Abstract
The majority of children, adolescents, and young adults diagnosed with cancer today will become long-term survivors. The threat to fertility that cancer treatments pose to young patients cannot be prevented in many cases, and thus research into methods for fertility preservation is developing, aiming at offering cancer patients the ability to have biologically related children in the future. This paper discusses the current status of fertility preservation methods when infertility risks are related to surgical oncologic treatments, radiation therapy, or chemotherapy. Several scientific groups and societies have developed consensus documents and guidelines for fertility preservation. Decisions about fertility and imminent potentially gonadotoxic therapies must be made rapidly. Timely and complete information on the impact of cancer treatment on fertility and fertility preservation options should be presented to all patients when a cancer treatment is planned.
Introduction
The number of reported new cancer cases is increasing every year. In the Swedish Cancer Registry, the validity of which relies on the inclusion of approximately 98% of cases having morphologic verification, the average annual increase has been 2.1% for men and 1.5% for women during the last two decades.Citation1 Only about half of this increase is explained by aging of the population and in many cases cancer patients are very young. Similar data have been observed in other European countries and in the US.Citation2 The good news is that the probability of surviving cancer today is high and is continually improving. Rates of survival today are above 80% for various cancer types, in particular for very young patients, such as those presenting with cancer in childhood or early adulthood.Citation3 Survivorship issues have therefore become highly relevant as well as quality of survival encompassing all health aspects.
The diagnosis of cancer at a young age, when individuals may have not yet started their families, poses unique challenges because treatments for cancer may induce ovarian or testicular failure by damaging ovarian follicles in females and spermatogonia in the testis in males. Gonadal failure may affect all aspects of reproductive health, including pubertal development, hormone production, and sexual function in adults. When cancer is treated by surgery, fertility may be impaired by removal or damage of the organs needed for reproduction.
The gonadotoxic effects of chemotherapy and radiation therapy are well recognized. These are dose-dependent and have been well characterized regarding the protocols used.Citation3–Citation12 Radiotherapy in females may also damage the uterus.Citation13,Citation14 Gonadotoxicity is particularly dependent on age in females, because the number of primordial follicles making up the female ovarian reserve is nonrenewable and diminishes steadily over the years until complete follicle depletion, which denotes menopause onset. Given that women who are older have a reduced reserve of eggs when compared with younger women, their risk of developing permanent ovarian failure is higher, whereas the risk may be relatively low in young women and girls following similar treatments.Citation14 If young patients present with apparently normal ovarian functioning after completion of cancer treatment, their reproductive period might be reduced and adequate reproductive counseling is recommended.Citation15
In males, spermatogenesis may still continue over several years if the spermatogonian cell population is not completely depleted. If a population of these germ stem cells remains after cancer treatment, regeneration of spermatozoa may continue for years.Citation16
The ability to start a family and have children is a key quality of life issue. Because infertility following cancer treatment has a recognized negative impact on quality of survival,Citation17–Citation21 several multidisciplinary groups and societies have made great effort in reviewing the currently available data on fertility preservation to produce guidelines for health care providers. Options to preserve fertility potential are currently available, and fertility preservation has emerged as a novel field where experience from disciplines such as oncology, surgery, reproductive medicine, psychology, and ethics are crossing over, and additional medical and paramedical disciplines are currently joining in. However, many inequalities for cancer patients in their access to counseling and fertility preservation have been reported.Citation22–Citation24
It is difficult to estimate the size of the population that may be interested in fertility preservation. Further, the risk of infertility differs depending on the type of cancer, treatment required, and age of the patient. The desired number of children is a very individual wish, and patients in similar clinical circumstances may have different wishes. For males, it is difficult to define a “reproductive age”, and regardless of age, a man could preserve sperm for the future, whereas the age-related follicle depletion in females, which occurs several years before onset of menopause, clearly denotes the end of the reproductive period.
Risk of cancer in young males and females
For men and women, the overall risk of developing cancer at some time during life is about 45% and 37%, respectively.Citation25 That risk increases with age in both sexes, and for people below 39 years of age, the risk is about 1/72 for men and 1/51 for women. In the age range of 40–59 years, the risk increases further to 1/12 for men and 1/11 for women. Given that the current trend in Western societies is to delay childbearing until later in life, there will be more young adults presenting with cancer who have not yet started their families and are interested in preserving their fertility. The most frequent cancer diagnoses in young adults and expected new cases in the US for the year 2014 are shown in .Citation26
Table 1 Ten leading cancer types for the estimated new cancer cases and deaths by sex, United States, 2014
Risk of infertility after cancer treatment and what can be offered
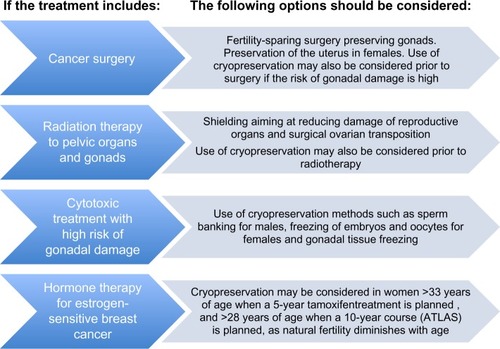
Local and systemic treatment modalities for cancer may affect fertility. This paper discusses fertility preservation approaches in oncologic surgery and in patients facing fertility threats due to radiation therapy and chemotherapy. Biological therapy is not discussed, given that its impact on reproduction is largely unknown. Hormonal treatment for cancer, which is targeted to specific hormone receptors, is discussed with regard to hormone-sensitive breast cancer. shows the strategies for fertility preservation depending on the type of cancer treatment.
Fertility-sparing surgery
Surgical techniques aiming at preserving the reproductive organs without compromising survival are relatively recent and the procedures are still evolving. Indications for fertility-sparing surgery include, in general, a well differentiated low-grade tumor in its early stages or with low malignant potential.
