Abstract
Basal cell carcinoma (BCC) is the most common human malignancy. Recent advances in our understanding of the critical biologic pathways implicated in the development and progression of BCC have led to the development of the first molecular targeted therapy for this disease. The hedgehog pathway is mutated in virtually all patients with BCC and recent trials with vismodegib, an inhibitor of this pathway, have shown significant responses. This review will discuss the importance of the hedgehog pathway in the pathogenesis of BCC and describe in detail the pharmacology of vismodegib in relation to its activity in advanced BCC.
Introduction
Basal cell carcinoma (BCC) is the most common human malignancy. Since it is not always included in cancer registries, the true incidence is unknown. It is estimated that approximately 3.5 million nonmelanoma skin cancers in 2.2 million patients are treated annually in the United States, the majority of which are BCC (American Cancer Society, Facts and Figures 2013). The American Academy of Dermatology estimates that approximately 2 million BCC are treated every year (http://www.aad.org/dermatology-a-to-z/diseases-and-treatments/a---d/basal-cell-carcinoma). Fortunately, these tumors are usually amenable to local therapy and only 1%–5.3% of lesions recur after initial resection.Citation1
Interestingly, even locally aggressive tumors seldom metastasize and metastatic BCC is quite rare. Since accurate incidence records of this disease are not available, the burden of metastatic disease is difficult to ascertain. Rates ranging from 0.0028% to 0.55% of patients with BCC developing metastases have been reported,Citation2–Citation7 but even these have been questioned.Citation8 The median age of patients with metastatic BCC at the time of diagnosis of the primary lesion is about 45–56 years, and metastases appear at a median of about 9 years later.Citation9–Citation11 Factors that may predispose to the development of metastatic BCC include male gender,Citation9 primary lesion in the ear regionCitation9,Citation11,Citation12 and face,Citation11 largeCitation11 and locally invasiveCitation13 primary tumors, recurrence following initial treatment,Citation11 and impairment of cell mediated immunity (eg, acquired immunodeficiency syndrome, therapeutic immunosuppression).Citation14,Citation15
Recent advances in our understanding of the biologic pathways that appear to be important in the development and progression of BCC have led to the development of the first molecular targeted therapy for this disease, vismodegib. The hedgehog (Hh) pathway is mutated in virtually all patients with BCC and inhibition of this pathway with vismodegib appears to result in significant clinical responses. The following sections will discuss the importance of the Hh pathway in the pathogenesis of BCC and describe in detail the pharmacology of vismodegib in relation to advanced BCC.
Hh pathway and its role in BCC
The Hh pathway is one of the most common signal transduction pathways used by mammalian cellsCitation16 for normal embryonic development of the neural tube, axial skeleton, skin, and hair,Citation17 and for wound healing during postnatal life.Citation18 While this pathway appears to be silenced in most adult tissues,Citation19 it has been shown to promote the proliferation of stem cells from various tissues in the adult. These include hematopoietic cells,Citation20 mammary,Citation21 mesenchymal,Citation22 and neuralCitation23 stem cells. This pathway also appears to play a role in the transition of hair follicles from the resting to the growth phase.Citation24 This could explain the alopecia noted in patients treated with the Hh pathway inhibitor, vismodegib. In addition, this pathway seems to be involved in the repair of various organs in the adult following injury.Citation25–Citation27
Recent studies have shown that aberrant Hh signaling pathway is involved in the pathogenesis, self-renewal of stem cells, and chemotherapeutic resistance of BCC.Citation28,Citation29 Besides BCC, Hh signaling is also activated in other malignancies such as medulloblastoma,Citation30,Citation31 colon,Citation32 prostate,Citation33,Citation34 breast,Citation35 lung,Citation36 and various hematologic malignancies.Citation37–Citation40 The Hh pathway dependent gene upregulation leads to increased cell proliferation and cell survival, and promotes bone metastases.Citation41 This pathway has been shown to regulate the epithelial–mesenchymal transition and dissemination of cancer stem cells in solid tumors,Citation42,Citation43 and enhance metastatic disease progression.Citation44 These observations suggest that targeted inhibition of Hh signaling may be an effective treatment strategy for several human cancers.
