Abstract
Botulinum toxin type A is a high molecular weight protein complex containing active neurotoxin and complexing proteins, the latter of which, it is believed, protect the neurotoxin when in the gastrointestinal tract, and may facilitate its absorption. Comparisons of conventional botulinum toxin type A drugs that include complexing proteins with the complexing protein-free formulation of Xeomin® strongly suggest that complexing proteins do not affect diffusion of the active neurotoxin. Studies of Xeomin have also shown that complexing proteins do not enhance product stability in storage. However, complexing proteins may stimulate antibody development against botulinum toxin type A. Numerous observational studies have been published showing that some patients receiving conventional botulinum toxin may develop neutralizing antibodies, leading to antibody-induced therapy failure. Studies have shown that Xeomin is not associated with the development of neutralizing antibodies in animal models or in patients. In conclusion, complexing proteins do not contribute to the stability of botulinum toxin type A drugs and do not contribute to their therapeutic effects, but may be associated with a secondary nonresponse due to the development of neutralizing antibodies.
Introduction
Commercial pharmaceutical preparations of botulinum toxin type A are an effective treatment for a large number of disorders resulting from increased muscle tone, such as cervical dystonia, hemifacial spasm, and blepharospasm.Citation1 In addition, botulinum toxin type A is effective for the treatment of axillary and palmar hyperhidrosisCitation2 and urologic disorders,Citation3 and is associated with a high level of patient satisfaction when used in facial esthetic procedures.Citation4
The active botulinum toxin (150 kDa) occurs naturally as part of a high molecular weight complex containing the neurotoxin moiety and a set of complexing proteins of clostridial origin.Citation5 The proteins are also called neurotoxin-associated proteins.Citation6 Conventional botulinum toxin type A drugs, including Botox® (Vistabel®, onabotulinumtoxin A; Allergan Inc, Irvine, CA) and Dysport® (Azzalure®, abobotulinumtoxinA; Ipsen Ltd, Berkshire, UK), contain these complexing proteins in addition to the neurotoxin.Citation7 More recently, a botulinum toxin type A-containing pharmaceutical preparation free from complexing proteins has been developed called Xeomin® (also known as incobotulinumtoxinA, NT 201, botulinum toxin type A [150 kDa], free from complexing proteins, Bocouture®; Merz Pharmaceuticals GmbH, Frankfurt, Germany). For the following discussion, the products will be referred to as Botox, Dysport, and Xeomin, regardless of their clinical indication. This new preparation is derived from a wild-type strain of Clostridium botulinum type A (ATCC 3502), and it contains only the active botulinum toxin type A with no complexing proteinsCitation7 and has similar biologic activity to Botox.Citation8 In comparative clinical trials, the efficacy and tolerability of Xeomin were noninferior to that of conventional botulinum toxin type A drugs.Citation9,Citation10 For example, the efficacy of Xeomin was compared with that of Botox in a 16-week randomized, double-blind, noninferiority trial in 463 patients with cervical dystonia.Citation9 Both treatments significantly improved the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) severity score compared with baseline, and noninferiority of Xeomin versus Botox was demonstrated.Citation9 The TWSTRS represents the standard method to quantify cervical dystonia and is a sum of subscores for the rating of maximal excursion of the head, duration, and other symptoms. No clinically relevant differences between the two treatments were observed for any secondary efficacy variable or with regard to adverse events. Similarly, a randomized, double-blind study of Xeomin and Botox in 300 patients with blepharospasm found that both treatments significantly reduced the Jankovic Rating Scale (JRS) score from baseline, displaying noninferiority of Xeomin.Citation10 The JRS is a standard rating scale which allows the quantification of typical symptoms of blepharospasm. Again, there were no significant differences between Xeomin and Botox on any efficacy or safety measure.
The purposes and effects of complexing proteins in botulinum toxin type A preparations are unclear, although suggested purposes include stabilization of the neurotoxin and enhancement of gastrointestinal uptake, and suggested effects include delay of botulinum toxin diffusion into adjacent tissuesCitation11 and formation of neutralizing antibodies against botulinum toxin type A, leading to subsequent treatment failure.Citation6,Citation12 This article reviews the literature and presents the latest thoughts on the role of complexing proteins in botulinum toxin type A preparations.