Fertility-sparing surgery in females
The global rates of fertility-sparing surgery in females are currently unknown. presents a compilation of data on female patients with gynecologic cancer undergoing conservative surgery aiming at preserving fertility. Young women presenting with borderline ovarian tumors may be offered a single oophorectomy, and this procedure appears to be safe with regard to oncologic outcome.Citation14 Another well-established surgical procedure for fertility preservation in young women is radical trachelectomy.Citation27 This may be offered in cases of early-stage invasive cervical cancer. Data on nearly 1,500 cases have been published, mostly from European countries, Japan, the US, Canada, China, and Argentina.Citation28–Citation33 A vaginal approach to radical trachelectomy was undertaken in about two thirds of these cases, and approximately 300 pregnancies resulting in live births, half being premature, have been reported.Citation34 An abdominal approach to trachelectomy was undertaken in the remaining 485 cases. Although 413 of these women were regarded as fertile after the procedure, only 113 (38%) attempted a pregnancy and 67 achieved a live birth (59.3%).Citation35 Cervical stenosis and subfertility are common after this type of surgery but, in general, the procedures appear to be safe, with no major complications recognized and no higher risk of recurrence having been observed. Recent reports suggest that patients with stage I cervical cancer 2–4 cm in diameter may also be offered a radical trachelectomy in selected cases, after negative nodal metastasis findings following laparoscopic pelvic and para-aortic lymphadenectomy for stagingCitation36 or whenever para-aortic nodes are not involved and frozen sections taken intraoperatively had provided safe results.Citation37 However, in these series, 45% of patients required immediate hysterectomy or chemoradiotherapy owing to high-risk features on final pathology.
Table 2 Fertility-sparing interventions in female patients
A recent European study has raised concerns regarding the need to centralize fertility-sparing surgery at accredited units to ensure a sufficient number of patients at each center aiming at maintaining quality of health care, given that the incidence observed after collecting data from several countries has been low.Citation38 Fertility-sparing surgery has also been reported in pregnant women, including abdominal radical trachelectomyCitation39 and vaginal trachelectomy with lymphadenectomy,Citation40 resulting in live births and preservation of fertility.
Fertility-sparing surgery in males
Oncologic surgery for prostate, bladder, or colon cancer may damage nerves and affect potency or ejaculation. In patients presenting with testicular cancer, hormone secretion and sperm production may be preserved by performing a partial orchiectomy, which has become an established method for selected patients. In particular, a conservative approach should be undertaken when the testicular mass is small and radical orchiectomy may result in anorchia.Citation41 The German Testicular Cancer Study Group reported a 98.6% disease-free survival rate at 7-year follow-up after conservative surgery for tumors <2 cm in 73 patients.Citation42 However, the benefits must be weighed against the risk of tumor recurrence in these patients. It may advisable to offer sperm banking prior to surgery, even to patients who may consider a partial orchiectomy at the outset (). Successful cases of sperm retrieval from the cancerous testicle in men with azoospermia at the time of radical orchiectomy have been reported, as well as sperm extraction from the epididymis and vas deferens of the orchiectomy specimen. Sperm obtained in this way has been cryopreserved and its use in fertility treatment has resulted in live births.Citation43,Citation44 Sperm may also be recovered from the contralateral noncancerous testicle at the time of orchiectomy in patients with azoospermia.Citation45
Fertility preservation options for females and males undergoing radiotherapy
Radiation therapy is used for several cancers in young patients and may be applied in a field affecting the reproductive organs in cases of Hodgkin’s disease, Ewing’s sarcoma, gynecologic cancer, and prostate cancer. In both sexes, the gonads are very sensitive to radiotherapy, and the extent of damage depends on the dose, fractionation schedule, and irradiation field.Citation46–Citation48 Total body irradiation given in conjunction with myeloablative conditioning prior to bone marrow transplantation is associated with a high risk of gonadal failure in both sexes.Citation49,Citation50 In prepubertal patients, failure of pubertal development may be the first sign of gonadal failure.Citation50 presents a compilation of current knowledge on the impact of radiation doses and age at radiotherapy in females and males.Citation48
Table 3 Radiation therapy protocols with high or intermediate impact on ovarian and testicular function
In females, all reproductive organs may suffer damage by direct irradiation if they are included in the irradiation field, but can also be damaged by scattered radiation, even after shielding. The degree and persistence of ovarian damage is influenced by the ovarian reserve and age at the time of exposure to radiotherapy. Thus, older women who present with a reduced number of follicles in the ovaries are at higher risk of permanent ovarian failure.Citation11 In general, a dose of about 2 Gy applied to the gonadal area may destroy up to 50% of the ovarian follicle reserve. Irradiation of the vagina is related to fertility and sexual issues due to loss of lubrication, anatomic impairment, and in some cases vaginal stenosis. Radiotherapy of the uterus in young women and girls causes tissue fibrosis, leading to restricted uterine capacity and blood flow, and damage to the uterus seems to be more pronounced in women who are younger at the time of radiotherapy.Citation51 Impaired uterine growth during pregnancy and unfavorable pregnancy outcomes, including spontaneous abortion, premature labor, and low birth weight offspring, have been reported in women who had undergone radiotherapy to the uterus.Citation13,Citation51
In males, the spermatogonia are extremely sensitive to radiation, regardless of age. Leydig cells, on the other hand, are highly sensitive to radiation before puberty onset,Citation52 whereas in adulthood, the cells become more resistant, and adult patients may retain Leydig cell function and testosterone production despite becoming azoospermic.
For females and males, both adult and prepubertal, shielding of the gonadal area is the standard procedure for reducing scatter radiation to the reproductive organs and to preserve fertility. Surgical ovarian transposition in females has been practiced and has been shown to reduce the risk of ovarian failure by about 50%.Citation53 Failure of this procedure is related to scatter radiation and damage to the blood vessels supplying the ovaries.Citation53
In adult patients, cryopreservation techniques, such as sperm banking for males and embryo and oocyte banking for females, both of which are now established methods, may also be considered prior to radiotherapy ().
Cranial irradiation for the treatment of brain tumors may induce infertility in both female and male patients by disruption of the hypothalamic-pituitary-gonadal axis and disturbance of gonadotropin secretion. In some cases, precocious puberty may also be induced by cranial irradiation in childhood, which has been attributed to cortical disruption and loss of inhibition by the hypothalamus.
Fertility preservation options for patients undergoing chemotherapy
Most chemotherapy protocols combine several agents and there is the possibility of a synergistic gonadotoxic effect.Citation54 In females, the primordial ovarian follicles, including oocytes and granulosa cells, are particularly sensitive to alkylating agents, and ovarian failure is common after alkylating treatment.Citation11,Citation15,Citation49,Citation50,Citation55 Apoptosis-induced chemotherapy has been demonstrated in vitroCitation53 and in vivoCitation56 using human ovarian tissue xenotransplanted into the SCID mouse. While claims have been made that chemotherapy may induce ovarian follicle depletion by massive activation of inactive follicles to grow in mice,Citation57 these have not been substantiatedCitation58 or supported by work using human material.
