Abstract
Objective
Bone marrow (BM) niche is a three-dimensional structure composed of a series of cells and it is one of the most controversial topics in hematological malignancies, leukemia, and even metastasis. Here, we review the relationship between Notch signaling and different fates of stem cells and other BM niche cells.
Methods
Relevant English-language literature were searched and retrieved from PubMed (2000–2013) using the terms Notch signaling, BM niche, and microRNAs (miRNAs).
Discussion
Notch signaling pathway is a signaling system involved in cellular processes such as proliferation, differentiation, and apoptosis. The notch signaling pathway components are associated with interaction between leukemic, metastatic, and normal cells and their microenvironment. miRNAs play an important role in expression and regulation of signaling molecules. It is necessary to evaluate the relationship between aberrant miRNA expression and notch signaling such as miR-128 and miR-30 in glioma and angiogenesis with notch signaling, respectively.
Conclusions
Characterizing malignant cells and future studies focus on better understanding the variety of cancers and apoptosis with activated Notch signaling pathway, may remain promising this signaling system as a safe and effective therapeutic target.
Introduction
Bone marrow (BM) stem cell niche is one of the most controversial topics in the hematological malignancies, leukemia, and even metastasis. BM niche is a three-dimensional structure composed of a series of cells, and is locating many important hematopoietic and non-hematopoietic stem cells.Citation1 BM is the place where the blood cells are produced, while BM niche is the starting zone for many hematological malignancies and metastasis site of many cancer cells of other organs. Thus, studying the structure of BM, causes of its malignancy, the reasons for migration of cells of other tissues towards BM, and even drug resistance of malignant cells in niche are important topics to notice.Citation2 Besides, another vital function of niche is controlling the fate of cells. Therefore, understanding the reactions as well as cellular and molecular mechanisms involved in niche is that important to allow the cells to affect the fate of each other (differentiation induction or inhibition, apoptosis, and inactivation of the cells). The study of cell signaling pathways during cell development has helped the researchers effectively.Citation3 Notch is a cell signaling pathway involved in a wide variety of cellular processes such as apoptosis, differentiation, tissue self-renewal, and migration regulation.Citation4–Citation6 In addition, the above-mentioned roles, Notch plays important roles in development of cancer and tissue metastasis as an oncogene or tumor suppressor.Citation3 Therefore, in this review, we present some concepts about the relationship between signaling of Notch molecule and different fates of stem cells and other cells in BM niche.
Notch signaling and its effect on BM components
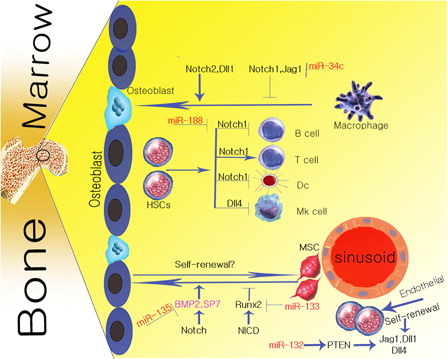
Notch is a signaling pathway controlling a wide range of cellular processes including proliferation, apoptosis, tissue self-renewal regulation, differentiation, and maintenance of precursor cells. These are essential functions of Notch signaling pathway in maintaining the normal homeostasis of BM.Citation7 Besides, abnormalities in Notch signaling pathway during the transformation of normal to malignant niche direct the cells towards oncogenes, tissue metastasis, and even human degenerative diseases.Citation3,Citation5 The Notch molecule has four Notch receptorsCitation1–Citation4 and five Delta-like 1 ligands, including Dll-1, Dll-3, Dll-4, Jagged-1, and Jagged-2.Citation8 Notch receptor is composed of three parts including extracellular, intracellular, and transmembrane domains with various cellular functions. The process triggering Notch signaling pathway is the release of the intracellular domain of Notch receptor into cytoplasm and its subsequent transport to the nucleus through a proteolytic cut by a gamma-secretase (GS) protein complex. This process is caused by coupling Notch receptor and ligands.Citation5,Citation9 The GS protein complex cut is formed during receptor–ligand coupling, and the second transmembrane of Notch receptor is the site of this cut. ADAM17/TAEC enzyme complex can also generate cuts in the intracellular domain of Notch (NICD) receptor. The set of events during the above-mentioned two cuts leads to releasing the NICD molecule. NICD forms a complex with any of CSL (CBF-1, suppressor of Hairless, Lag-2), MAML-1, and P300/CβP transcription regulators in the nucleus, as well as inducing expression of downstream Notch transcriptional factors, especially Hes-1 (hairy and enhancer of split-1).Citation10 Hes-1 expression is induced by Notch, and plays a role in regulating stem cell characteristics in the niche. Notch signaling has various effects on the main cellular components of BM microenvironment, including hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), osteoclasts, and osteoblasts ().
Figure 1. The figure shows the relationship between the main components of BM and Notch signaling pathway.
Osteoblasts
Osteoblasts are among BM niche cells derived from MSCs.Citation1 The inductive and inhibitory roles of Notch signaling in the development of osteoblasts have been demonstrated in many studies.Citation11 During osteoblastogenesis, the inhibitory effect of Notch is observed during reaction with Forkhead box transcription factor O (Foxo) 1 and Wnt signaling pathway. These processes inhibits the function of nuclear factor of activated T cells (NFAT) and β-catenin.Citation11 The binding of NICD to runt-related transcription factor 2 (Runx2) has also been noted that inhibits maturing osteoblasts.Citation11,Citation12 The stimulating effect of Notch in differentiation of osteoblasts is induced by bone morphogenetic protein 2 (BMP-2).Citation13 In addition, Notch can enhance osteoblastic proliferation by increasing the frequency of SP7 transcription factor.Citation11 Meanwhile, mir-133 and mir-135 are molecules that inhibit Runx2 and BMP-2 functions, respectively, in the osteoblastic differentiation pathway.Citation12 Therefore, it can be concluded that besides controlling MSCs to osteoblasts differentiation by Notch signaling pathway, the short-term and persistent activity of this signaling pathway will result in stimulation and inhibition of osteoblasts.Citation13 In this regard, studies on a murine model of overexpression of the Notch1 intracellular domain have indicated inhibiting the osteoblastic differentiation during osteopenia.Citation14
Osteoclasts
Osteoclasts are among BM niche cells derived from macrophage lineages in response to receptor activator of NF-kB ligand (RANKL) and macrophage colony-stimulating factor.Citation15 Similar to its role in osteoblasts, the Notch signaling pathway may function as a positive or negative regulator of osteoclastic development.Citation15,Citation16 Deletion of Notch receptors such as Notch1 has been found to increase osteoclastic development.Citation15 It has also been shown that Notch2 and Dll1 are important stimulating factors in the osteoclastogenesis through Notch signaling pathway.