Structure and role of complexing proteins
In all naturally occurring serotypes of botulinum toxin (types A–G), the neurotoxin is noncovalently associated with complexing proteins and thus forms toxin complexes.Citation13,Citation14 Complexing proteins are encoded in two gene clusters located close to each other on the C. botulinum chromosome.Citation15,Citation16 The first cluster encodes botulinum toxin itself plus a nontoxic, nonhemagglutinin (NTNHA) protein, while the second encodes three hemagglutinin (HA) proteins (HA1, HA2, and HA3), with HA3 being cleaved in serotype A post-translationally into two smaller components (HA3a and 3b). In botulinum toxin serotypes A–D and G, these components form two different toxin complexes, ie, a medium toxin complex comprising botulinum toxin and NTNHA (300 kDa) and a large toxin complex that also includes the three HA molecules (500–600 kDa).Citation5 In contrast, serotypes E and F produce only the medium toxin complex.Citation13 Serotype A also forms a third complex with a higher molecular weight (900 kDa).Citation5 The detailed molecular structure of botulinum toxin type D large toxin complex has been visualized and comprises a 14-subunit complex of neurotoxin, NTNHA, three HA3 molecules (a 70 kDa molecule, also known as HA-70), three HA2 (also known as HA-17), and six HA1 (also known as HA-33, ).Citation13 A denaturing capillary electrophoresis method was used to determine the subunits forming the very large/or higher molecular weight toxin complex of botulinum toxin type A, concluding that it contains single copies of the 150 kDa neurotoxin and NTNHA subunits, as well as 5–6 HA-17, 4–5 HA-23, 3–4 HA-48, and 8–9 HA-34 subunits, with a total mass of 880–1000 kDa.Citation17
Figure 1 Arrangement of components in botulinum toxin type D complex. Botulinum toxin is highlighted in red, the nontoxic, nonhemagglutinin protein in green, three HA-70 in yellow, six HA-33 in blue, and three HA-17 in cyan. The catalytic zinc ion in botulinum toxin is indicated by the orange circle and the arrow. Copyright© 2009 The American Society for Biochemistry and Molecular Biology. All rights reserved. Reproduced with permission from Hasegawa K, Watanabe T, Suzuki T, et al. A novel subunit structure of Clostridium botulinum serotype D toxin complex with three extended arms. J Biol Chem. 2007;282:24777–24783.Citation13
In nature, complexing proteins appear to have a number of functions. Based on differences in oral toxicity between toxin complexes of different sizes, it was initially suggested that the function of complexing proteins was the protection of the botulinum toxin moiety when in the gastrointestinal tract.Citation18 This was subsequently confirmed in biochemical analyses (protease resistance) of different toxin species.Citation19 It has also been suggested that complexing proteins may stabilize the biologic activity of the neurotoxin in vivo and facilitate adherence to muscle tissue.Citation20 In addition, complexing proteins may have a role in limiting the diffusion of botulinum toxin out of the target tissues, due to the large size of the toxin complex.Citation11,Citation21,Citation22 However, it was found that there was no difference in the diffusion of the free or complexed form after injection into the muscle.Citation23 Orally ingested botulinum toxin must cross the epithelium of the gastrointestinal tract before it can affect muscle, and a role for complexing proteins in the uptake and transcytosis of botulinum toxin through the intestinal epithelium has been suggested.Citation14,Citation24 In the therapeutic setting, where botulinum toxin type A is not delivered orally, the roles of complexing proteins in protection from gastric pH extremes, resistance against stomach and intestinal proteases, and transport across the intestinal epithelium are not relevant to clinical efficacy. However, it should be noted that the lack of complexing proteins in Xeomin leads to a marked decrease in oral bioavailability and toxicity.Citation25