The presence of a rich ovarian reserve with high numbers of follicles in the ovaries, typical during childhood and young adulthood in females, is in itself relatively protective against chemotherapy-induced follicle damage. Girls and very young women who have undergone chemotherapy have a lower risk of ovarian failure and permanent infertility than older women after such treatment.Citation11,Citation15 Recent follow-up of survivors of cancer in childhood and adolescence corroborates this.Citation55 Experimental data from prepubertal and adult mouse models treated with cyclophosphamide do not support the notion that a prepubertal stage would be protective for the primordial follicles.Citation58
Toxicity of chemotherapy to growing oocytes has also been demonstrated,Citation59 and the clinical recommendation to avoid conception in the 6-month period immediately following completion of chemotherapy is widely practiced. The high risk of teratogenesis during or immediately following chemotherapy has been shown to diminish thereafter, with DNA integrity returning over time after cancer treatment.Citation60 No increase in childhood malignancies or genetic malformations have been found in surveys of more than 4,000 children of cancer survivors.Citation61–Citation63 Of note, chemotherapeutic agents such as doxorubicin and cyclophosphamide may induce double-stranded DNA breaks.Citation64 Oocytes are now known to have the ability to repair double-stranded DNA breaks and some oocytes may eventually repair these mutagenic DNA breaks and survive. This underscores further the importance of giving the ovaries some time to recover after chemotherapy.
In male patients, younger age or prepubertal status does not provide protection against the damage done to the gonads by alkylating agents.Citation54 Given that most chemotherapeutic agents are given as part of a combination regimen, it has been difficult to quantify the gonadotoxicity of individual drugs. shows the impact of chemotherapeutic agents on the female and male gonads.
Table 4 Chemotherapy agents with high or intermediate gonadotoxic impact in females and males
For patients with a high risk of becoming infertile due to cancer treatment, evidence-based guidelines for fertility preservation following a systematic review and formal procedures for creation of guidelines have been provided by several groups, including the American Society of Clinical Oncology,Citation53 which have recently been updated,Citation65 the National Institute for Health and Clinical Excellence in the UK,Citation66 the Scottish Intercollegiate Guidelines Network,Citation3 and the Clinical Oncological Society of Australia,Citation67 among others. Reproductive science societies such as the American Society for Reproductive Medicine,Citation68 the European Society of Human Reproduction and Embryology,Citation68 and the International Society of Fertility Preservation,Citation69 have also provided practice guidelines and now promote clinical, educational, and research activities regarding preservation of fertility. Nevertheless, the field of fertility preservation is rapidly evolving, in particular because assisted reproductive technologies, as well as cryopreservation, transplantation, and in vitro culture methods, are developing rapidly, and new treatment options may be available in the near future. When facing gonadotoxic chemotherapy, the options of freezing sperm for males and freezing of embryos for females have been established methods for many years. Recently, due to improvements in freezing of oocytes by vitrification, the American Society of Reproductive Medicine has also introduced vitrification of oocytes as an established method and the updated American Society of Clinical Oncology guidelines reflect this change.Citation65 The UK Association of Clinical Embryologists also recommends offering both vitrification of embryos or oocytes for routine fertility preservation.Citation70 Additional options, such as freezing of ovarian tissue and prepubertal testicular tissue, are still considered experimental, but are the only options that can be offered to prepubertal children.Citation53
Sperm banking for male patients
Cryopreservation of ejaculated semen is the recommended fertility preservation method for adult males and pubertal boys, and should be offered routinely to cancer patients.Citation71 Mature spermatozoa can be found at Tanner III stage with a testis volume above 5 mL; nevertheless, production of spermatozoa is generally effective only at the age of 13–14 years.Citation72 Successful sperm cryopreservation has been reported in adolescent patients from the age of 13 years, with a high prevalence of normal sperm counts and semen volume.Citation73,Citation74 Cryopreservation of at least three semen samples with an abstinence period of at least 48 hours in between samples is recommended for male patients interested in preserving fertility.Citation75
In cases of ejaculation failure, search for spermatozoa in a urine sample could be proposed. Testicular sperm extraction can also be performed to retrieve spermatozoa in young men and adolescents.Citation74 The use of mature sperm isolated from testicular tissue has been demonstrated to be effective for in vitro fertilization (IVF) and intracytoplasmic sperm injection.Citation44,Citation76 Other methods described for retrieval of spermatozoa in adolescents include penile vibratory stimulation and electro-ejaculation.
Cryopreservation of embryos or oocytes for females
Retrieval of oocytes for freezing or IVF of the retrieved eggs and freezing of embryos are now established methods for adult females. In most countries, women with a partner choose to have embryos frozen. In some countries, women can use donor sperm if they do not have a partner to fertilize their eggs and then freeze the embryos. Ovarian stimulation with gonadotropins is needed to obtain more than one oocyte per cycle and is a key component in the success of IVF cycles as well as cycles aiming to preserve fertility. Although these methods are widely available, the costs inherent in fertility drugs and IVF procedures seem to be a barrier limiting access of patients to preservation of fertility,Citation22 and time constraints may also be a limitation for female patients.Citation20 Use of random-start antagonist protocols, the feasibility of which is supported by demonstration of up to three major follicle-recruiting waves during a normal menstrual cycle,Citation77 has challenged the concept that antral follicles observed in the luteal phase are mostly atretic. Random-start protocols have thus proved to be efficient for fertility preservation while shortening the delay to egg retrieval to about 2 weeks.Citation78,Citation79
After freezing of embryos, success rates for transfer of thawed embryos are currently similar to those for fresh embryos if they remain intact after thawing, and this treatment can lead to a 59% pregnancy rate and a 26% live birth rate.Citation80
The freezing methods used for unfertilized oocytes have advanced remarkably in recent years, with the development of vitrification techniques that have improved oocyte survival and fertilization rates, approaching those reported for fresh oocytes. Worldwide, an increasing number of pregnancies and children born after fertilization of frozen-thawed oocytes has been reported, and although overall pregnancy rates are still relatively lower than those with embryo-freezing,Citation81–Citation83 pregnancy rates and live births after thawing and fertilizing frozen eggs are currently reaching those obtained after embryo cryopreservation.Citation84 In a recent meta-analysis of individual patient data, raw data from 1,805 women from 10 studies who underwent egg-freezing and attempted pregnancy were reanalyzed. Using these data, the authors were able to calculate specific success rates based on age, number of eggs, and method of freezing.Citation85 The formula used can be found at http://www.i-fertility.net/index.php/probability-calc.