Therefore, Notch1 and Jagged-1 are responsible for the inhibitory role of Notch in differentiation of osteoclasts.Citation13 In addition, in osteoclast precursor level, Notch will inhibit the final differentiation of osteoclasts by decreasing the expression of c-Fms.Citation16 It can be concluded that the role of Notch signaling pathway during osteoclastogenesis is dependent on the relationship between the expression of specific Notch ligands and receptors.
Hematopoietic stem cells
As an essential regulator of hematopoietic differentiation, Notch is involved in regulating the function and maintaining the phenotype of HSCs.Citation17 Expression of Notch receptors and ligands by HSCs and stromal cells, respectively, terminates the differentiation of HSC during the reaction between the Notch ligands and receptors.Citation18 The hormones are also involved in the regulation of BM niche by interacting with Notch ligands. In this connection, parathyroid hormone (PTH) increases the expansion of HSCs by increasing the expression of Jagged-1.Citation1,Citation6,Citation19 In addition to the hormonal role in self-renewal of HSCs, interaction between HoxB4 (homeobox B4) and Notch1 also plays a role in increasing the number of these cells.Citation20 Notch signaling in interaction with other signaling pathways, especially Wnt signaling, will lead to β-catenin stabilization in BM stromal cells, and will enhance maintenance and self-renewal of HSC.Citation21 Besides the role of Notch in maintenance of hematopoietic progenitors, deletion of Notch1 alone or Notch1 and Notch2 can lead to increased differentiation of lymphoid HSCs.Citation17 BM vascular niche plays an important role in maintenance of HSCs in association with Notch. Endothelial cells in the niche will lead to reconstitution of hematopoiesis by secreting angiocrine factors such as Notch ligands through replenishment of long-term hematopoietic stem cells (LT-HSCs) pool.Citation21 Components of Notch signaling pathway, including Notch1 are vital for development of embryonic stem cells from endothelial cells. It has been shown that knockdown of the components of this signaling pathway (including blocking the cutting of the intracellular domain of Notch) can lead to decreased repopulation of HSC pool.Citation22 Lack of Notch signaling pathway causes impaired maintenance of HSC both in vivo and in vitro.Citation20 In summary, it is now evident that Notch signaling can regulate the specification of HSC such as proliferation, differentiation, and maintenance.
Mesenchymal stem cells
These cells are multipotent stem cells with the capacity to differentiate into various cell lines, including osteoblast, cartilage, neural precursors, bone, fat, and other cell types.Citation1 Notch1–3 receptors are identified as MSCs receptors. However, Jagged-1 is the sole ligand significantly expressed in MSCs.Citation23 Studies have indicated the prominent role of Notch signaling in targeted differentiation of MSCs into different cell lines.Citation1,Citation24 In BM, Notch keeps MSCs in an undifferentiated state by reacting with transcription factors and maintaining the pool of mesenchymal progenitors. For example, Notch signaling pathway inhibits osteoblastic differentiation of MSCs by inhibiting Runx2 transcription factor.Citation25 Zanoti showed that overexpression of Notch1 molecule with Prx1 enhancer causes proliferation induction of precursor mesenchymal cells.Citation1 The presence of oxygen affects the differentiation function of MSCs, and its deficiency plays a role in the induction of Notch1 expression during osteogenic differentiation of MSCs. On the other hand, GS inhibitors (GSIs) reduce proliferation of MSCs and increase their differentiation into osteoblasts.Citation26 In hypoxic conditions, Notch inhibits osteogenic differentiation of MSC through increase in Notch1 protein by hypoxia-inducible factor 1α.Citation25 So, we can conclude that the Notch signaling pathway not only inhibits MSC differentiation, but will also enhance their proliferation.
shows cross-talk between components of Notch signaling pathway and BM components. Notch signaling pathway in BM is responsible for a separate role in each of the niche components such as MSCs, HSCs, osteoblasts, and osteoclasts. Notch regulates BM proliferation, differentiation, development, and maintenance through interaction with target genes of each of the BM components ().
Table 1. Cross-talk between Notch pathway and BM components
Notch signaling in stem cell niche
Notch signaling pathway plays a major role in regulating and controlling the key features of BM stem cells such as self-renewal, quiescence, and apoptosis. Notch ligands are the main components of the stem cell niche, boosting and maintaining stem cells by activating Notch receptors expressed by stem cells.Citation1,Citation5,Citation17 Concurrent increase in the expression of Notch Jagged-1 ligand by endosteal cells and activation of osteoblasts by PTH in the stem cell niche, leads to an increased number and self-renewal of stem cells.Citation17,Citation21 Following BM myeloablation, endothelial cells regenerate the damage by expressing Notch ligands,Citation27 suggesting the critical role of this signaling pathway in regulating the self-renewal of stem cells in the niche.
The important role of Notch signaling pathway in quiescence regulation of stem cells has been studied. However, the role of Notch in regulating quiescence in HSC is different from its role in other stem cells.Citation28–Citation30 Evidences have shown that unlike the incremental role of Notch signaling in quiescence regulation of neural and muscle stem cells, it is not necessary for maintaining the quiescence of HSC.Citation28 In addition to its role in maintaining the quiescence of stem cells, Hes-1 oscillation by Notch in neural stem cells causes these cells to quit the quiescent state.Citation29 Notch signaling has also been evaluated in satellite cells (SCs), which are stem cells in skeletal muscles. Notch3 and downstream targets of Notch-like Hes-1 and Hey1 (Hairy/enhancer-of-split related with YRPW motif protein 1) have been found to be highly expressed in quiescent SCs. Therefore, Notch signaling is essential to maintain SCs quiescence in skeletal muscle stem cells.Citation30
In this regard, nervous system plays an important role in migration of stem and progenitor cells, so that norepinephrine signaling controls the mobilization of these cells. Thus, the injection of β-2 adrenergic agonist will lead to increased mobilization of stem and progenitor cells to niche.Citation31 The role of Notch during development of the central nervous system is also interesting to note. Notch is responsible for maintenance of neural progenitor cells. Therefore, reduced Notch activity is in line with a decrease in neural progenitors and increased neuronal differentiation.Citation32 Given the above facts, it can be stated that Notch signaling may play a significant role in quiescence regulation of stem cells. However, the role of this pathway is not so prominent in maintenance of HSC quiescence.