While it is conceivable that complexing proteins are involved in botulinum toxin stability and in limiting botulinum toxin diffusion from the injection site (thereby minimizing adverse events), comparison of the complexing protein-free drug Xeomin with conventional botulinum toxin type A drugs suggests that this is not the case.Citation26–Citation28 To evaluate the role of complexing proteins, the stability of the 900 kDa botulinum toxin type A was analyzed using ion-exchange chromatography under various physiologic pH conditions.Citation26,Citation27 The 900 kDa toxin complex eluted as a single fraction at pH 6.0, but increasingly dissociated into several fractions as the pH value of the eluent increased.Citation27 At physiologic pH values, the active 150 kDa neurotoxin is efficiently released in less than one minute from the 900 kDa complex by a shift of the complex association-dissociation equilibrium to unbound 150 kDa neurotoxin.Citation29 This is in contrast with the time to onset of therapeutic effect, which is measured in days. Therefore, it is unlikely that complexing proteins are essential for the stability of the 900 kDa toxin complex at physiologic pH or for limiting diffusion of the 150 kDa botulinum toxin type A after injection of conventional botulinum toxin type A drugs. The rapid dissociation of neurotoxin from the toxin complexes under physiologic conditions may also explain the similar adverse effect profile of Xeomin and conventional botulinum toxin type A drugs that contain complexing proteins.Citation9,Citation10,Citation30 Although the injected product is different, the rapid dissociation would lead to the generation of the same active agent, ie, the 150 kDa botulinum toxin type A, eliciting the same diffusion characteristics and therapeutic effects. Indeed, earlier in vivo studies using different botulinum toxin type A drugs and a preparation of the free botulinum toxin type A (150 kDa) have shown that diffusion from the injection site does not differ between preparations.Citation31 Similarly, studies using radiolabeled botulinum toxin type A have shown that there was no difference in the distribution of the free botulinum toxin type A or complexed botulinum toxin type A at or outside the injection site, even when using high doses.Citation23 In the past, some confusion has occurred when comparing diffusion characteristics between different botulinum toxin type A drugs in the clinical setting.Citation22 For example, a review comparing the area of anhidrotic effect between Botox and Dysport concluded that the diffusion of the two drugs differed as a result of numerous factors, including molecular size, dosing, and injection technique.Citation21 However, the comparison failed to take into account the different potencies of the two drugs, which resulted in different anhidrotic areas.Citation22,Citation28 When botulinum toxin type A efficacy was evaluated by anhidrotic area across a range of Botox:Dysport dose ratios (1:2.5, 1:3, 1:4)Citation32 and electromyography using a 1:3 Botox:Dysport dose ratio,Citation33 the different drugs were found to produce similar results. In contrast, one report demonstrated that Dysport had a greater area of diffusion in the forehead than Botox.Citation34
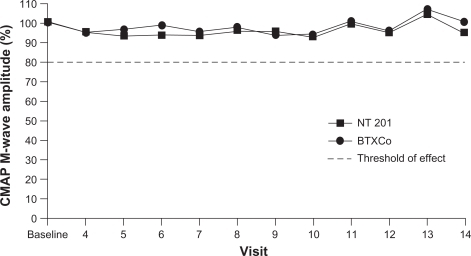
Further evidence for a lack of effect of complexing proteins on botulinum toxin type A diffusion comes from clinical studies in which compound muscle action potential (CMAP) M-wave amplitudes were measured in patients receiving Xeomin or Botox. In a Phase I study, 14 healthy male volunteers received repeated injections of Xeomin or Botox into the extensor digitorum brevis muscle of each foot.Citation35 There were no statistically significant differences between the two treatments in terms of degree of paralysis, onset of action, or duration of paralysis (although time to onset of action was slightly earlier with Xeomin). This indicates their similar efficacy and diffusion profiles, neither showing any effect in adjacent muscles. In another Phase I study of similar design, no significant differences between Xeomin and Botox were observed in 32 healthy male volunteers.Citation36 Furthermore, measurements in adjacent muscles found that CMAP M-wave amplitudes were greater than 80% of baseline at all post-injection visits (),Citation36 suggesting no difference in diffusion-induced reduction of muscle activity. The diffusion of different botulinum toxin type A drugs was also investigated in a mouse study using a high-sensitivity test for muscle expression of neural cell adhesion molecule, which does not occur under physiologic conditions.Citation37 Results of the neural cell adhesion molecule assay showed that injection of Botox, Dysport, and Xeomin (1:4:1 ratio) led to limited diffusion of botulinum toxin type A into adjacent muscles, with no significant differences between the formulations.