Concerns when women with breast cancer are interested in preserving fertility
Counseling a breast cancer patient regarding her options for fertility preservation should include several important factors other than just the impact of chemotherapy on ovarian reserve. The age of the patient, the number of children desired, concerns regarding type of tumor, presence of BRCA mutation, hormone sensitivity, and concerns regarding the possibility of pregnancy after treatment for breast cancer may vary greatly between patients, so there is no “one-size-fits-all” approach.Citation86 Early referral has been demonstrated to improve the outcome of embryo and oocyte cryopreservation in women with breast cancer by enabling early initiation of chemotherapy. Early referral for fertility preservation may also improve the likelihood of success by allowing, in some cases, repeated stimulation cycles aiming at obtaining a larger number of oocytes or embryos for cryopreservation before treatment of the cancer. Women with breast cancer and BRCA gene mutations seem to present with reduced ovarian reserve at a young age.Citation87
Women with hormone-sensitive tumors who are embarking on a 5-year course of tamoxifen should be informed about the negative impact of increasing age on fertility. The recent ATLAS (Adjuvant Tamoxifen: Longer Against Shorter) study indicated that 10 years of treatment with tamoxifen is superior to a 5-year course, and this will result in a larger proportion of breast cancer patients needing preservation of fertility due to the enforced delay of childbearing. Tamoxifen itself does not affect the ovarian reserve, but the delay in attempting pregnancy for 5–10 years may render some patients infertile by virtue of the impact of aging on their already diminished ovarian reserve. Many breast cancer patients, in particular women who are older than 33 years of age at the time of starting on tamoxifen, may benefit from fertility preservation even when they do not receive chemotherapyCitation88 (). However, patients presenting with hormone-positive breast cancer have most likely been excluded from any options to preserve fertility, in particular those requiring gonadotropin stimulation, because the supraphysiologic elevation of circulating estradiol levels occurring during gonadotropin stimulation is undesirable and has been regarded as potentially harmful.Citation88 In fact, because estrogen can have receptor-independent actions, even women who are estrogen receptor-negative are not generally offered the option of embryo or oocyte freezing.
Natural cycle IVF without hormone stimulation has been regarded as a safe alternative in these patients. However, it has reduced efficacy when compared with gonadotropin-stimulated cycles, because it yields only one oocyte or embryo per cycle and the rate of cycle cancellation is high. Ovarian stimulation by selective estrogen receptor modulators and aromatase inhibitors alone or in combination with gonadotropins has been proposed to reduce the potentially harmful impact of high estrogen levels.Citation88 Both tamoxifen and letrozole have been investigated and implemented in protocols for ovarian stimulation in women with breast cancer. Stimulation protocols using letrozole alongside gonadotropins have been demonstrated to be the most effective, because they are associated with a higher number of oocytes recovered and fertilized when compared with tamoxifen protocols.Citation89 Letrozole is currently used in the treatment of anovulatory infertility in many countries, and its use for fertility treatments aiming at conception has not revealed any increased risks to the fetus.Citation90 Follow-up of breast cancer patients who have undergone ovarian stimulation with letrozole for fertility preservation has not identified any detrimental effects on survival in the short-term.Citation91,Citation92
Freezing immature oocytes may also be an option for preservation of female fertility in cases where there is not time available for stimulation. Oocytes are retrieved in the natural cycle and frozen at an immature stage or after maturation in vitro.Citation93 In vitro maturation of oocytes for fertility preservation has also been reported for immature eggs obtained after gonadotropin stimulation.Citation94
Cryopreservation of gonadal tissue
Cryopreservation of ovarian tissue as a method of fertility preservation for adult females has been demonstrated to be effective, with successful recovery of fertility and live births having been reported after retransplantation of the tissue.Citation95–Citation97 In prepubertal children facing gonadotoxic treatments, cryopreservation of gonadal tissue has also been undertaken for fertility preservation.Citation98 Methods for freezing adult and prepubertal gonadal tissue have been developed and improved over the years, but methods for using such tissues in fertility treatment by tissue retransplantation or in vitro culture and maturation of gametes are still under development.Citation53,Citation65,Citation99,Citation100
Cryopreservation and transplantation of ovarian tissue for females
Ovarian tissue freezing aims at preserving eggs within primordial follicles in the ovarian cortex. Retrieval of ovarian tissue may be performed by laparoscopy, can be planned shortly after diagnosis of malignant disease has been established, and does not require hormonal stimulation.Citation98 It is preferable to retrieve ovarian tissue for cryopreservation before a gonadotoxic treatment is initiated; however, it may still be worthwhile after the first courses of chemotherapy in young women and girls, who normally have a high number of primordial follicles in their ovaries.Citation14
Transplantation of frozen-thawed ovarian cortical tissue has been demonstrated to provide recovery of ovarian functionCitation101 and restore spontaneous fertility.Citation97,Citation102 Hormonal stimulation with gonadotropins and success after in vitro fertilization have also been reported in women who have undergone retransplantation of ovarian tissue.Citation96 Ovarian tissue can be transplanted orthotopically, ie, at the anatomic intrapelvic ovarian site, or heterotopically, ie, at other places, including extrapelvic sites.Citation103,Citation104 The techniques used for transplantation are currently being improved, in particular aiming at reducing initial ischemic follicle loss in the transplanted tissue, which is a concern.Citation105,Citation106
Autotransplantation of ovarian tissue in patients who have suffered from systemic hematologic malignancy is not recommended due to the high risk of retransmission of malignancy, and only patients with a cancer diagnosis associated with a negligible or no risk of ovarian compromise should be considered for future autotransplantation.Citation107–Citation109 Methods for detection of cancer cells in the ovarian tissue of patients who have suffered from hematologic malignancy are under development, including immunohistochemistry or the polymerase chain reaction, which can be attempted if there is a genetic marker.Citation110,Citation111 Investigation for residual malignant cells in ovarian tissue has been done by xenotransplantation in the immunodeficient SCID mouse, but this approach has not been shown to be of clinical use. Ovarian tissue cryopreservation and transplantation has been shown not to interfere with appropriate genomic imprinting in mouse pups,Citation112 but further studies in other animal models are needed.
Future possibilities: in vitro culture and maturation of ovarian follicles
Ovarian follicles cultured or isolated within a piece of thawed tissue will be the option for patients with hematologic and ovarian malignancies, given that tissue retransplantation is considered unsafe due to the risk of reintroducing malignant cells. Although many improvements have been reported for in vitro culture of follicles at early stages aiming at developing them into competent mature follicles, these methods are still in development.Citation100,Citation113–Citation115 The normality of imprinted genes of cryobanked oocytes cultured and matured in vitro has yet to be verified experimentally.