Notch signaling also plays an important role in apoptosis. Cross-talk between tumor necrosis factor α (TNFα) and Notch signaling controls endothelial cell apoptosis.Citation33 TNFα is a cytokine found in considerable quantities in many tumors.Citation34 In endothelial cells, TNFα causes up-regulation and down-regulation of Notch2 mRNA and Notch4 mRNA, respectively.Citation33 Research has shown that survivin is an anti-apoptotic factor in the cells.Citation35 Up-regulation of Notch2 molecule causes reduced levels of survivin in the cells, sensitizing the susceptible cells to apoptosis. Such a reaction between Notch2 molecule and survivin is consistent with reduced Notch activity by TNFα signaling. Thus, inhibition of Notch signaling by TNFα inhibits endothelial cell apoptosis.Citation33 Chadwick et al.Citation36 also reported that sustained activation of Notch signaling pathway by aberrant expression of Notch 1 intracellular domain (N1ICD) through up-regulation of P21 and Bcl2L1 can induce apoptosis in human CD34+ hematopoietic progenitor cells. Therefore, increased or decreased activity of Notch signaling pathway plays an important role in apoptosis regulation in stem cells.
Notch also interacts with other apoptotic pathways during apoptosis. Notch pathway and apoptotic nuclear factor-kappa B (NF-kB) pathway have been introduced as important regulators of apoptosis, so that Notch1 inactivation results in apoptosis induction through NF-kB inactivation. Thus, it can be stated that Notch1 has an inhibitory function during apoptosis.Citation37 Interaction between miRNA-206 and 3′-untranslated region of Notch3 molecule leads to overexpression of microRNA (miRNA), decreased expression of Notch3 and blocking its anti-apoptotic activity, and finally apoptotic cell death.Citation38
Endothelial cells are present in the BM vascular niche, and are involved in HSC maintenance, proliferation, and differentiation by providing a niche for HSC.Citation39 Vascular cell-adhesion molecule-1 (VCAM-1) is expressed by endothelial cells. In addition to regulation of megakaryocyte maturation and platelet production, VCAM-1 plays an important role in homing of stem cells in the BM during cross-talk between HSCs and endothelial cells.Citation40 BM endothelial cells secrete soluble factors such as PTEN (Phosphatase and tensin homolog), and are involved in regulating the self-renewal of HSCs.Citation40 Mir-132 enhances the function of endothelial cells via up-regulating PTEN activity.Citation41 In addition to the above-mentioned roles, endothelial cells play an important role in malignant niche. Secretion of cytokines and proangiogenic factors such as vascular endothelial growth factor (VEGF) and initiation of endothelial cell growth by tumor cells cause stimulation, chemotaxis, and binding of endothelial cells to endothelium. This process results in physiological regeneration and repairing of vessels in the tumor.Citation42 Endothelial cell functions to augment the survival of leukemic stem cells including acute lymphoblastic leukemia (ALL) cells by modulating bcl-2 anti-apoptotic protein.Citation40 The role of Notch signaling pathway in endothelial progenitor cell biology has also been studied. Notch signaling plays an important role in the function and kinetics of the BM-derived endothelial progenitor cells.Citation43 In other words, maintenance, proliferation, and differentiation of endothelial progenitor cells and their role in vascular regeneration because of the Notch receptor–ligand interaction between these cells and the BM are regulated.Citation44 In addition, the role of Notch signaling and endothelial progenitor cells in tumor angiogenesis is parallel. Therefore, we suggest further studies on the Notch signaling pathway between endothelial progenitor cells and the BM niche. These may shed light on these cellular mechanisms and therapeutic significance of these cells in vascular regeneration.Citation45 Endothelial cells stimulate Notch signaling to enhance long-term self-renewal of HSCs by angiocrine expression of Notch ligands including Jagged1–2, DII1, and DII4. This process inhibits the excessive differentiation of hematopoietic progenitor cells.Citation20 Considering the extensive functions of endothelial cells in tumor growth, they can be called as cellular vectors that selectively target the tumor and deliver cytotoxic genes with anti-tumor effects in cells.Citation46
Notch in metastatic niche
Metastatic niche is a microenvironment to support the growth of cancer cells extravasated in tissues other than their tissue of origin.Citation47 In metastatic niche, certain signaling pathways are activated in stem cells, which play a significant role in development of cancer cells in that niche.Citation48 The Notch ligands and receptors play an important role in providing proper conditions for bone metastatic niche during interaction with growth factors released by osteoblasts and osteoclasts.Citation47 Transforming growth factor-β (TGFβ) is secreted by bone matrix through osteoclastic activity, which stimulates JAG-1 expression. The role of JAG-1 as a Notch ligand is triggering sustained proliferation of metastatic cells and subsequent breakdown of bone tissue.Citation47,Citation49 Connective tissue growth factor functions as a target for TGFβ, and is responsible for angiogenesis and invasion during metastasis.Citation49 Notch signaling pathway activation mediated by JAG associated with osteoblasts in metastatic niche is resulted from interleukin-6 (IL-6) secretion as a pro-proliferative cytokine during BM metastasis and tumor proliferation induction.Citation47,Citation49 The role of Jagged-1 ligand of Notch molecule in metastasis is so important that its overexpression will significantly increase tumor growth in damaged areas of bone.Citation49 In other words, it can be stated that Jagged-1 is responsible for osteolytic bone metastasis, and TGFβ-Jagged1-Notch-IL-6 interaction is a signaling network involved in development of bone metastasis.
Tenascin C is another important molecule in metastatic niche for the cells involved in tumor initiation. This molecule supports survival of metastasis initiating cells by increasing the response of these cells to Notch signaling pathway in metastatic niche.Citation50
Mechanisms of cancer metastasis cooperate or intervene with signaling pathways required for each stage of metastasis, and study of these mechanisms may result in therapeutic approaches.Citation5 Patel et al.Citation51 studied the reaction between Notch–Jagged and claimed that it plays a crucial role in the engagement catalyst of cancer cells in addition to modulation of cell–cell connections. Cancer cells exploit the Notch–Jagged reaction in activation of osteoclasts originating from hematopoietic lineage and osteoblasts derived from MSCs. This mechanism accelerates and enhances the growth and invasion of cancer cells in the bone, eventually breaking down the bone tissue.Citation51
Notch signaling pathway is important during angiogenesis, and its activation has been shown during specific stages in tumor angiogenesis.Citation52 Notch signaling affects angiogenesis directly or indirectly through receptors for VEGF.Citation53 VEGF, which is secreted in hypoxic conditions, has been introduced as the best angiogenic stimulus.Citation54 The two types of endothelial cells required for angiogenesis sprouting have been entitled ‘tip’ cell and ‘stalk’ cell.Citation53 During angiogenesis regulation stages, endothelial tip cells increase expressing DII4 and decrease Notch activity in response to a stimulatory gradient.Citation54 The response of endothelial stalk cells to such Notch signaling is exerted via down-regulation of VEGF receptors such as VEGFR-2 and VEGF-3 to inhibit extra tube formation during angiogenesis process.Citation53,Citation54 Therefore, Notch plays an important role in angiogenesis regulation by regulating the signaling between VEGF and DII4 receptors.