Figure 2 Mean values of abductor hallucis muscle compound muscle action potential M-wave amplitudes are above threshold of effect after injection of NT 201 (Xeomin®) or BTXCo (Botox®) into the extensor digitorum brevis, indicating no relevant diffusion-induced effect in adjacent muscles.
Copyright© 2007. Adapted with permission from Wolters Kluwer Health. Wohlfarth K, Muller C, Sassin I, et al. Neurophysiological double-blind trial of a botulinum neurotoxin type a free of complexing proteins. Clin Neuropharmacol. 2007;30:86–94.Citation36
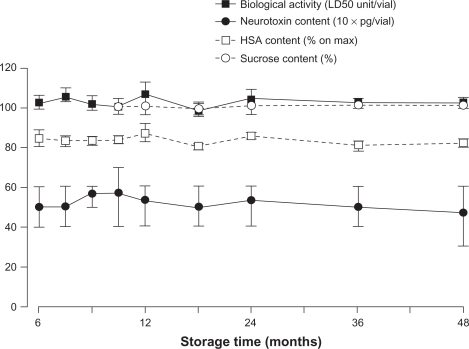
Another postulated effect of complexing proteins in a therapeutic context could be to enhance the stability of the botulinum toxin type A drug during storage. However, studies of Xeomin do not corroborate this proposal.Citation38 The stability of Xeomin was evaluated in long-term storage studies and in short-term temperature-stress studies.Citation38 The studies evaluated the neurotoxin content (via enzyme-linked immunosorbent assay), sucrose content (via enzymatic assay), and human serum albumin content (via high-pressure liquid chromatography) of vials containing Xeomin stored at 5°C or 25°C, as well as the biologic activity of the neurotoxin (mouse median lethal dose [LD50]). After 48 months of storage at room temperature or in a refrigerator, no significant changes in neurotoxin, sucrose, or human serum albumin content or, most importantly, biologic activity, were observed in the Xeomin samples (, data on file, Merz Pharmaceuticals GmbH).Citation38 In contrast, all other licensed botulinum toxin type A drugs containing complexing proteins require refrigerated storage.Citation39,Citation40 Furthermore, storage studies showed that Xeomin was stable for at least 18 months at 30°C and for at least six months at 40°C (data on file, Merz Pharmaceuticals GmbH).Citation38 In short-term temperature-stress studies, all parameters tested remained within the release specifications when Xeomin was stored at 60°C for one month. At 80°C, the expected reduction of biologic activity occurred within five days, although proteolytic activity had not fallen to below one-third of the initial value after 10 days, with a decline over time considerably slower than for biologic activity. Therefore, it appears that the binding and/or translocation domains of the neurotoxin are more sensitive to temperature changes than the light-chain protease (data on file, Merz Pharmaceuticals GmbH).Citation38 Overall, these results demonstrate that complexing proteins are not required to maintain the stability of botulinum toxin type A during storage.
Figure 3 Xeomin® is stable at room temperature (25°C) over 48 months.Citation38
Reproduced with permission from Merz Pharmaceuticals GmbH, Frankfurt, Germany.