Prepubertal testicular tissue cryopreservation
In adult males, mature spermatozoa can be recovered from testicular tissue biopsies and several cryopreservation protocols have been developed.Citation116 In prepubertal boys, there is only the possibility to preserve spermatogonia, because no mature sperm yet exist. Success has been reported in cryopreservation methods for prepubertal testicular tissue,Citation117 but when preserving prepubertal tissue, more research is still needed aiming at using the frozen-thawed tissue to obtain mature spermatozoa in vitro. Research suggests that in vitro spermatogenesis is likely to be the safest option for boys suffering from hematologic malignancies, which might be retransmitted by retransplantation. Isolation of spermatogonia from contaminating leukemia cells by flow cytometry of a human testis cell suspension has been reported,Citation118 and spermatogonial transplantation into the testes may be an option in the future.Citation119
Cryopreservation of gonadal tissue offers hope for survivors of childhood cancer, but also requires careful oversight. Experimental methods for fertility preservation should only be offered to patients at specialized centers working with ethically approved research protocols, and only in cases where the recognized risks associated with the procedure are minimal. Whenever possible, these procedures should be performed with other necessary procedures, such as port placement or biopsies to reduce the need for additional surgery.Citation98
Preventing gonadal damage in females or males by gonadal suppression
It has been hypothesized that administration of gonadotropin-releasing hormone analogs to suppress female gonadal function transiently during chemotherapy could prevent ovarian follicle destruction by inducing a prepubertal hormonal milieu, aiming to maintain the follicles in a dormant state. However, the pool of primordial follicles is normally nonproliferating. The data show that these follicles lack follicle-stimulating hormone receptorsCitation120 and that their initial recruitment is not controlled by gonadotropins,Citation121 thus hormonal manipulation by suppressing gonadotropin release is not likely to affect them.Citation120 In mouse models, prepubertal status has not been protective against the follicle depletion induced by cyclophosphamide at doses similar to those used therapeutically in humans.Citation58
In females, the vast majority of clinical studies investigating gonadal protection by gonadotropin-releasing hormone analogs during chemotherapy have been small, retrospective, and uncontrolled. A significant number of these studies used resumption of menses as a surrogate marker for fertility and many reported a higher frequency of menstrual cycles in women who have received gonadotropin-releasing hormone analogs, but none has demonstrated a beneficial effect regarding fertility recovery. The current data indicate that infertility is increased after chemotherapy, even if menstrual cycles are resumed.Citation122 A recent review of 12 trials, both randomized and nonrandomized, in women with breast cancer found the benefit of cotreatment with gonadotropin-releasing hormone analogs to be uncertain in female fertility.Citation123
In males, a young age or prepubertal status do not provide protection against damage to the gonads by cytotoxic agents.Citation54,Citation124 Suppression of gonadotropins and testosterone secretion does not seem to affect the kinetics of developing cells and only blocks completion of spermatogenesis,Citation125 and experimental studies using animal models have reported conflicting results.Citation126 Clinical studies of males with Hodgkin’s lymphoma or testicular cancerCitation126 have not found any fertility-protecting effects after using these agents. Based on the preponderance of evidence, gonadotropin-releasing hormone analogs should not be considered as a proven method of fertility preservation in females or males. However, there are other promising pharmacologic agents, such as the Sphingosine-1-phosphate, which may protect ovarian reserve against chemotherapy.Citation127
Current deficiencies in provision of information and fertility preservation
Despite the evidence that fertility loss in survivors of cancer is related to psychologic distress and impaired quality of life,Citation20,Citation128–Citation130 many cancer patients of reproductive age still do not receive adequate information or referral to a reproductive specialist for fertility preservation.Citation23,Citation71,Citation131,Citation132 This is in contrast with data indicating that approximately three of four cancer patients younger than 35 years and childless at the time of cancer treatment may be interested in having children in the future.Citation18 In males, it has been shown that the expected number of patients who should bank sperm before cancer treatment is consistently lower than the expected number of cancer cases in young men.Citation71 Reluctance to delay initiation of cancer treatment, difficulties in communicating, lack of knowledge, and concerns regarding the costs of freezing sperm have been discussed, among other reasons.Citation71,Citation132 Regarding females, access to fertility preservation seems to be more difficult than for males, regardless of economic issues,Citation23 which are a recognized barrier for females in some countries.Citation22
To allow cancer patients to make informed decisions regarding their reproductive potential, information on the impact of cancer treatment on fertility and on the available options for fertility preservation should be presented to them in a timely manner. Several multidisciplinary groups, most of them based on oncology and reproductive societies, have organized educational activities and provide resources for both health care personnel and patients regarding fertility preservation, as well as numerous nonprofit patient associations.
Conclusion
The majority of children, adolescents, and young adults diagnosed with cancer today will become long-term survivors. Many surveys of cancer survivors have found that these patients are at increased risk of emotional distress if they become infertile as a result of their treatment. Health care providers should be prepared to discuss the negative impact of cancer therapy on reproductive health with their patients in the same way as any other risks of cancer treatment are discussed. The possibility of using fertility preservation methods should be presented to all patients even if they are ambivalent at the time. Recent reports indicate that fertility preservation may be offered in a safe context, without any risk of delaying initiation of cancer treatment. In this context, prompt referral may increase the cancer patient’s chances of receiving appropriate counseling and improve the success of fertility preservation. Interdisciplinary collaboration between oncologists and reproductive endocrinologists, development of local clinical guidelines, and educational activities should be encouraged.
Acknowledgments
KARW is supported by The Swedish Research Council and Stockholm County Council. KO is supported by the National Institute of Health (grants RO1 HD053112 and R21 HD061259).
Disclosure
The authors report no conflicts of interest in this work.