In one study, it was found that co-culture of breast cancer cells and fibroblasts leads to increased expression of chemokine (C–C motif) ligand 2 (CCL2). This chemokine will induce the expression of Notch1 through increased self-renewal of cancer stem cells (CSCs). In other words, the absence of CCL2 regulates Notch1 via inhibition of tumorigenesis and expression of Notch1.Citation55 Up-regulation of Notch2 in breast cancer is linked to increased survival, and blocking the expression of Notch3 is involved in reducing the invasiveness of cancer cells.Citation56 Besides, the individual function of Notch ligands and receptors, interaction of cytokines with this signaling pathway plays a role in maintenance of carcinoma. IL-6 will increase self-renewal and hypoxic survival in breast carcinoma cells by regulation of Notch signaling.Citation57 It has also been found that TGFβ1 cause increased bone metastasis by increasing the expression of Notch3 and Hes-1.Citation56 Notch receptors also play critical roles during angiogenesis, so that the signaling regulation function of VEGF and DII4 receptors is prominent in angiogenesis.Citation53,Citation54 In addition to involvement of Notch receptors in metastasis, activation of its signaling pathway by Jagged-1 ligand causes osteolytic bone metastasis in breast cancer via regulation of TGFβ-SMAD signaling. As a result, Jagged-1 is known as an important clinical factor in breast cancer metastasis to bone.Citation58
In completion of the above findings, it can be concluded that manipulation of Notch signaling pathway would be a supplementary and complementary therapeutic approach. This pathway will be promising for researchers in further studies on the role of this signaling pathway in cancer treatment because of targeting cancer metastasis.Citation5
Notch in leukemic niche
In addition to the role of Notch in controlling cell function in normal BM niche, aberrant expression of this signaling pathway can result in various neoplasias including hematological malignancies.Citation3,Citation59 In hematological malignancies, the role of Notch in disease progress includes apoptosis inhibition and induction of unchecked cancer cell proliferation. Moreover, many studies and clinical evidences have indicated tumor suppressor and/or oncogenic role of Notch signaling pathway in solid and hematological malignancies.Citation5 Besides hematological malignancies, alteration of this signaling pathway has been reported in human breast tumors, melanoma progression, and medulloblastoma.Citation59
Disruption of Notch signaling pathway has been found in myeloma.Citation60 The role of Notch during progression and development of multiple myeloma (MM) is induced by aberrant expression of Notch ligands and receptors in various stages of the disease.Citation60,Citation61 Sustained expression of Notch1–3 and Jagged-1–2 ligands by malignant MM cells and expression of Dll and Jagged ligands by BM stromal cells are two distinct mechanisms in the activation of Notch signaling pathway in MM cells.Citation62,Citation63 Cross-talk between Notch ligands including Jagged-2 and BM stroma cells such as MRC5 fibroblasts play significant roles in the activation of Notch in MM cells.Citation63 In this regard, Jagged-2 can activate Notch signaling in MCR5 fibroblasts by increased production of IL-6, VEGF, and insulin-like growth factor-1, which are essential inducers of malignant plasma cells in BM niche.Citation64 Skeletrophin is an ubiquitin ligand aberrantly expressed in MM cells, resulting in dysregulation of Jagged-2 ligand and subsequent activation of Notch in resulting malignant stromal cells. The latter activation causes increased progression of disease by accelerating the growth and number of malignant MM stem cells.Citation62,Citation63 Contact between MM cells and BM stromal cells causes increased clonogenic growth and self-renewal of malignant CD138+ cells in patients following Dll1 expression in BM stromal cells.Citation63 Therefore, Notch plays an important role in the stability of malignant plasma cells in BM niche through dysregulation of its components. It should be expressed both in MM and BM stromal cells to play its role.Citation62,Citation63
Dysregulation of Notch signaling pathway has also been observed in T-cell ALL (T-ALL).Citation65 Mutations are another factor involving Notch signaling pathway in hematological neoplasias. t(7;9) chromosomal translocation causing N1ICD expression in T-cell receptor-β indicates the role of Notch1 in most of the T-ALL patients.Citation3 Notch1 mutations in T-ALL constitute activated alleles causing abnormally high levels of Notch signaling. Exons 26 and 27 are the most frequent mutation hotspots in Notch1 molecule, another one being the second PEST (proline, glutamic acid, serine, and threonine) of Notch1 molecule.Citation65 Notch signaling pathway aberration has also been seen in acute myeloid leukemia, which is associated with Jagged-1 and Notch1 overexpression.Citation5 B-cell chronic lymphocytic leukemia (B-CLL) is among the most common lymphoproliferative disorders. In the B-CLL, dysregulation of Notch signaling pathway is indicated by continuous expression of Notch receptors 1–2 and the related ligands of Jagged-1 and Jagged-2.Citation66,Citation67 shows the relationship between various cancer types and metastatic diseases with dysregulation of components of Notch signaling pathway. As the table shows, the Notch signaling is deregulated during cancers and malignancies. Notch pathway components, including oncogene or tumor suppressor, in these malignancies implicated in the pathogenesis via interaction with the target genes. In addition, interacting miRNA and Notch molecules are also responsible for resistance to therapy. However, better understanding of the components involved in the Notch in various malignancies and using underutilized treatment strategies such as GSI, antibody against the involved Notch components, and inhibiting their expression may be considered as important points during treatment ().