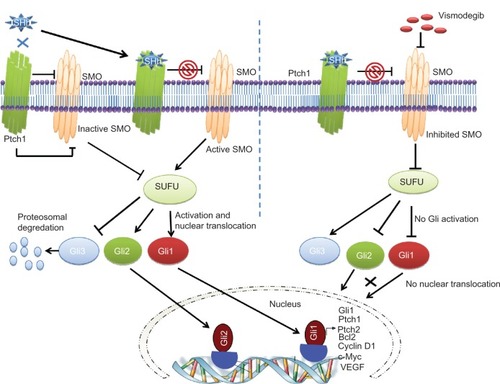
The signaling cascade of the Hh pathway in mammalian cells is initiated by binding of one of three Hh ligands (sonic, Indian, or desert Hh) to the 12 pass transmembrane receptor patched 1 (Ptch1) (). This causes internalization and degradation of Ptch1, thereby releasing smoothened (SMO), a 7 pass transmembrane G protein coupled receptor like protein. This protein then enters the primary cilia and disrupts the cytoplasmic complex containing Suppressor of Fused (SUFU)-glioma-associated oncogene homologue (Gli). The SUFU regulates transcriptional factors (Glis) through posttranslational modifications like phosphorylation, sumoylation, and selective proteolysis.Citation45 Release of Gli transcription factors (Gli 1–3) from SUFU inhibition results in nuclear translocation and upregulation of Gli1 associated target genes that include Gli1, Ptch1, cyclin D1, c-Myc, vascular endothelial growth factor, Bcl-2, and snail, depending upon cell typeCitation46 and degradation of the repressor transcription factor Gli3. Depending on the Gli2 posttranscriptional and posttranslational processing events, Gli2 has been shown to act as either a positive or negative regulator of Hh pathway signaling.Citation47 Deregulated Hh signaling has been associated with either mutations in pathway genes or overexpression of signaling molecules in either tumor cells themselves or in cells within the supportive tumor microenvironment.Citation46,Citation48–Citation51
Figure 1 A schematic diagram showing inhibition of Hedgehog (Hh) signaling by vismodegib.
Notes: The extracellular Hh ligands (Sonic, Indian, or Desert Hh) bind to Patched 1 (Ptch1), relieving the inhibition of Smoothened (SMO) by Ptch1. SMO disrupts the cytoplasmic complex containing the Suppressor of Fused (SUFU)-glioma-associated oncogene homologue (Gli) complex resulting in degradation of Gli3 and translocation of Gli1 and Gli2 to the nucleus to upregulate target genes like Ptch1, Gli1, c-Myc, Bcl2, vascular endothelial growth factor, and cyclin D1. Vismodegib binds to the extracellular domain of SMO, markedly inhibiting the release and translocation of Gli1 and Gli2 to the nucleus.
Abbreviations: Gli, glioma-associated oncogene homologue; Ptch1, patched 1; SHh, sonic hedgehog; SMO, smoothened; SUFU, Suppressor of Fused; VEGF, vascular endothelial growth factor.
The first link between the Hh pathway and BCC came from the discovery of loss-of-function mutations of Ptch1 gene in Gorlin syndrome.Citation29,Citation52 Patients with Gorlin syndrome are strongly predisposed to the development of BCC at an early age. Recent molecular and genetic studies have shown that almost all BCC tumors contain genetic alterations in components of the Hh signaling pathway and these tumors have elevated Ptch and Gli1 messenger (m)RNA levels.Citation53,Citation54 This ligand-independent mechanism of Hh activation, driven by specific Hh gene mutations within the tumor cells, is termed Type I Hh signaling.Citation46 It has been estimated that almost 90% of sporadic BCCs have loss-of-function mutation in at least one allele of Ptch1 and in addition, around 10% have activating mutations in the SMO, rendering it resistant to Ptch1 inhibition.Citation28,Citation55–Citation57 In contrast, mutations in other downstream molecules of the Hh pathway appear to be rare in BCC. In a study of 42 patients with sporadic BCC, only two patients had mutations in SUFU, one of which was a missense mutation and the other a silent mutation.Citation58 No mutation was seen in Gli1.
Pharmacology of vismodegib
Structure
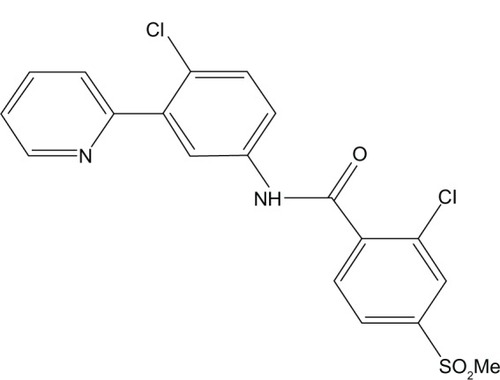
Vismodegib is an orally active SMO inhibitor (2-chloro-N-[4-chloro-3-pyridin-2-yl-phenyl]-4-methanesulfonyl benzamide) with a molecular weight 421.30 g/mol ().
