannettino AC. Tug of war in the haematopoietic stem cell niche: do myeloma plasma cells compete for the HSC niche? Blood Cancer J. 2012;2:e91.
- Roman-Gomez J, Cordeu L, Agirre X, Jimenez-Velasco A, San Jose-Eneriz E, Garate L, et al. Epigenetic regulation of Wnt-signaling pathway in acute lymphoblastic leukemia. Blood [Clinical Trial Comparative Study Multicenter Study Research Support, Non-U.S. Gov't Retracted Publication] 2007;109(8):3462–9.
- Thanendrarajan S, Kim Y, Schmidt-Wolf IG. Understanding and Targeting the Wnt/beta-Catenin Signaling Pathway in Chronic Leukemia. Leuk Res Treatment. 2011; doi: 10.4061/2011/329572.
- Rosenbauer F, Koschmieder S, Steidl U, Tenen DG. Effect of transcription-factor concentrations on leukemic stem cells. Blood. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. Review] 2005;106(5):1519–24.
- Gao SM, Xing CY, Chen CQ, Lin SS, Dong PH, Yu FJ. miR-15a and miR-16-1 inhibit the proliferation of leukemic cells by down-regulating WT1 protein level. J Exp Clin Cancer Res. [Research Support, Non-U.S. Gov't] 2011;30:110.
- Helbling D, Mueller BU, Timchenko NA, Hagemeijer A, Jotterand M, Meyer-Monard S, et al. The leukemic fusion gene AML1-MDS1-EVI1 suppresses CEBPA in acute myeloid leukemia by activation of Calreticulin. Proc Natl Acad Sci USA. [Research Support, Non-U.S. Gov't] 2004;101(36):13312–7.
- Perrotti D, Cesi V, Trotta R, Guerzoni C, Santilli G, Campbell K, et al. BCR-ABL suppresses C/EBPalpha expression through inhibitory action of hnRNP E2. Nat Genet. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] 2002;30(1):48–58.
- Helbling D, Mueller BU, Timchenko NA, Schardt J, Eyer M, Betts DR, et al. CBFB-SMMHC is correlated with increased calreticulin expression and suppresses the granulocytic differentiation factor CEBPA in AML with inv(16). Blood. [Research Support, Non-U.S. Gov't] 2005;106(4):1369–75.
- Zhang P, Zhang X, Iwama A, Yu C, Smith KA, Mueller BU, et al. PU.1 inhibits GATA-1 function and erythroid differentiation by blocking GATA-1 DNA binding. Blood [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] 2000;96(8):2641–8.
- Ohnishi K. PML-RARalpha inhibitors (ATRA, tamibaroten, arsenic troxide) for acute promyelocytic leukemia. Int J Clin Oncol. [Review] 2007;12(5):313–7.
- Scandura JM, Boccuni P, Cammenga J, Nimer SD. Transcription factor fusions in acute leukemia: variations on a theme. Oncogene [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. Review] 2002;21(21):3422–44.
- Li X, Xu YB, Wang Q, Lu Y, Zheng Y, Wang YC, et al. Leukemogenic AML1-ETO fusion protein upregulates expression of connexin 43: the role in AML 1-ETO-induced growth arrest in leukemic cells. J Cell Physiol. [Research Support, Non-U.S. Gov't] 2006;208(3):594–601.
- Liu S, Klisovic RB, Vukosavljevic T, Yu J, Paschka P, Huynh L, et al. Targeting AML1/ETO-histone deacetylase repressor complex: a novel mechanism for valproic acid-mediated gene expression and cellular differentiation in AML1/ETO-positive acute myeloid leukemia cells. J Pharmacol Exp Ther. [Research Support, N.I.H., Extramural] 2007;321(3):953–60.
- Mitani K. Molecular mechanisms of leukemogenesis by AML1/EVI-1. Oncogene. [Review] 2004;23(24):4263–9.
- Zhu YM, Zhao WL, Fu JF, Shi JY, Pan Q, Hu J, et al. NOTCH1 mutations in T-cell acute lymphoblastic leukemia: prognostic significance and implication in multifactorial leukemogenesis. Clin Cancer Res. [Research Support, Non-U.S. Gov't] 2006;12(10):3043–9.
- Shih Ie M, Wang TL. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res. [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, Non-P.H.S. Review] 2007;67(5):1879–82.
- Staal FJ, Langerak AW. Signaling pathways involved in the development of T-cell acute lymphoblastic leukemia. Haematologica [Comment Research Support, Non-U.S. Gov't] 2008;93(4):493–7.
- Lane SW, Wang YJ, Lo Celso C, Ragu C, Bullinger L, Sykes SM, et al. Differential niche and Wnt requirements during acute myeloid leukemia progression. Blood [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] 2011;118(10):2849–56.
- Schrottner P, Leick M, Burger M. The role of chemokines in B cell chronic lymphocytic leukaemia: pathophysiological aspects and clinical impact. Ann Hematol. [Research Support, Non-U.S. Gov't Review] 2010;89(5):437–46.
- Abroun S. Chemokines in homeostasis and cancers. Cell 2007;10.
- Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood [Research Support, Non-U.S. Gov't Review] 2006;107(5):1761–7.
- Fukuda S, Onishi C, Pelus LM. Trafficking of acute leukemia cells–chemokine receptor pathways that modulate leukemia cell dissemination. 2011.
- Kittang AO, Hatfield K, Sand K, Reikvam H, Bruserud O. The chemokine network in acute myelogenous leukemia: molecular mechanisms involved in leukemogenesis and therapeutic implications. Curr Top Microbiol Immunol. [Review] 2010;341:149–72.
- Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. [Review] 2007;1768(4):952–63.
- Katoh M. Integrative genomic analyses of CXCR4: transcriptional regulation of CXCR4 based on TGFbeta, Nodal, Activin signaling and POU5F1, FOXA2, FOXC2, FOXH1, SOX17, and GFI1 transcription factors. Int J Oncol. 2010;36(2):415–20.
- Babashah S, Soleimani M. The oncogenic and tumour suppressive roles of microRNAs in cancer and apoptosis. Eur J Cancer. [Review] 2011;47(8):1127–37.
- Garg M. MicroRNAs, stem cells and cancer stem cells. World J Stem Cells. 2012;4(7):62–70.
- Valastyan S, Weinberg RA. Roles for microRNAs in the regulation of cell adhesion molecules. J Cell Sci. 2011;124(7):999–1006.
- Babashah S, Sadeghizadeh M, Tavirani MR, Farivar S, Soleimani M. Aberrant microRNA expression and its implications in the pathogenesis of leukemias. Cell Oncol (Dordr). [Research Support, Non-U.S. Gov't] 2012;35(5):317–34.
- Sionov RV. MicroRNAs and Glucocorticoid-Induced Apoptosis in Lymphoid Malignancies. ISRN Hematol. 2013; doi: 10.1155/2013/348212.
- Hatzimichael E, Georgiou G, Benetatos L, Briasoulis E. Gene mutations and molecularly targeted therapies in acute myeloid leukemia. Am J Blood Res. 2013;3(1):29–51.