Antibody formation and its consequences
While complexing proteins do not appear to have an effect on botulinum toxin type A stability or limit diffusion from the injection site, it is possible that they stimulate antibody production against the active neurotoxin. In a mouse study using formalin treated botulinum toxin type B (toxoid), the amount of neutralizing antibodies produced was greater when the botulinum toxin type B was complexed with a large toxin complex, compared with the toxoid supplemented with hemagglutins, or when the neurotoxoid was administered alone.Citation11 Further analysis showed that HA1 and HA3b were responsible for the adjuvant action, with HA2 producing no increase in antibody production. The mechanism of increased immune response to HA1 and HA3b appeared to be mediated by an increase in interleukin-6, leading to increased numbers of CD19-positive cells. In vitro enzyme-linked immunosorbent assay analysis of antibody binding to botulinum toxin type A large toxin complex showed that HA1 accounted for most of the immunogenic response.Citation12 Although AttassiCitation41 commented that a formalin-treated neurotoxin (toxoid) was used and the experiment does not reflect the therapeutic situation in detail, Lee et al showed in the vaccination experiment that two HAs could enhance the antibody titer against the antigen and act as adjuvants.Citation12
Antibodies against botulinum toxin type A can lead to treatment failure. In patients receiving therapeutic treatment with conventional botulinum toxin type A drugs, case reports have been published describing patients with complete antibody-induced treatment failure using the original Botox formulation (20 U/ng) for hemifacial spasm (cumulative dose, 96 U)Citation42 or cervical dystonia (cumulative dose, 2540 U).Citation43 Similarly, four of 25 patients receiving Botox for urologic disorders developed high botulinum toxin type A antibody titers, which were associated with complete therapy failure in three cases (12% of patients overall), and a further four patients had borderline antibody titers (32% of patients overall).Citation44 In a pooled analysis of data from patients with poststroke upper or lower limb spasticity receiving Botox, neutralizing antibodies were detected in one of 191 patients (0.5%); this patient (cumulative dose, 960 U) showed no clinical response to treatment at any time.Citation45 Of 880 patients treated with botulinum toxin type A for a variety of indications, five (0.6%) were positive for neutralizing antibodies, of whom four with cervical dystonia (cumulative Botox exposure, 1200–3100 U) remained responsive, while the other patient had poststroke spasticity and developed antibodies after a single injection of 200 U Botox, with no response to botulinum toxin type A.Citation46 In a recent study, four of 326 patients with cervical dystonia (1.2%) developed neutralizing antibodies (up to 15 cycles of Botox; median, nine cycles; mean cumulative dose, 1576 U), three of whom were clinically unresponsive.Citation47 Another study found that five of 42 patients (11.9%) developed botulinum toxin type A antibodies following treatment with Botox (mean cumulative dose, 4610 ± 1936 U) and/or Dysport (14,033 ± 7566 U) for spasticity of various etiologies.Citation48 Of these five patients, three had low titers (<0.3 mU/mL), one had an intermediate titer (0.6 mU/mL) and one had a high titer (>1.0 mU/mL) of antibodies.Citation48 Two of the patients with low titers remained clinically responsive to therapy, while the other three patients were unresponsive to Botox and/or Dysport. Thus, the formation of neutralizing antibodies can result in partial or complete clinical unresponsiveness to botulinum toxin type A.Citation49
The presence of complexing proteins in botulinum toxin type A drugs may lead to an increased risk of development of neutralizing antibodies. Indeed, the original formulation of Botox was six times more likely to elicit the production of antibodies than the newer formulation, which contains fewer complexing proteins and reduced inactive neurotoxin.Citation50 When data were analysed from 149 patients with cervical dystonia who had received treatment with Botox, neutralizing antibodies were detected in four of 42 patients (9.5%) who had received only the older formulation (100 U/25 ng neurotoxin), compared with none of the 119 patients who had received only a newer formulation (with a higher specific biologic activity of 100 U/5 ng botulinum toxin type A, P < 0.004).Citation50 Complexing proteins may also be associated with development of non-neutralizing antibodies, with an estimated 40% of patients developing titers of antibodies against C. botulinum HA and NTNH molecules, although they do not impact upon the efficacy of the neurotoxin.Citation51 Clearly, injection with complexed botulinum toxin represents a substantial increase in foreign protein load in addition to the 150 kDa botulinum toxin type A moiety.
Preliminary experiments with Xeomin suggest that the absence of complexing proteins is indeed associated with reduced immunogenicity. In a study in Cynomolgus monkeys, repeated four-weekly injections with 4, 8, or 16 U/kg Xeomin or control were not associated with the development of neutralizing antibodies in any animal, despite clear evidence of biologic activity of the neurotoxin, particularly in the highest dose group.Citation52 However, this study lacked a positive control group and, therefore, confirmation of these data are required to show that complexing proteins result in antibody formation.