References
- Swedish Official StatisticsCancer Incidence in Sweden 2011 Updated 2012. Available from: http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/18919/2012-12-19.pdfAccessed October 30, 2013
- International Agency for Research on CancerEUCAN. Cancer Factsheets2013 Available from: http://eco.iarc.fr/EUCAN/Accessed October 30, 2013
- Health Improvement ScotlandLong term follow up of survivors of childhood cancer. A national clinical guideline2013 Available from: http://www.sign.ac.uk/pdf/sign132.pdfAccessed October 30, 2013
- United Kingdom Childhood Cancer StudySubfertility risk consensus document, 2005 Available from: http://www.ukccs.org/Accessed October 30, 2013
- BursteinHJWinerEPPrimary care for survivors of breast cancerN Engl J Med2000343151086109411027744
- GoodwinPJEnnisMPritchardKITrudeauMHoodNRisk of menopause during the first year after breast cancer diagnosisJ Clin Oncol19991782365237010561298
- GriggAThe impact of conventional and high-dose therapy for lymphoma on fertilityClin Lymphoma200452848815453922
- HowellSJShaletSMSpermatogenesis after cancer treatment: damage and recoveryJ Natl Cancer Inst Monogr200534121715784814
- WallaceWHThomsonABKelseyTWThe radiosensitivity of the human oocyteHum Reprod200318111712112525451
- WallaceWHThomsonABSaranFKelseyTWPredicting age of ovarian failure after radiation to a field that includes the ovariesInt J Radiat Oncol Biol Phys200562373874415936554
- WallaceWHAndersonRAIrvineDSFertility preservation for young patients with cancer: who is at risk and what can be offered?Lancet Oncol20056420921815811616
- FleischerRTVollenhovenBJWestonGCThe effects of chemotherapy and radiotherapy on fertility in premenopausal womenObstet Gynecol Surv201166424825421756407
- WoJYViswanathanANImpact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patientsInt J Radiat Oncol Biol Phys20097351304131219306747
- WallbergKAKerosVHovattaOClinical aspects of fertility preservation in female patientsPediatr Blood Cancer200953225426019340856
- Rodriguez-WallbergKAPrinciples of cancer treatment: impact on reproductionAdv Exp Med Biol20127321822210248
- PaceyAAFertility issues in survivors from adolescent cancersCancer Treat Rev200733764665517499440
- SchoverLRPsychosocial aspects of infertility and decisions about reproduction in young cancer survivors: a reviewMed Pediatr Oncol1999331535910401498
- SchoverLRRybickiLAMartinBABringelsenKAHaving children after cancer. A pilot survey of survivors’ attitudes and experiencesCancer199986469770910440699
- DuffyCAllenSMedical and psychosocial aspects of fertility after cancerCancer J2009151273319197170
- RosenARodriguez-WallbergKARosenzweigLPsychosocial distress in young cancer survivorsSemin Oncol Nurs200925426827719879433
- Howard-AndersonJGanzPABowerJEStantonALQuality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic reviewJ Natl Cancer Inst2012104538640522271773
- QuinnGPVadaparampilSTBell-EllisonBAGwedeCKAlbrechtTLPatient-physician communication barriers regarding fertility preservation among newly diagnosed cancer patientsSoc Sci Med200866378478918023955
- ArmuandGMRodriguez-WallbergKAWettergrenLSex differences in fertility-related information received by young adult cancer survivorsJ Clin Oncol201230172147215322585695
- LetourneauJMSmithJFEbbelEERacial, socioeconomic, and demographic disparities in access to fertility preservation in young women diagnosed with cancerCancer2012118184579458822451228
- National Cancer InstituteSEER Cancer Statistics Review 1975–2007 Updated 2011. Available from: http://seer.cancer.gov/archive/csr/1975_2007/Accessed October 30, 2013
- SiegelRMaJZouZJemalACancer statistics. 2014CA: A Cancer Journal for Clinicians20146492924399786
- DargentDMathevetPRadical laparoscopic vaginal hysterectomyJ Gynecol Obstet Biol Reprod (Paris)19922167097101430921
- MoricePUzanCGouySEffects of radiotherapy (external and/or internal) and chemotherapy on female fertilityBull Acad Natl Med20101943481492 French21171243
- LiouWSYapOWChanJKWestphalLMInnovations in fertility preservation for patients with gynecologic cancersFertil Steril20058461561157316359944
- Abu-RustumNRSonodaYFertility-sparing surgery in early-stage cervical cancer: indications and applicationsJ Natl Compr Canc Netw20108121435143821147906
- PlanteMGregoireJRenaudMCRoyMThe vaginal radical trachelectomy: an update of a series of 125 cases and 106 pregnanciesGynecol Oncol2011121229029721255824
- LiJLiZWangHRadical abdominal trachelectomy for cervical malignancies: surgical, oncological and fertility outcomes in 62 patientsGynecol Oncol2011121356557021334051
- TestaRRamirezPTFerreyraHAbdominal radical trachelectomy: a safe and feasible option for fertility preservation in developing countriesJ Low Genit Tract Dis201317437838423609587
- SpeiserDKöhlerCSchneiderAManglerMRadical vaginal trachelectomy: a fertility-preserving procedure in early cervical cancer in young womenDtsch Arztebl Int20131101728929523671476
- ParejaRRendónGJSanz-LomanaCMMonzónORamirezPTSurgical, oncological, and obstetrical outcomes after abdominal radical trachelectomy – a systematic literature reviewGynecol Oncol20131311778223769758
- VercellinoGFPiekJMSchneiderALaparoscopic lymph node dissection should be performed before fertility preserving treatment of patients with cervical cancerGynecol Oncol2012126332532922704949
- WethingtonSLSonodaYParkKJExpanding the indications for radical trachelectomy: a report on 29 patients with stage IB1 tumors measuring 2 to 4 centimetersInt J Gynecol Cancer20132361092109823714706
- KesicVRodolakisADenschlagDFertility preserving management in gynecologic cancer patients: the need for centralizationInt J Gynecol Cancer20102091613161921119372
- UngárLSmithJRPálfalviLDel PrioreGAbdominal radical trachelectomy during pregnancy to preserve pregnancy and fertilityObstet Gynecol20061083 Pt 281181417018513
- SioutasASchedvinsKLarsonBGemzell-DanielssonKThree cases of vaginal radical trachelectomy during pregnancyGynecol Oncol2011121242042121284995
- SabaneghESJrRaghebAMMale fertility after cancerUrology200973222523119036419
- HeidenreichAWeissbachLHöltlWOrgan sparing surgery for malignant germ cell tumor of the testisJ Urol200116662161216511696727
- BanielJSellaASperm extraction at orchiectomy for testis cancerFertil Steril200175226026211172824
- ChoiBBGoldsteinMMoomjyMPalermoGRosenwaksZSchlegelPNBirths using sperm retrieved via immediate microdissection of a solitary testis with cancerFertil Steril2005845150816275252
- SchraderMMüllerMSof ikitisNStraubBKrauseHMillerK“Onco-tese”: testicular sperm extraction in azoospermic cancer patients before chemotherapy-new guidelines?Urology200361242142512597960
- GosdenRGWadeJCFraserHMSandowJFaddyMJImpact of congenital or experimental hypogonadotrophism on the radiation sensitivity of the mouse ovaryHum Reprod19971211248324889436690
- SpeiserBRubinPCasarettGAspermia following lower truncal irradiation in Hodgkin’s diseaseCancer19733236926984726968