Preclinical evidence
Vismodegib was shown to be effective in suppressing Gli in the Hh responsive tumor cell line CALU-6 when implanted in nude mice.Citation59 It was also effective in producing complete regression of the Hh pathway dependent medulloblastoma allograft model generated from Ptch+/− mice.Citation59
Pharmacokinetics
Vismodegib has an unusual pharmacokinetic profile. It demonstrates saturable absorption with a dose dependent increase in absorption until a dose of 540 mg, beyond which the degree of absorption does not appear to increase.Citation60 This could explain the plateau of the rise in concentration of the drug within the first 14 days. The drug reaches its steady state concentration in the plasma in about 7–14 days after initiation of therapy. Vismodegib is highly protein bound to albumin and α1-acid glycoprotein. Plasma concentrations of total vismodegib appear to be strongly correlated with α1-acid glycoprotein levels.Citation60,Citation61 Food does not seem to affect the degree of absorption of vismodegib.
Vismodegib undergoes oxidation mediated by the cytochrome P450 (CYP) enzymes CYP2C9, CYP3A4, and CYP3A5, and the oxidative metabolites are excreted in the feces.Citation62,Citation63 In addition, vismodegib undergoes glucuronidation and pyridine ring cleavage to a smaller extent.Citation62 A study in dogs and rats found that a small proportion of the drug was metabolized by an uncommon pyridine ring opening.Citation64 These metabolites were excreted in the feces as well. In pharmacokinetic studies, vismodegib had an elimination half-life of approximately 12 days after a single dose. However this duration decreased to 4 days after repeated once-daily administration, suggesting an increased clearance with repeated administration.Citation65
Although it has not been specifically studied, based on the pharmacokinetic profile, it is unlikely that renal dysfunction affects the safety profile of vismodegib significantly. Hence, patients with renal dysfunction are not likely to need a modification of their dose. The need for dose modification in patients with hepatic dysfunction is currently unknown.
Pharmacodynamics
Vismodegib binds to, and inactivates, SMO. This prevents its translocation when Ptch1 is stimulated by Hh ligands and leads to the inhibition of the downstream signaling pathways and a decrease in the production of downstream proliferation factors ().Citation66
Exposure to vismodegib leads to the downregulation of Gli1. In a Phase I trial of vismodegib in patients with locally advanced or metastatic solid tumors there was >2 fold downmodulation of Gli1 expression in skin biopsies from almost 75% patients, compared with pretreatment specimens.Citation66 In the same study, a similar proportion of patients with BCC showed >2 fold down-modulation of Gli1 expression. However, the down-modulation of Gli1 expression was not associated with plasma concentrations of the drug.
Adverse effects
Vismodegib is relatively well tolerated. The most common adverse reactions seen in clinical trials have been muscle spasms (70%), alopecia (60%), dysgeusia (55%), weight loss (45%), fatigue (40%), nausea (30%), diarrhea (30%), decreased appetite (25%), constipation (20%), arthralgias (15%), vomiting (12%), and ageusia (10%).Citation67 Approximately 30% of premenopausal women developed amenorrhea while on vismodegib. Other laboratory abnormalities noted by patients on clinical trials included hyponatremia (4%), hypokalemia (1%), and azotemia (2%). A recent report described the development of keratoacanthomas in two patients, who did not have a history of previous squamous cell carcinomas, while being treated with vismodegib.Citation68 While the authors were not able to establish a causal relationship with vismodegib, clinicians need to be alert to these findings.
The vismodegib package insert contains a black box warning regarding the risk of severe birth defects and embryonic fetal death. This is based on rat studies, which demonstrated that vismodegib administered at a dose of 10 mg/kg/day showed teratogenic effects.Citation69 This dose corresponds to an exposure of approximately 20% of the human exposure at the recommended dose of 150 mg daily. Similarly, there is a potential risk of exposure to the drug through semen. Hence, sexually active individuals receiving vismodegib should be advised of the need for contraception (Source: Erivedge™ package insert. http://www.gene.com/download/pdf/erivedge_prescribing.pdf).