The immunogenicity of Xeomin in comparison with that of Botox and Dysport was evaluated in New Zealand white rabbits.Citation53 After repeated intradermal injection, Xeomin did not induce the formation of neutralizing antibodies, unlike Botox and Dysport.Citation53 In this study, both Xeomin and Botox were administered intradermally into female New Zealand white rabbits at 16 U/animal for eight administrations every 2–8 weeks (data on file, Merz Pharmaceuticals GmbH). A final administration of Xeomin 25 U/animal was given 10 weeks after the eighth injection. Conversely, Dysport was given twice weekly at 40 U/kg of animal for five administrations, with a lowered dose of 20 U/kg of animal for the final administration, over a period of 13 weeks (cumulative dose 220 U/kg of animal; data on file, Merz Pharmaceuticals GmbH). Unlike the two conventional botulinum toxin type A drugs, Xeomin was not associated with the development of neutralizing antibodies in this animal model. Indeed, 15 rabbits developed neutralizing antibodies after six injections of Dysport, while four rabbits had neutralizing antibodies following nine injections of Botox (data on file, Merz Pharmaceuticals GmbH). Thus, Xeomin shows low immunogenicity even using doses four to five times higher than the equivalent clinically recommended highest doses for humans in therapeutic indications.Citation52,Citation53 While this suggests that Xeomin presents a lower risk of generating antibodies, it remains to be confirmed whether these results are reflected in different species, such as humans, who may differ in their immune response. However, emerging data in humans do support these results. For example, in a recent study of up to 89 weeks in patients with upper limb spasticity who received one injection of Xeomin or placebo followed by up to five injections of Xeomin, no patient developed neutralizing antibodies throughout the study.Citation54 In the clinical development program of Xeomin in the US, 12 of 1080 subjects developed antibodies against the neurotoxin but each of these patients was previously treated with a botulinum toxin product which contained complexing proteins and so may have already been primed by this treatment.Citation55 For several reasons this outcome cannot be compared with other studies, eg, a different assay was used with a different sensitivity and specificity. Treatment with Xeomin may result in a reduced incidence of antibody-induced therapy failure after long-term treatment compared with conventional botulinum toxin type A drugs but, in order to finally resolve this issue, the results of long-term comparative trials are required.
Conclusion
Complexing proteins do not contribute to the diffusion properties of botulinum toxin products. Furthermore, they are not required to stabilize the neurotoxin in the pharmaceutical formulation. Xeomin does not contain complexing proteins, yet retains safety and efficacy profiles equivalent to conventional botulinum toxin type A formulations. It would therefore appear that complexing proteins do not contribute to the therapeutic effect of botulinum toxin type A treatment. Studies in animals suggest that complexing proteins may increase formation of antibodies against botulinum toxin type A, which can lead to the termination of therapy in some patients, although further studies in human subjects are required to verify this finding.
Disclosures
DD has received consultation fees from Allergan, Ipsen, Merz, and Elan. JF is an employee of Merz Pharmaceuticals. Editorial support for the preparation of this manuscript was provided by Ogilvy 4D; funding was provided by Merz Pharmaceuticals GmbH.