- Rodriguez-WallbergKAOktayKFertility preservation medicine: options for young adults and children with cancerJ Pediatr Hematol Oncol201032539039620502358
- SklarCGrowth and endocrine disturbances after bone marrow transplantation in childhoodActa Paediatr Suppl199541157618563071
- ThibaudERodriguez-MaciasKTrivinCEspérouHMichonJBraunerROvarian function after bone marrow transplantation during childhoodBone Marrow Transplant19982132872909489652
- GreenDMSklarCABoiceJDJrOvarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor StudyJ Clin Oncol200927142374238119364956
- ShaletSMTsatsoulisAWhiteheadEReadGVulnerability of the human Leydig cell to radiation damage is dependent upon ageJ Endocrinol198912011611652493061
- LeeSJSchoverLRPartridgeAHAmerican Society of Clinical Oncology recommendations on fertility preservation in cancer patientsJ Clin Oncol200624182917293116651642
- HudsonMMReproductive outcomes for survivors of childhood cancerObstet Gynecol201011651171118320966703
- BartonSENajitaJSGinsburgESInfertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohortLancet Oncol201314987388123856401
- OktemOOktayKA novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserveCancer Res20076721101591016217974956
- Kalich-PhilosophLRonessHCarmelyACyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertilitySci Transl Med20135185185ra62
- Rodriguez-WallbergKAAlonso de MenaSMalmELarssonAKuiperRHassanMPre-pubertal status does not protect against follicle depletion induced by cyclophosphamide in mice. A randomized studyHum Reprod201328Suppl 1P321
- MeirowDEpsteinMLewisHNugentDGosdenRGAdministration of cyclophosphamide at different stages of follicular maturation in mice: effects on reproductive performance and fetal malformationsHum Reprod200116463263711278209
- SimonBLeeSJPartridgeAHRunowiczCDPreserving fertility after cancerCA Cancer J Clin200555421122816020423
- DoddsLMarrettLDTomkinsDJGreenBShermanGCase-control study of congenital anomalies in children of cancer patientsBMJ199330768971641688343744
- LangagergaardVGislumMSkriverMVBirth outcome in women with breast cancerBr J Cancer200694114214616306874
- DalbergKErikssonJHolmbergLBirth outcome in women with previously treated breast cancer – a population-based cohort study from SwedenPLoS Med200639e33616968117
- SoleimaniRHeytensEDarzynkiewiczZOktayKMechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromiseAging (Albany NY)20113878279321869459
- LorenAWManguPBBeckLNAmerican Society of Clinical OncologyFertility preservation for patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline UpdateJ Clin Oncol201331192500251023715580
- National Institute for Health Care and ExcellenceFertility: assessment and treatment for people with fertility problems Available from: http://publications.nice.org.uk/fertility-cg156Accessed October 30, 2013
- Cancer Council Australia [homepage on the Internet]SydneyCancer Council Australia Available from: http://www.cancer.org.auAccessed November 19, 2013
- ReproductiveFacts.org [homepage on the Internet]Birmingham, ALThe American Society for Reproductive Medicine Available from: http://www.asrm.orgAccessed November 19, 2013
- International Society for Fertility Presentation [homepage on the Internet]KansasInternational Society for Fertility Presentation Available from: http://www.isfp-fertility.orgAccessed November 19, 2013
- BrisonDCuttingRClarkeHWoodMACE consensus meeting report: oocyte and embryo cryopreservation Sheffield 17.05.11Hum Fertil (Camb)2012152697422524465
- PaceyAAEiserCBanking sperm is only the first of many decisions for men: what health care professionals and men need to knowHum Fertil (Camb)201114420821722088127
- GuérinJFTesticular tissue cryoconservation for prepubertal boy: indications and feasibilityGynecol Obstet Fertil20053310804808 French16139551
- BahadurGLingKLHartRSemen quality and cryopreservation in adolescent cancer patientsHum Reprod200217123157316112456617
- MenonSRivesNMousset-SiméonNFertility preservation in adolescent males: experience over 22 years at Rouen University HospitalHum Reprod2009241374418945713
- MeseguerMMolinaNGarcía-VelascoJARemohíJPellicerAGarridoNSperm cryopreservation in oncological patients: a 14-year follow-up studyFertil Steril200685364064516500332
- MoreyAFDeshonGEJrRozanskiTADresnerMLTechnique of biopty gun testis needle biopsyUrology19934233253268379035
- BaerwaldARAdamsGPPiersonRAA new model for ovarian follicular development during the human menstrual cycleFertil Steril200380111612212849812
- von WolffMThalerCJFrambachTOvarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phaseFertil Steril20099241360136518930226
- SönmezerMTürkçüoğluICoşkunUOktayKRandom-start controlled ovarian hyperstimulation for emergency fertility preservation in letrozole cyclesFertil Steril20119562125. e9e1121292255
- MarrsRPGreeneJStoneBAPotential factors affecting embryo survival and clinical outcome with cryopreserved pronuclear human embryosAm J Obstet Gynecol200419061766177115284794
- OktayKCilAPBangHEfficiency of oocyte cryopreservation: a meta-analysisFertil Steril2006861708016818031
- Practice Committee of American Society for Reproductive MedicinePractice Committee of Society for Assisted Reproductive TechnologyOvarian tissue and oocyte cryopreservationFertil Steril200890Suppl 5S241S24619007638
- Practice Committees of American Society for Reproductive MedicineSociety for Assisted Reproductive TechnologyMature oocyte cryopreservation: a guidelineFertil Steril2013991374323083924
- NoyesNPorcuEBoriniAOver 900 oocyte cryopreservation babies born with no apparent increase in congenital anomaliesReprod Biomed Online200918676977619490780
- CilAPBangHOktayKAge-specific probability of live birth with oocyte cryopreservation: an individual patient data meta-analysisFertil Steril20131002492499. e323706339
- Rodriguez-WallbergKAOktayKFertility preservation and pregnancy in women with and without BRCA mutation-positive breast cancerOncologist201217111409141723006497
- TitusSLiFStobezkiRImpairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humansSci Transl Med20135172172ra21
- Rodriguez-WallbergKAOktayKFertility preservation in women with breast cancerClin Obstet Gynecol201053475376221048442
- OktayKBuyukELibertellaNAkarMRosenwaksZFertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservationJ Clin Oncol200523194347435315824416
- TulandiTMartinJAl-FadhliRCongenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrateFertil Steril20068561761176516650422
- AzimAACostantini-FerrandoMOktayKSafety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled studyJ Clin Oncol200826162630263518509175
- OktayKLeeSKimJYLong-term outcomes and safety of letrozole-FSH protocol in women with breast cancer undergoing fertility preservation: a prospective-controlled studyFertil Steril2010944 Suppl 1S11