Drug resistance
Resistance mechanisms to vismodegib have not been extensively studied, which is understandable given the relatively short clinical experience with this drug. However, anecdotal reports of acquired resistance to vismodegib are beginning to emerge. In the only case reported thus far, a 26 year old man with treatment refractory metastatic medulloblastoma who was treated with vismodegib responded initially, but developed progressive disease after 3 months, suggesting the development of resistance. A molecular analysis of the biopsy specimen obtained at disease progression showed a heterozygous G to C missense mutation at position 1697, which is responsible for a change in codon 473 from aspartic acid to histidine (D473H).Citation70 This mutation prevents the binding of vismodegib to SMO, thereby leading to resistance.
This was further studied in vitro by Dijkgraaf et al who replaced the aspartic acid at position 473 of the SMO gene with every other amino acid and found that vismodegib did not bind to SMO in any of the mutant variations, thereby suggesting that D473 is critical for SMO binding by vismodegib.Citation71 It is unclear, however, if D473 is directly involved in the binding of vismodegib or if its main role is to maintain the necessary SMO conformation for binding.
Other potential resistance mechanisms identified in vitro include mutations at E518.Citation71 Substitution of glutamic acid at this site conferred complete resistance to vismodegib. Other possible mechanisms of resistance include focal amplifications of the transcription factor Gli2 and the target gene Ccnd1, which exert their actions downstream of SMO.Citation71 There are initial data on resistance to vismodegib in BCC, which are described below.
Mechanisms to overcome this resistance are currently being studied. Dijkgraaf et alCitation71 screened multiple chemically diverse Hh pathway inhibitors and were able to identify several compounds that were active against the two known SMO resistance mutations, SMO-D473H and SMO-E518K. Available data also suggest that Hh pathway resistant medulloblastoma allografts may respond to inhibition of phosphoinositide-3-kinase.Citation71
Clinical data on vismodegib
The Phase I trial of vismodegib included 68 patients with different malignancies, of which 33 had advanced BCC.Citation66 Vismodegib was well tolerated with only six patients (8.8%) developing grade 4 adverse events (hyponatremia, fatigue, pyelonephritis, presyncope, resectable pancreatic adenocarcinoma, and paranoia with hyperglycemia). A maximum tolerated dose was not reached and based on achievement of maximal plasma concentration and response, the recommended Phase II dose was 150 mg daily. Interestingly, 20 patients (19 with BCC and one with medulloblastoma) had a response based on Response Evaluation Criteria in Solid Tumors (RECIST) criteria.Citation72 At the time of publication, the median duration of response in patients with advanced BCC was 12.8 months (range 3.7–26.4 months). This led to further investigation of vismodegib in BCC.
A multicenter, international, two cohort, nonrandomized Phase II study, administered 150 mg of oral vismodegib daily to patients with either metastatic or locally advanced BCC (inoperable disease or for whom surgery was inappropriate, ie, multiple recurrences and a low likelihood of cure, or substantial anticipated disfigurement).Citation73 The response rate was 30% for patients with metastatic BCC and 43% (21% complete response) in patients with locally advanced BCC. The median duration of response was 7.6 months in both cohorts. The most common adverse events were muscle spasms, alopecia, dysgeusia, weight loss, and fatigue. Serious adverse events were reported in 25% of patients. Seven deaths were reported, although their relationship to the study drug was unclear. The results of these studies led to the approval of vismodegib for the treatment of metastatic BCC and locally advanced BCC in patients who are not candidates for surgery or radiation based on the location or size of the lesion.
More recently, vismodegib has been studied for its potential to prevent the development of BCC in patients with basal cell nevus syndrome. In a randomized placebo controlled trial, 42 patients with the basal cell nevus syndrome were randomly assigned to either vismodegib (150 mg daily) or placebo for 18 months.Citation74 The primary end point of this study was the rate of development of new BCC that were eligible for surgical resection. Vismodegib significantly reduced the rate of new surgically eligible basal cell carcinomas when compared to placebo (2 versus 29 new surgically eligible BCC per group annually; P < 0.001). Vismodegib also reduced the size of existing BCC (median decrease 71%) when compared to placebo (median decrease 21%; P = 0.003). Patients receiving vismodegib had fewer surgeries for BCC (mean of −0.31), compared with those receiving placebo (mean −4.4; P < 0.001). Palmar and plantar pits, pathognomonic signs of the basal cell nevus syndrome, also disappeared with vismodegib. However, almost half of the patients had to discontinue treatment due to drug related adverse events.