References
- TruongDDresslerDHallettMManual of Botulinum Toxin TherapyCambridge, UKCambridge University Press2009
- BhidayasiriRTruongDDEvidence for effectiveness of botulinum toxin for hyperhidrosisJ Neural Transm200811564164517885725
- Smith complexing protein. Botulinum toxin in the treatment of OAB, BPH and ICToxicon20095463964619268490
- FagienSCarruthersJDA comprehensive review of patient-reported satisfaction with botulinum toxin type A for aesthetic proceduresPlast Reconstr Surg20081221915192519050545
- InoueKFujinagaYWatanabeTMolecular composition of Clostridium botulinum type A progenitor toxinsInfect Immun199664158915948613365
- SharmaSKSinghBRImmunological properties of Hn-33 purified from type A Clostridium botulinumJ Nat Toxins2000935736211126514
- JostWHBlümelJGrafeSBotulinum neurotoxin type A free of complexing proteins (XEOMIN) in focal dystoniaDrugs20076766968317385940
- DresslerDEquivalent potency of Xeomin® and Botox®Mov Disord200823Suppl2021
- BeneckeRJostWHKanovskyPA new botulinum toxin type A free of complexing proteins for treatment of cervical dystoniaNeurology2005641949195115955951
- RoggenkamperPJostWHBihariKEfficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasmJ Neural Transm200611330331215959841
- AokiKRRanouxDWisselJUsing translational medicine to understand clinical differences between botulinum toxin formulationsEur J Neurol200613Suppl 4101917112345
- LeeJCYokotaKArimitsuHProduction of anti-neurotoxin antibody is enhanced by two subcomponents, HA1 and HA3b, of Clostridium botulinum type B 16S toxin-haemagglutininMicrobiology20051513739374716272395
- HasegawaKWatanabeTSuzukiTA novel subunit structure of Clostridium botulinum serotype D toxin complex with three extended armsJ Biol Chem2007282247772478317581814
- FujinagaYTransport of bacterial toxins into target cells: Pathways followed by cholera toxin and botulinum progenitor toxinJ Biochem200614015516016954533
- HauserDEklundMWBoquetPOrganization of the botulinum neurotoxin C1 gene and its associated non-toxic protein genes in Clostridium botulinum C 468Mol Gen Genet19942436316408028579
- MintonNPMolecular genetics of clostridial neurotoxinsCurr Top Microbiol Immunol19951951611948542753
- LietzowMAGielowETLeDSubunit stoichiometry of the Clostridium botulinum type A neurotoxin complex determined using denaturing capillary electrophoresisProtein J20082742042519020965
- OhishiISugiiSSakaguchiGOral toxicities of Clostridium botulinum toxins in response to molecular sizeInfect Immun197716107109326664
- ChenFKuziemkoGMStevensRCBiophysical characterization of the stability of the 150-kilodalton botulinum toxin, the nontoxic component, and the 900-kilodalton botulinum toxin complex speciesInfect Immun199866242024259596697
- JohnsonEABradshawMClostridium botulinum and its neurotoxins: A metabolic and cellular perspectiveToxicon2001391703172211595633
- De AlmeidaATde BoulleKDiffusion characteristics of botulinum neurotoxin products and their clinical significance in cosmetic applicationsJ Cosmet Laser Ther20079Suppl 1172217885882
- PickettADoddSRzanyBConfusion about diffusion and the art of misinterpreting data when comparing different botulinum toxins used in aesthetic applicationsJ Cosmet Laser Ther20081018118318608706
- Tang-LiuDDAokiKRDollyJOIntramuscular injection of 125I-botulinum neurotoxin-complex versus 125I-botulinum-free neurotoxin: Time course of tissue distributionToxicon20034246146914529727
- JinYTakegaharaYSugawaraYDisruption of the epithelial barrier by botulinum haemagglutinin (HA) proteins – differences in cell tropism and the mechanism of action between HA proteins of types A or B and HA proteins of type CMicrobiology2009155354519118344
- BlümelJNT 201 – a new botulinum neurotoxin A. A preparation free of complexing proteins demonstrating the safety and benefit of decreased total clostridial protein burdenJ Parkinsonism Relat Disord200511Suppl 2267
- EiseleKHTaylorHDissociation of the 900 kDa neurotoxin complex from C. Botulinum under physiological conditionsToxicon2008511018045635
- EiseleKHGreinSManderGJRole of complexing proteins in pharmaceutical botulinum neurotoxin formulationsPresented at International Master Course on Aging SkinParis, France2009 Jan 8–11