Table 2. Dysregulation of Notch signaling in different cancer and metastasis disease
Notch and miRNA
The miRNAs are small single-stranded RNA molecules regulating gene expression by inhibition of translation or by mRNA degradation.Citation7 Research has demonstrated the relationship between miRNA and Notch signaling pathway during regulation of bone and hematopoietic cell homeostasis as well as in various malignancies. In the process of bone homeostasis, miR-34c regulates osteoclasts by targeting the components of Notch signaling pathway.Citation7,Citation85–Citation90 In this regard, miR-34c inhibits osteoclastogenesis through the inhibition of Jag1 and Notch1–2.Citation7 In addition, miRNA and Notch signaling have been found to play an important role in the expansion of natural killer cells (NK cells). In this regard, miR-181 promotes human NK cell development by enhancing the activity of Notch.Citation85 Besides the stimulatory role of miR-181 in Notch activity, its inhibitory role has been demonstrated during B-cell development.Citation86 Notch is a target molecule of miR-326, which is involved in maintenance of glioma stem cell proliferation and stemness.Citation87 Forced expression of miR-150 adversely affects the proliferation of T-cell lines by reducing Notch3 expression during differentiation of T-cells.Citation88
Due to the involvement of miRNA in apoptosis and cell proliferation during development of human malignancies, miRNAs are deemed to be involved in development of cancer along with aberrant activation of Notch signaling pathway. In medulloblastoma, miR-199b-5p acts as an inhibitory regulator of Notch signaling through inhibition of Hes-1.Citation89 DCAMKL-1 is up-regulated microtubule kinase that plays an important role in pancreatic and colorectal cancers. In his study, Sureban reported that DCAMKL-1 inhibits colorectal cancer by increasing miR-144 through down-regulation of Notch-1 molecule.Citation90 The miR-34a is a different miRNA that inhibits cancer cells through interaction with Notch signaling. MiR-34a in choriocarcinoma cells inhibits translation through inhibition of Notch-1 expression.Citation91 Besides the role of Notch-1 in colorectal cancer, Mei et al. showed involvement of Notch molecule in glioblastoma, but Notch-1 is inhibited by miR-146a in this type of cancer.Citation92 miR-19 is among miRNAs causing leukemogenesis in Notch-1-induced T-ALL.Citation93
miRNA function in differentiation regulation of osteoblasts and osteoclasts is prominent besides its role in Notch signaling pathway.Citation94–Citation96 In particular, miR-637 and miR-23a-27a-24-2 related cluster will inhibit differentiation of osteoblasts.Citation94 Differentiation of osteoblasts is concomitant with reduced expression of miR-206.Citation95 The inhibition of differentiation of osteoclasts has been declared by miRNAs such as miR-146 and miR-29b.Citation96,Citation97 Finally, miRNA molecules and Notch signaling are involved in proliferation, development, and general homeostasis of resident cells in normal BM niche through their interactions. This interaction is impaired in malignant BM niche, i.e. their role in the regulation of neoplastic niche is out of control. It is assumed that changing expression of miR molecules and subsequent Notch signaling deregulation increases the likelihood of progress of malignancies in the BM. Detection of important miRNAs in BM microenvironment requires further studies to compare the miRNAs expressed in normal and neoplastic niches in order to suggest appropriate treatment targets.
shows a diagram of the cross-talk between miRNA molecules and Notch signaling pathway components.
Table 3. Interaction of miRNA and Notch signaling in normal and neoplasia regulation
Therapy and drug resistance of Notch in niche
Aberrant expression or dysregulation of Notch signaling pathway components has been reported in a variety of malignancies. Therefore, this important cell signaling pathway has been increasingly considered in the treatment of various cancer types and metastasis.Citation73 Inhibiting Notch receptors and ligands by different therapeutic strategies such as GSIs, monoclonal antibodies, glucocorticoids, and natural agents has been discussed in the treatment of malignancies.Citation73 Since GS plays an important role in Notch signaling, it can be considered as a therapeutic target.Citation98 In this regard, GSIs reduce tumor growth and induce apoptosis in several human cancers as anti-tumor agents. However, non-specificity of GSIs and cytotoxicity in some tissues, particularly the gastrointestinal tract are factors restricting the use of these therapeutic agents.Citation73 Development of antibodies that selectively inhibit Notch receptors and gut toxicity may be another therapeutic approach. However, the effects of antibodies in treatment, like that of T-ALL, have not been reported to be as strong as GSIs.Citation71 The use of GSIs alone showed limited efficacy in the treatment of leukemic diseases like T-ALL. In this regard, compensatory outcomes have been achieved in combined treatments. Combined treatment of GSI with other inhibitors such as cyclin-dependent kinase inhibitors and inhibitors of PI3K-AKT-mTOR pathway and glucocorticoids has resulted in better anti-leukemic effects.Citation65 Natural agents are compounds in fruits and vegetables as well as flavoring agent in foods, which play an important role in malignancies such as pancreatic cancer. They inhibit growth and apoptosis induction by inactivating Notch pathway.Citation73
Besides therapeutic roles of Notch, laboratory evidences suggest it as an anti-cancer drug-resistant entity. CSCs and epithelial–mesenchymal transition (EMT) phenotype are drug-resistant factors. Elimination of CSCs and EMT-type cells by Notch will overcome the drug resistance of cancer cells.Citation72 Cross-talk between Notch and miRNA is also effective in overcoming the drug resistance phenotype of cancer cells. miR-34, miR-1, miR-21, and miR-200 are miRNAs involved in reducing drug resistance of cancer cells by regulating Notch signaling.Citation72
Discussion
Notch signaling pathway is a signaling pathway involved in cellular processes such as proliferation, differentiation, and apoptosis. The relationship between Notch signaling pathway with miRNA and stem cells has also been reported in many studies. In addition to regulating cellular functions, aberrant expression of this signaling pathway has been observed in a number of cancers and metastatic diseases. Aberrant expression of miRNA molecules and disruption of Notch signaling pathway are important factors contributing to malignancy. The genes involved in Notch signaling pathway are the targets for miRNAs implicated in malignancy. For example, three miRNA molecules associated with Notch signaling have been mentioned to be down-regulated in glioma, including miR-128, miR-218, and miR-26a.Citation99 Uncontrolled expression of DLL4 in endothelial cells will lead to excessive sprouting. Given the role of miR-30 in angiogenesis, it can be assumed that DLL4 enables the critical role of angiogenesis in cancer development as a target for miR-30.Citation100 Therefore, this important cell signaling pathway has been increasingly considered in the treatment of a variety of cancer types and metastasis. In conclusion, future studies on a variety of cancers and apoptosis can aim at Notch signaling pathway as a safe and effective therapeutic target.
Disclaimer statements
Contributors NS, KJ, and FR conceived the manuscript and revised it; NS, SA, MeShB, MaShB, and MS wrote the manuscript. SA, SS, MG, and FS prepares the figure and tables.
Funding None.
Conflicts of interest None.
Ethics approval None.
Acknowledgements
We wish to thank all our colleagues in Shafa Hospital and Allied Health Sciences School, Ahvaz Jundishapur University of Medical Sciences.
References
- Saki N, Abroun S, Farshdousti Hagh M, Asgharei F. Neoplastic bone marrow niche: hematopoietic and mesenchymal stem cells. Cell J. 2011;13(3):131–6.
- Shiozawa Y, Pienta KJ, Taichman RS. Hematopoietic stem cell niche is a potential therapeutic target for bone metastatic tumors. Clin Cancer Res. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. 2011;17(17):5553–8.
- Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. [Research Support, Non-U.S. Gov't Review]. 2007;28(3):339–63.
- Weber JM, Calvi LM. Notch signaling and the bone marrow hematopoietic stem cell niche. Bone [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review]. 2010;46(2):281–5.
- Hu YY, Zheng MH, Zhang R, Liang YM, Han H. Notch signaling pathway and cancer metastasis. Adv Exp Med Biol. [Research Support, Non-U.S. Gov't Review]. 2012;727:186–98.
- Maillard I, Koch U, Dumortier A, Shestova O, Xu L, Sai H, et al. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. 2008;2(4):356–66.
- Bae Y, Yang T, Zeng HC, Campeau PM, Chen Y, Bertin T, et al. miRNA-34c regulates Notch signaling during bone development. Hum Mol Genet. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. 2012;21(13):2991–3000.
- Wang Z, Li Y, Banerjee S, Sarkar FH. Exploitation of the Notch signaling pathway as a novel target for cancer therapy. Anticancer Res. [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, Non-P.H.S. Review]. 2008;28(6A):3621–30.
- Perdigoto CN, Bardin AJ. Sending the right signal: Notch and stem cells. Biochim Biophys Acta [Research Support, Non-U.S. Gov't Review]. 2013;1830(2):2307–22.
- Yeung TM, Chia LA, Kosinski CM, Kuo CJ. Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell Mol Life Sci. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review]. 2011;68(15):2513–23.
- Canalis E. Notch signaling in osteoblasts. Sci Signal. [Review]. 2008;1(17):e17.
- Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, et al. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci USA [Research Support, N.I.H., Extramural]. 2008;105(37):13906–11.
- Regan J, Long F. Notch signaling and bone remodeling. Curr Osteoporos Rep. [Research Support, N.I.H., Extramural]. 2013;11(2):126–9.
- Canalis E, Parker K, Feng JQ, Zanotti S. Osteoblast lineage-specific effects of notch activation in the skeleton. Endocrinology [Research Support, N.I.H., Extramural]. 2013;154(2):623–34.
- Bai S, Kopan R, Zou W, Hilton MJ, Ong CT, Long F, et al. NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem. [Research Support, N.I.H., Extramural]. 2008;283(10):6509–18.
- Yamada T, Yamazaki H, Yamane T, Yoshino M, Okuyama H, Tsuneto M, et al. Regulation of osteoclast development by Notch signaling directed to osteoclast precursors and through stromal cells. Blood [Research Support, Non-U.S. Gov't]. 2003;101(6):2227–34.
- Bigas A, Espinosa L. Hematopoietic stem cells: to be or Notch to be. Blood [Research Support, Non-U.S. Gov't Review]. 2012;119(14):3226–35.
- Hayashi N, Takahashi K, Abe Y, Kashiwakura I. Placental/umbilical cord blood-derived mesenchymal stem cell-like stromal cells support hematopoietic recovery of X-irradiated human CD34+ cells. Life Sci. [Research Support, Non-U.S. Gov't]. 2009;84(17–18):598–605.
- Wilson A, Radtke F. Multiple functions of Notch signaling in self-renewing organs and cancer. FEBS Lett. [Review]. 2006;580(12):2860–8.
- Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. 2010;6(3):251–64.
- Chotinantakul K, Leeanansaksiri W. Hematopoietic stem cell development, niches, and signaling pathways. Bone Marrow Res. 2012;2012:270425.
- Jacobsen SE. Defining ‘stemness’: Notch and Wnt join forces?. Nat Immunol. [Comment News]. 2005;6(3):234–6.
- Docheva D, Haasters F, Schieker M. Mesenchymal stem cells and their cell surface receptors. Curr Rheumatol Rev. 2008;4(3):155–60.
- Wang Y, Tu W, Lou Y, Xie A, Lai X, Guo F, et al. Mesenchymal stem cells regulate the proliferation and differentiation of neural stem cells through Notch signaling. Cell Biol Int. [Research Support, Non-U.S. Gov't]. 2009;33(11):1173–9.
- Xu N, Liu H, Qu F, Fan J, Mao K, Yin Y, et al. Hypoxia inhibits the differentiation of mesenchymal stem cells into osteoblasts by activation of Notch signaling. Exp Mol Pathol. [Research Support, Non-U.S. Gov't]. 2013;94(1):33–9.
- Xie J, Wang W, Si JW, Miao XY, Li JC, Wang YC, et al. Notch signaling regulates CXCR4 expression and the migration of mesenchymal stem cells. Cell Immunol. [Research Support, Non-U.S. Gov't]. 2013;281(1):68–75.
- Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. [Research Support, Non-U.S. Gov't Review]. 2011;208(3):421–8.
- Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Review]. 2013;14(6):329–40.
- Moore N, Lyle S. Quiescent, slow-cycling stem cell populations in cancer: a review of the evidence and discussion of significance. J Oncol. 2011;2011. doi: 10.1155/2011/396076.
- Bjornson CR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, Non-P.H.S.]. 2012;30(2):232–42.
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. 2006;124(2):407–21.
- Mason HA, Rakowiecki SM, Gridley T, Fishell G. Loss of notch activity in the developing central nervous system leads to increased cell death. Dev Neurosci. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. 2006;28(1–2):49–57.
- Gu JW, Rizzo P, Pannuti A, Golde T, Osborne B, Miele L. Notch signals in the endothelium and cancer ‘stem-like’ cells: opportunities for cancer therapy. Vasc Cell 2012;4:7.
- Joussen AM, Doehmen S, Le ML, Koizumi K, Radetzky S, Krohne TU, et al. TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol Vis. [Research Support, Non-U.S. Gov't]. 2009;15:1418–28.
- Chiou SK, Jones MK, Tarnawski AS. Survivin – an anti-apoptosis protein: its biological roles and implications for cancer and beyond. Med Sci Monit. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Review]. 2003;9(4):PI25–9.
- Chadwick N, Nostro MC, Baron M, Mottram R, Brady G, Buckle AM. Notch signaling induces apoptosis in primary human CD34+ hematopoietic progenitor cells. Stem Cells [Research Support, Non-U.S. Gov't]. 2007;25(1):203–10.
- Wang Z, Zhang Y, Li Y, Banerjee S, Liao J, Sarkar FH. Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol Cancer Ther. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. 2006;5(3):483–93.
- Song G, Zhang Y, Wang L. MicroRNA-206 targets notch3, activates apoptosis, and inhibits tumor cell migration and focus formation. J Biol Chem. [Research Support, N.I.H., Extramural]. 2009;284(46):31921–7.