Recently, an interesting phenomenon of tumor regrowth was reported in patients treated with vismodegib.Citation75 In a retrospective analysis of 28 patients on vismodegib, Chang et al noted tumor regrowth within or adjacent to (≤1 cm) the prior tumor bed in 6 (21%) patients.Citation75 Surprisingly, none of the eight patients with metastatic BCC in their analysis demonstrated regrowth after initial shrinkage. Hence, close clinical examination of patients, even examination of those who have a response to therapy, is warranted to detect the development of secondary resistance to vismodegib. These findings would also suggest that vismodegib and other agents targeting the Hh pathway are unlikely to cure BCC, and surgery still would be considered the best curative option for patients who can undergo the procedure without major cosmetic deformity.
Conclusion
The Hh pathway is critical to the development of BCC. Mutations in this pathway are seen in virtually all patients with BCC. Inhibition of this pathway with the SMO inhibitor, vismodegib, leads to significant responses in patients with metastatic and locally advanced BCC. While vismodegib can delay the development of BCC in patients with the basal cell nevus syndrome, its adverse effect profile tempers its use in this setting. Finally, there are reports of resistance to vismodegib and those mechanisms are currently being studied.
Acknowledgments
The authors on this manuscript, in part, were supported by the VA Career Development Award (AKG) and NIH grants (UO1 EDRN CA 111294, P50 SPORE CA127297, and U54 TMEN CA160163) (SKB).
Disclosure
The authors report no conflicts of interest in this work.
References
- ThissenMRNeumannMHSchoutenLJA systematic review of treatment modalities for primary basal cell carcinomasArch Dermatol19991351177118310522664
- PaverKPoyzerKBurryNDeakinMLetter: The incidence of basal cell carcinoma and their metastases in Australia and New ZealandAustralas J Dermatol197314534753676
- CadeSMalignant Disease and its Treatment by RadiumBaltimoreWilliams and Wilkins Company1940
- CotranRSMetastasizing basal cell carcinomasCancer1961141036104013695862
- LakshmipathiTHuntKMMetastasizing basal-cell carcinomaBr J Dermatol1967792672706025574
- ScanlonEFVolkmerDDOviedoMAKhanedarJDVictorTAMetastatic basal cell carcinomaJ Surg Oncol1980151711807421276
- WronkowskiZPrzerzuty raka podstawnokomorkowego skory [Metastases in dermal basal cell carcinoma.]Nowotwory1968185155 Polish5669013
- WadheraAFazioMBriccaGStantonOMetastatic basal cell carcinoma: a case report and literature review. How accurate is our incidence data?Dermatol Online J200612716962022
- von DomarusHStevensPJMetastatic basal cell carcinoma. Report of five cases and review of 170 cases in the literatureJ Am Acad Dermatol198410104310606736323
- WeissGJKornRLMetastatic basal cell carcinoma in the era of hedgehog signaling pathway inhibitorsCancer20121185310531922511370
- SnowSNSahlWLoJSMetastatic basal cell carcinoma. Report of five casesCancer1994733283358293396
- LoJSSnowSNReiznerGTMohsFELarsonPOHruzaGJMetastatic basal cell carcinoma: report of twelve cases with a review of the literatureJ Am Acad Dermatol1991247157191869642
- BerlinJMWarnerMRBailinPLMetastatic basal cell carcinoma presenting as unilateral axillary lymphadenopathy: report of a case and review of the literatureDermatol Surg2002281082108412460309
- SitzK VKeppenMJohnsonDFMetastatic basal cell carcinoma in acquired immunodeficiency syndrome-related complexJAMA19872573403433795417
- WallingH WFoskoS WGeraminejadPAWhitakerDCArpeyCJAggressive basal cell carcinoma: presentation, pathogenesis, and managementCancer Metastasis Rev20042338940215197337
- MerchantJLHedgehog signalling in gut development, physiology and cancerJ Physiol201259042143222144577
- TangJYSoPLEpsteinEHJrNovel Hedgehog pathway targets against basal cell carcinomaToxicol Appl Pharmacol200722425726417276471
- BarakatMTHumkeE WScottM PLearning from Jekyll to control Hyde: Hedgehog signaling in development and cancerTrends Mol Med20101633734820696410
- InghamP WMcMahonA PHedgehog signaling in animal development: paradigms and principlesGenes Dev2001153059308711731473
- BhardwajGMurdochBWuDSonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulationNat Immunol2001217218011175816
- LiuSDontuGMantleIDHedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cellsCancer Res2006666063607116778178
- JamesAWPangSAskarinamAAdditive effects of sonic hedgehog and Nell-1 signaling in osteogenic versus adipogenic differentiation of human adipose-derived stromal cellsStem Cells Dev2012212170217822264144
- AhnSJoynerALIn vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehogNature200543789489716208373
- PaladiniRDSalehJQianCXuGXRubinLLModulation of hair growth with small molecule agonists of the hedgehog signaling pathwayJ Invest Dermatol200512563864616185261
- MichelottiGAXieGSwiderskaMSmoothened is a master regulator of adult liver repairJ Clin Invest Epub482013
- ZarogoulidisPZarampoukaKHuangHHedgehog signaling pathway: the must, the maybe and the unknownJ Thorac Dis2013519519723585948
- WangSHyunJYounBJungYHedgehog signaling regulates the repair response in mouse liver damaged by irradiationRadiat Res2013179697523181588
- GailaniMRStahle-BackdahlMLeffellDJThe role of the human homologue of Drosophila patched in sporadic basal cell carcinomasNat Genet19961478818782823
- HahnHChristiansenJWickingCA mammalian patched homolog is expressed in target tissues of sonic hedgehog and maps to a region associated with developmental abnormalitiesJ Biol Chem199627112125121288647801
- ZurawelRHAllenCChiappaSAnalysis of Ptch/SMO/SHH pathway genes in medulloblastomaGenes Chromosomes Cancer200027445110564585
- BarEEChaudhryAFarahMHEberhartCGHedgehog signaling promotes medulloblastoma survival via Bc/IIAm J Pathol200717034735517200206
- BermanDMKarhadkarSSMaitraAWidespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumoursNature200342584685114520411
- KarhadkarSSBovaGSAbdallahNHedgehog signalling in prostate regeneration, neoplasia and metastasisNature200443170771215361885
- FanLPepicelliC VDibbleCCHedgehog signaling promotes prostate xenograft tumor growthEndocrinology20041453961397015132968
- VorechovskyIBenediktssonK PToftgardRThe patched/hedgehog/ smoothened signalling pathway in human breast cancer: no evidence for H133Y SHH, Ptch and SMO mutationsEur J Cancer19993571171310505029
- WatkinsDNBermanDMBurkholderSGWangBBeachyPABaylinSBHedgehog signalling within airway epithelial progenitors and in small-cell lung cancerNature200342231331712629553
- HegdeG VMungerCMEmanuelKTargeting of sonic hedgehog-GLI signaling: a potential strategy to improve therapy for mantle cell lymphomaMol Cancer Ther200871450146018524848
- HegdeG VPetersonKJEmanuelKHedgehog-induced survival of B-cell chronic lymphocytic leukemia cells in a stromal cell microenvironment: a potential new therapeutic targetMol Cancer Res200861928193619074837
- DierksCGrbicJZirlikKEssential role of stromally induced hedgehog signaling in B-cell malignanciesNat Med20071394495117632527
- WarzechaJBonkeLKoehlUThe hedgehog inhibitor cyclopamine induces apoptosis in leukemic cells in vitroLeuk Lymphoma2008492383238619052992
- KatohYKatohMHedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activationCurr Mol Med2009987388619860666
- VarnatFDuquetAMalerbaMHuman colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansionEMBO Mol Med2009133835120049737
- FeldmannGDharaSFendrichVBlockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancersCancer Res2007672187219617332349
- RasheedZAYangJWangQPrognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinomaJ Natl Cancer Inst201010234035120164446
- HuiCCAngersSGli proteins in development and diseaseAnnu Rev Cell Dev Biol20112751353721801010
- ScalesSJde SauvageFJMechanisms of Hedgehog pathway activation in cancer and implications for therapyTrends Pharmacol Sci20093030331219443052
- SasakiHNishizakiYHuiCNakafukuMKondohHRegulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signalingDevelopment19991263915392410433919
- WickingCMcGlinnEThe role of hedgehog signalling in tumorigenesisCancer Lett20011731711578802
- Ruiz i AltabaATherapeutic inhibition of Hedgehog-GLI signaling in cancer: epithelial, stromal, or stem cell targets?Cancer Cell20081428128318835029