- PickettADiffusion of type A botulinum toxin in vivo is not related to the size of the toxin complexPresented at International Master Course on Aging SkinParis, France2009 Jan: 8–11
- BrinMFDosing, administration, and a treatment algorithm for use of botulinum toxin A for adult-onset spasticity. Spasticity Study GroupMuscle Nerve Suppl19976S208S2209826992
- DresslerDRoutine use of Xeomin® in patients previously treated with Botox®: Long term resultsEur J Neurol200916Suppl 22520002739
- DoddSLRowellBAVrabasISA comparison of the spread of three formulations of botulinum neurotoxin A as determined by effects on muscle functionEur J Neurol1998518118610210830
- HexselDDal’FornoTHexselCA randomized pilot study comparing the action halos of two commercial preparations of botulinum toxin type ADermatol Surg200834525918053050
- KarsaiSAdrianRHammesSA randomized double-blind study of the effect of Botox and Dysport/Reloxin on forehead wrinkles and electromyographic activityArch Dermatol20071431447144918025375
- Trindade de AlmeidaARMarquesEde AlmeidaJPilot study comparing the diffusion of two formulations of botulinum toxin type A in patients with forehead hyperhidrosisDermatol Surg200733Suppl3743
- JostWHKohlABrinkmannSEfficacy and tolerability of a botulinum toxin type A free of complexing proteins (NT 201) compared with commercially available botulinum toxin type A (Botox®) in healthy volunteersJ Neural Transm200511290591315526142
- WohlfarthKMullerCSassinINeurophysiological double-blind trial of a botulinum neurotoxin type a free of complexing proteinsClin Neuropharmacol200730869417414940
- CarliLMontecuccoCRossettoOAssay of diffusion of different botulinum neurotoxin type A formulations injected in the mouse legMuscle Nerve20094037438019618426
- GreinSManderGJTaylorHVXeomin® is stable without refrigeration: Complexing proteins are not required for stability of botulinum toxin type A preparationsToxicon20085113 Abstr 36.
- Botox®. Summary of product characteristicsIrvine, CAAllergan Inc2010
- Dysport®. Summary of product characteristicsSlough, UKIpsen Ltd2009
- AtassiMZOn the enhancement of anti-neurotoxin antibody production by subcomponents HA1 and HA3b of Clostridium botulinum type B 16S toxin-haemagglutininMicrobiology2006152Pt 71891189516804163
- DresslerDNew formulation of Botox. Complete antibody-induced therapy failure in hemifacial spasmJ Neurol200425136015015023
- DresslerDAdib SaberiFNew formulation of Botox: Complete antibody induced treatment failure in cervical dystoniaJ Neurol Neurosurg Psychiatry20077810810917172580
- Schulte-BauklohHBigalkeHMillerKBotulinum neurotoxin type A in urology: Antibodies as a cause of therapy failureInt J Urol20081540741518452456
- YablonSABrashearAGordonMFFormation of neutralizing antibodies in patients receiving botulinum toxin type A for treatment of poststroke spasticity: A pooled-data analysis of three clinical trialsClin Ther20072968369017617291
- YablonSThe development of toxin-neutralizing antibodies with botulinum toxin type A (botulinum toxin type A) treatmentNeurotox Res20069238
- BrinMFComellaCLJankovicJLong-term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assayMov Disord2008231353136018546321
- MullerKMixEAdib SaberiFPrevalence of neutralising antibodies in patients treated with botulinum toxin type A for spasticityJ Neural Transm200911657958519352590
- DresslerDPharmacological aspects of therapeutic botulinum toxin preparationsNervenarzt200677912921 German.16810528
- JankovicJVuongKDAhsanJComparison of efficacy and immunogenicity of original versus current botulinum toxin in cervical dystoniaNeurology2003601186118812682332
- GoschelHWohlfarthKFrevertJBotulinum A toxin therapy: Neutralizing and non-neutralizing antibodies – therapeutic consequencesExp Neurology199714796102
- EiseleKHTaylorHVBlümelJImmunogenicity of NT201 (Xeomin®) in Cynomolgus monkeys following high-dose injectionsMov Disord200823Suppl 1S15
- BlümelJFrevertJSchwaierAComparative antigenicity of three preparations on botulinum neurotoxin A in the rabbitNeurotox Res20069238
- KanovskyPPlatzTComesGGrafeSSassinINT 201, botulinum neurotoxin free from complexing proteins (Xeomin®) provided sustained efficacy and was safe in spasticity: 89 weeks long-term dataJ Neurol Sci2009285S75S76
- Xeomin®: Summary of Product CharacteristicsGreensboro, NCMerz Pharmaceuticals2010