- Azizidoost S, Babashah S, Rahim F, Shahjahani M, Saki N. Bone marrow neoplastic niche in leukemia. Hematology. 2013;19(4):232–8
- Doan P, Chute J. The vascular niche: home for normal and malignant hematopoietic stem cells. Leukemia. 2012;26(1):54–62.
- Anand S. A brief primer on microRNAs and their roles in angiogenesis. Vasc Cell. 2013;5(1):2.
- Stoelting S, Heinze G, Nadrowitz R, Wagner T, Peters SO. Bone marrow-derived endothelial cells contribute to angiogenesis in murine WEHI and JC tumors. Anticancer Res. [Evaluation Studies]. 2008;28(2A):771–7.
- Kwon S-M, Eguchi M, Wada M, Iwami Y, Hozumi K, Iwaguro H, et al. Specific Jagged-1 signal from bone marrow microenvironment is required for endothelial progenitor cell development for neovascularization. Circulation. 2008;118(2):157–65.
- Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood. 2000;95(5):1671–9.
- Kwon S-M, Alev C, Asahara T. The role of notch signaling in endothelial progenitor cell biology. Trends Cardiovasc Med. 2009;19(5):170–3.
- Ferrari N, Glod J, Lee J, Kobiler D, Fine HA. Bone marrow-derived, endothelial progenitor-like cells as angiogenesis-selective gene-targeting vectors. Gene Ther. 2003;10(8):647–56.
- Irmisch A, Huelsken J. Metastasis: New insights into organ-specific extravasation and metastatic niches. Exp Cell Res. 2013;319(11):1604–10.
- Descot A, Oskarsson T. The molecular composition of the metastatic niche. Exp Cell Res. 2013;319(11):1679–86.
- Tao J, Erez A, Lee B. One NOTCH further: Jagged 1 in bone metastasis. Cancer Cell [Comment]. 2011;19(2):159–61.
- Oskarsson T, Massague J. Extracellular matrix players in metastatic niches. Embo J. 2012;31(2):254–6.
- Patel LR, Camacho DF, Shiozawa Y, Pienta KJ, Taichman RS. Mechanisms of cancer cell metastasis to the bone: a multistep process. Future Oncol. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Review]. 2011;7(11):1285–97.
- Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Review]. 2006;107(6):2223–33.
- Kofler NM, Shawber CJ, Kangsamaksin T, Reed HO, Galatioto J, Kitajewski J. Notch signaling in developmental and tumor angiogenesis. Genes Cancer. 2011;2(12):1106–16.
- Garcia A, Kandel JJ. Notch: a key regulator of tumor angiogenesis and metastasis. Histol Histopathol. [Research Support, U.S. Gov't, P.H.S. Review]. 2012;27(2):151–6.
- Tsuyada A, Chow A, Wu J, Somlo G, Chu P, Loera S, et al. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. 2012;72(11):2768–79.
- Zhang Z, Wang H, Ikeda S, Fahey F, Bielenberg D, Smits P, et al. Notch3 in human breast cancer cell lines regulates osteoblast-cancer cell interactions and osteolytic bone metastasis. Am J Pathol. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.]. 2010;177(3):1459–69.
- Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. [Research Support, Non-U.S. Gov't]. 2007;117(12):3988–4002.
- Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.]. 2011;19(2):192–205.
- Dreesen O, Brivanlou AH. Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review]. 2007;3(1):7–17.
- Abroun S, Saki N, Fakher R, Asghari F. Biology and bioinformatics of myeloma cell. Lab Hematol. [Review]. 2012;18(4):30–41.
- Basak GW, Srivastava AS, Malhotra R, Carrier E. Multiple myeloma bone marrow niche. Curr Pharm Biotechnol. [Review]. 2009;10(3):345–6.
- Mirandola L, Apicella L, Colombo M, Yu Y, Berta DG, Platonova N, et al. Anti-Notch treatment prevents multiple myeloma cells localization to the bone marrow via the chemokine system CXCR4/SDF-1. Leukemia. 2013;27(7):1558–66.
- Colombo M, Mirandola L, Platonova N, Apicella L, Basile A, Figueroa AJ, et al. Notch-directed microenvironment reprogramming in myeloma: a single path to multiple outcomes. Leukemia [Research Support, Non-U.S. Gov't Review]. 2013;27(5):1009–18.
- Kim SY, Min HJ, Park HK, Oh B, Kim TY, She CJ, et al. Increased copy number of the interleukin-6 receptor gene is associated with adverse survival in multiple myeloma patients treated with autologous stem cell transplantation. Biol Blood Marrow Transplant. [Research Support, Non-U.S. Gov't]. 2011;17(6):810–20.
- Tzoneva G, Ferrando AA. Recent advances on NOTCH signaling in T-ALL. Curr Top Microbiol Immunol. [Review]. 2012;360:163–82.
- Seke Etet PF, Vecchio L, Nwabo Kamdje AH. Interactions between bone marrow stromal microenvironment and B-chronic lymphocytic leukemia cells: any role for Notch, Wnt and Hh signaling pathways? Cell Signal. [Review]. 2012;24(7):1433–43.
- Galluzzo P, Bocchetta M. Notch signaling in lung cancer. Expert Rev Anticancer Ther. [Research Support, N.I.H., Extramural Review]. 2011;11(4):533–40.
- van den Brandt J, Kwon SH, McPherson KG, Petrovic S, Zettl A, Muller-Hermelink HK, et al. Unexpected features of acute T lymphoblastic lymphomas in Notch1IC transgenic rats. Eur J Immunol. [Research Support, Non-U.S. Gov't]. 2006;36(8):2223–34.
- Aster JC, Blacklow SC, Pear WS. Notch signalling in T-cell lymphoblastic leukaemia/lymphoma and other haematological malignancies. J Pathol. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review]. 2011;223(2):262–73.
- Jundt F, Probsting KS, Anagnostopoulos I, Muehlinghaus G, Chatterjee M, Mathas S, et al. Jagged1-induced Notch signaling drives proliferation of multiple myeloma cells. Blood [Research Support, Non-U.S. Gov't]. 2004;103(9):3511–5.
- Yin L, Velazquez OC, Liu ZJ. Notch signaling: emerging molecular targets for cancer therapy. Biochem Pharmacol. [Research Support, Non-U.S. Gov't Review]. 2010;80(5):690–701.
- Kawamata S, Du C, Li K, Lavau C. Overexpression of the Notch target genes Hes in vivo induces lymphoid and myeloid alterations. Oncogene. 2002;21(24):3855–63.
- Kannan S, Sutphin RM, Hall MG, Golfman LS, Fang W, Nolo RM, et al. Notch activation inhibits AML growth and survival: a potential therapeutic approach. J Exp Med. [Research Support, Non-U.S. Gov't]. 2013;210(2):321–37.