- Ruiz i AltabaASanchezPDahmaneNGli and hedgehog in cancer: tumours, embryos and stem cellsNat Rev Cancer2002236137212044012
- YauchRLGouldSEScalesSJA paracrine requirement for hedgehog signalling in cancerNature200845540641018754008
- JohnsonRLRothmanALXieJHuman homolog of patched, a candidate gene for the basal cell nevus syndromeScience1996272166816718658145
- UndenABZaphiropoulosPGBruceKToftqardRStahle-BackdahlMHuman patched (Ptch) mRNA is overexpressed consistently in tumor cells of both familial and sporadic basal cell carcinomaCancer Res199757233623409192803
- DahmaneNLeeJRobinsPHellerPRuiz i AltabaAActivation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumoursNature19973898768819349822
- AszterbaumMRothmanAJohnsonRLIdentification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndromeJ Invest Dermatol19981108858889620294
- ReifenbergerJWolterMWeberRGMissense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous systemCancer Res199858179818039581815
- EpsteinEHBasal cell carcinomas: attack of the hedgehogNat Rev Cancer2008874375418813320
- ReifenbergerJWolterMKnobbeCBSomatic mutations in the Ptch, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomasBr J Dermatol2005152435115656799
- RobargeKDBruntonSACastanedoGMGDC-0449-a potent inhibitor of the hedgehog pathwayBioorg Med Chem Lett2009195576558119716296
- GrahamRALumBLCheetiSPharmacokinetics of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with locally advanced or metastatic solid tumors: the role of alpha-1-acid glycoprotein bindingClin Cancer Res2011172512252021300760
- GiannettiAMWongHDijkgraafGJIdentification, characterization, and implications of species-dependent plasma protein binding for the oral Hedgehog pathway inhibitor vismodegib (GDC-0449)J Med Chem2011542592260121438527
- GrahamRALumBLMorrisonGA single dose mass balance study of the Hedgehog pathway inhibitor vismodegib (GDC-0449) in humans using accelerator mass spectrometryDrug Metab Dispos2011391460146721602311
- WongHChenJZChouBPreclinical assessment of the absorption, distribution, metabolism and excretion of GDC-0449 (2-chloro-N-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(methylsulfonyl) benzamide), an orally bioavailable systemic Hedgehog signalling pathway inhibitorXenobiotica20093985086119845436
- YueQChenYHMulderTAbsorption, distribution, metabolism, and excretion of [14C]GDC-0449 (vismodegib), an orally active hedgehog pathway inhibitor, in rats and dogs: a unique metabolic pathway via pyridine ring openingDrug Metab Dispos20113995296521363998
- LoRussoPMJimenoADyGPharmacokinetic dose-scheduling study of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with locally advanced or metastatic solid tumorsClin Cancer Res2011175774578221753154
- LoRussoPMRudinCMReddyJCPhase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumorsClin Cancer Res2011172502251121300762
- KeatingGMVismodegib: in locally advanced or metastatic basal cell carcinomaDrugs2012721535154122788238
- AasiSSilkissRTangJYNew onset of keratoacanthomas after vismodegib treatment for locally advanced Basal cell carcinomas: a report of 2 casesJAMA Dermatol201314924224323426496
- AxelsonMLiuKJiangXUS Food and drug administration approval: vismodegib for recurrent, locally advanced, or metastatic Basal cell carcinomaClin Cancer Res2013192289229323515405
- YauchRLDijkgraafGJAlickeBSmoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastomaScience200932657257419726788
- DijkgraafGJAlickeBWeinmannLSmall molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistanceCancer Res20117143544421123452
- TherassePArbuckSGEisenhauerEAWandersJKaplanRSRubinsteinLNew guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of CanadaJ Natl Cancer Inst2000922051610655437
- SekulicAMigdenMROroAEEfficacy and safety of vismodegib in advanced basal-cell carcinomaN Engl J Med20123662171217922670903
- TangJ YMackay-WigganJMAszterbaumMInhibiting the hedgehog pathway in patients with the basal-cell nevus syndromeN Engl J Med20123662180218822670904
- ChangALOroAEInitial assessment of tumor regrowth after vismodegib in advanced Basal cell carcinomaArch Dermatol20121481324132522910979