- ChianRCGilbertLHuangJYLive birth after vitrification of in vitro matured human oocytesFertil Steril200991237237618514195
- OktayKBuyukERodriguez-WallbergKASahinGIn vitro maturation improves oocyte or embryo cryopreservation outcome in breast cancer patients undergoing ovarian stimulation for fertility preservationReprod Biomed Online201020563463820219430
- DonnezJDolmansMMDemylleDLivebirth after orthotopic transplantation of cryopreserved ovarian tissueLancet200436494431405141015488215
- DonnezJSilberSAndersenCYChildren born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live birthsAnn Med201143643745021226660
- OktayKTürkçüoğluIRodriguez-WallbergKAFour spontaneous pregnancies and three live births following subcutaneous transplantation of frozen banked ovarian tissue: what is the explanation?Fertil Steril2011952804. e7e1020801441
- Rodriguez-WallbergKABorgstromBSheikhiMLundqvistMLHovattaOCryopreservation of oocytes and gonadal tissue in a large fertility preservation programme at a teaching hospitalHum Reprod201025Suppl 1i104
- von WolffMDonnezJHovattaOCryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy – a technique in its infancy but already successful in fertility preservationEur J Cancer20094591547155319264478
- SmitzJDolmansMMDonnezJCurrent achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservationHum Reprod Update201016439541420124287
- OktayKKarlikayaGOvarian function after transplantation of frozen, banked autologous ovarian tissueN Engl J Med200034225191910877641
- AndersenCYRosendahlMByskovAGTwo successful pregnancies following autotransplantation of frozen/thawed ovarian tissueHum Reprod200823102266227218603535
- OktayKBuyukEVeeckLEmbryo development after heterotopic transplantation of cryopreserved ovarian tissueLancet2004363941283784015031026
- SonmezerMOktayKOrthotopic and heterotopic ovarian tissue transplantationBest Pract Res Clin Obstet Gynaecol201024111312619853515
- Rodriguez-WallbergKAOktayKRecent advances in oocyte and ovarian tissue cryopreservation and transplantationBest Pract Res Clin Obstet Gynaecol201226339140522301053
- SoleimaniRHeytensEOktayKEnhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplantsPLoS One2011296(4)e1947521559342
- DolmansMMLuyckxVDonnezJAndersenCYGreveTRisk of transferring malignant cells with transplanted frozen-thawed ovarian tissueFertil Steril20139961514152223541406
- GreveTClasen-LindeEAndersenMTCryopreserved ovarian cortex from patients with leukemia in complete remission contains no apparent viable malignant cellsBlood2012120224311431622709693
- RosendahlMGreveTAndersenCYThe safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literatureJ Assist Reprod Genet2013301112423263841
- MeirowDHardanIDorJSearching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patientsHum Reprod20082351007101318344563
- GreveTWielengaVTGrauslundMOvarian tissue cryopreserved for fertility preservation from patients with Ewing or other sarcomas appear to have no tumour cell contaminationEur J Cancer20134981932193823452988
- SauvatFCapitoCSarnackiSImmature cryopreserved ovary restores puberty and fertility in mice without alteration of epigenetic marksPLoS One200834e197218414667
- CarlssonIBScottJEVisserJARitvosOThemmenAPHovattaOAnti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitroHum Reprod20062192223222716720622
- TelferEEMcLaughlinMDingCThongKJA two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activinHum Reprod20082351151115818326514
- TelferEEZelinskiMBOvarian follicle culture: advances and challenges for human and nonhuman primatesFertil Steril20139961523153323635350
- BaertYVan SaenDHaentjensPIn’t VeldPTournayeHGoossensEWhat is the best cryopreservation protocol for human testicular tissue banking?Hum Reprod20132871816182623569082
- KerosVHultenbyKBorgströmBFridströmMJahnukainenKHovattaOMethods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatmentHum Reprod20072251384139517259225
- DoveySLValliHHermannBPEliminating malignant contamination from therapeutic human spermatogonial stem cells2013123418331843
- GoossensEVan SaenDTournayeHSpermatogonial stem cell preservation and transplantation: from research to clinicHum Reprod201328489790723427228
- OktayKBriggsDGosdenRGOntogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian folliclesJ Clin Endocrinol Metab19978211374837519360535
- McGeeEAHsuehAJInitial and cyclic recruitment of ovarian folliclesEndocr Rev200021220020110782364
- LetourneauJMEbbelEKatzPThe prevalence of self-reported reproductive impairment in young female cancer survivors throuhgout CaliforniaFertil Steril2010944510
- TurnerNHPartridgeASannaGDi LeoABiganzoliLUtility of gonadotropin-releasing hormone agonists for fertility preservation in young breast cancer patients: the benefit remains uncertainAnn Oncol20132492224223523709175
- MeistrichMLMale gonadal toxicityPediatr Blood Cancer200953226126619326418
- MeistrichMLWilsonGYeWSKurdogluBParchuriNTerryNHProtection from procarbazine-induced testicular damage by hormonal pretreatment does not involve arrest of spermatogonial proliferationCancer Res1994544102710348313358
- MeistrichMLShettyGHormonal suppression for fertility preservation in males and femalesReproduction2008136669170118515310
- LiFTuranVLiermanSCuvelierCDe SutterPOktayKSphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle deathHum Reprod201429110711324221908
- SkinnerRWallaceWHLevittGAUK Children’s Cancer Study Group Late Effects GroupLong-term follow-up of people who have survived cancer during childhoodLancet Oncol20067648949816750499
- ByrneJFearsTRSteinhornSCMarriage and divorce after childhood and adolescent cancerJAMA198926219269326992810602
- PartridgeAHGelberSPeppercornJWeb-based survey of fertility issues in young women with breast cancerJ Clin Oncol200422204174418315483028
- FormanEJAndersCKBeheraMAPilot survey of oncologists regarding treatment-related infertility and fertility preservation in female cancer patientsJ Reprod Med200954420320719438160
- MerrickHWrightEPaceyAAEiserCFinding out about sperm banking: what information is available online for men diagnosed with cancer?Hum Fertil (Camb)201215312112822746362
- SonodaYChiDSCarterJBarakatRRAbu-RustumNRInitial experience with Dargent’s operation: the radical vaginal trachelectomyGynecol Oncol2008108121421917996284
- MorrisRTGershensonDMSilvaEGFollenMMorrisMWhartonJTOutcome and reproductive function after conservative surgery for borderline ovarian tumorsObstet Gynecol200095454154710725486
- MoricePCamatteSWicart-PoqueFResults of conservative management of epithelial malignant and borderline ovarian tumoursHum Reprod Update20039218519212751780
- TangirJZeltermanDMaWSchwartzPEReproductive function after conservative surgery and chemotherapy for malignant germ cell tumors of the ovaryObstet Gynecol2003101225125712576247
- SeliETangirJFertility preservation options for female patients with malignanciesCurr Opin Obstet Gynecol200517329930815870565