- Gan ZH, Chen Y. [Notch signaling pathway and multiple myeloma]. Zhongguo Shi Yan Xue Ye Xue Za Zhi [Review]. 2009;17(5):1380–3.
- Wang Z, Li Y, Ahmad A, Azmi AS, Banerjee S, Kong D, et al. Targeting Notch signaling pathway to overcome drug resistance for cancer therapy. Biochim Biophys Acta [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, Non-P.H.S. Review]. 2010;1806(2):258–67.
- Wang Z, Ahmad A, Li Y, Azmi AS, Miele L, Sarkar FH. Targeting notch to eradicate pancreatic cancer stem cells for cancer therapy. Anticancer Res. [Research Support, N.I.H., Extramural Review]. 2011;31(4):1105–13.
- Ristorcelli E, Lombardo D. Targeting Notch signaling in pancreatic cancer. Expert Opin Ther Targets [Research Support, Non-U.S. Gov't Review]. 2010;14(5):541–52.
- Ma J, Dong C, Ji C. MicroRNA and drug resistance. Cancer Gene Ther. [Review]. 2010;17(8):523–31.
- Mittal S, Subramanyam D, Dey D, Kumar RV, Rangarajan A. Cooperation of Notch and Ras/MAPK signaling pathways in human breast carcinogenesis. Mol Cancer [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.]. 2009;8:128.
- Al-Hussaini H, Subramanyam D, Reedijk M, Sridhar SS. Notch signaling pathway as a therapeutic target in breast cancer. Mol Cancer Ther. [Review]. 2011;10(1):9–15.
- Santagata S, Demichelis F, Riva A, Varambally S, Hofer MD, Kutok JL, et al. JAGGED1 expression is associated with prostate cancer metastasis and recurrence. Cancer Res. 2004;64(19):6854–7.
- Scorey N, Fraser SP, Patel P, Pridgeon C, Dallman MJ, Djamgoz MB. Notch signalling and voltage-gated Na+ channel activity in human prostate cancer cells: independent modulation of in vitro motility. Prostate Cancer Prostatic Dis. [Research Support, Non-U.S. Gov't]. 2006;9(4):399–406.
- Wang Z, Li Y, Banerjee S, Kong D, Ahmad A, Nogueira V, et al. Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-kappaB signaling pathways. J Cell Biochem. [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, Non-P.H.S.]. 2010;109(4):726–36.
- Lino MM, Merlo A, Boulay JL. Notch signaling in glioblastoma: a developmental drug target? BMC Med. [Research Support, Non-U.S. Gov't Review]. 2010;8:72.
- Cichocki F, Felices M, McCullar V, Presnell SR, Al-Attar A, Lutz CT, et al. Cutting edge: microRNA-181 promotes human NK cell development by regulating Notch signaling. J Immunol. [Research Support, N.I.H., Extramural]. 2011;187(12):6171–5.
- Allman D, Aster JC, Pear WS. Notch signaling in hematopoiesis and early lymphocyte development. Immunol Rev. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. Review]. 2002;187:75–86.
- Singh SK, Vartanian A, Burrell K, Zadeh G. A microRNA link to glioblastoma heterogeneity. Cancers 2012;4(3):846–72.
- Ghisi M, Corradin A, Basso K, Frasson C, Serafin V, Mukherjee S, et al. Modulation of microRNA expression in human T-cell development: targeting of NOTCH3 by miR-150. Blood [Research Support, Non-U.S. Gov't]. 2011;117(26):7053–62.
- Wang Z, Li Y, Kong D, Ahmad A, Banerjee S, Sarkar FH. Cross-talk between miRNA and Notch signaling pathways in tumor development and progression. Cancer Lett. 2010;292(2):141–8.
- Sureban SM, May R, Mondalek FG, Qu D, Ponnurangam S, Pantazis P, et al. Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144 and inhibits colorectal cancer tumor growth via a Notch-1 dependent mechanism. J Nanobiotechnol. 2011;9(1):40.
- Pang RT, Leung CO, Lee C-L, Lam KK, Ye T-M, Chiu PC, et al. MicroRNA-34a is a tumor suppressor in choriocarcinoma via regulation of Delta-like1. BMC Cancer. 2013;13(1):25.
- Mei J, Bachoo R, Zhang CL. MicroRNA-146a inhibits glioma development by targeting Notch1. Mol Cell Biol. 2011;31(17):3584–92.
- Coskun E, Neumann M, Schlee C, Liebertz F, Heesch S, Goekbuget N, et al. MicroRNA profiling reveals aberrant microRNA expression in adult ETP-ALL and functional studies implicate a role for miR-222 in acute leukemia. Leuk Res. 2013;37(6):647–56.
- Lian JB, Stein GS, van Wijnen AJ, Stein JL, Hassan MQ, Gaur T, et al. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. [Research Support, N.I.H., Extramural Review]. 2012;8(4):212–27.
- Inose H, Ochi H, Kimura A, Fujita K, Xu R, Sato S, et al. A microRNA regulatory mechanism of osteoblast differentiation. Proc Natl Acad Sci USA [Research Support, Non-U.S. Gov't]. 2009;106(49):20794–9.
- Nakasa T, Shibuya H, Nagata Y, Niimoto T, Ochi M. The inhibitory effect of microRNA-146a expression on bone destruction in collagen-induced arthritis. Arthritis Rheum. [Research Support, Non-U.S. Gov't]. 2011;63(6):1582–90.
- Rossi M, Pitari MR, Amodio N, Di Martino MT, Conforti F, Leone E, et al. miR-29b negatively regulates human osteoclastic cell differentiation and function: implications for the treatment of multiple myeloma-related bone disease. J Cell Physiol. [Research Support, Non-U.S. Gov't]. 2013;228(7):1506–15.
- Tammam J, Ware C, Efferson C, O'Neil J, Rao S, Qu X, et al. Down-regulation of the Notch pathway mediated by a gamma-secretase inhibitor induces anti-tumour effects in mouse models of T-cell leukaemia. Br J Pharmacol. 2009;158(5):1183–95.
- Liu F, Xiong Y, Zhao Y, Tao L, Zhang Z, Zhang H, et al. Identification of aberrant microRNA expression pattern in pediatric gliomas by microarray. Diagnostic Pathol. 2013;8(1):158.
- Bridge G, Monteiro R, Henderson S, Emuss V, Lagos D, Georgopoulou D, et al. The microRNA-30 family targets DLL4 to modulate endothelial cell behavior during angiogenesis. Blood. 2012;120(25):5063–72.
