Abstract
After decades without promising new treatments for advanced and metastatic melanoma, ipilimumab was the first systemic therapy approved for use in this patient population. A fully human monoclonal antibody that blocks cytotoxic T-lymphocyte antigen 4 (CTLA-4) to augment antitumor T-cell responses, ipilimumab significantly extended overall survival in clinical trials. Because ipilimumab is associated with a set of immune-related adverse events that likely reflect the agent’s mechanism of action, a management guide has been established. Nurses play a significant role in initially identifying these adverse reactions and assisting in patient education, treatment, and follow-up. Herein, we discuss commonly asked questions related to ipilimumab therapy and treatment of adverse events, and how nurses can be prepared to answer these questions as they arise from patients and caregivers.
Keywords:
Introduction
For patients diagnosed with unresectable stage III or IV (advanced) melanoma, historical benchmark data from a meta-analysis estimate a 25% 1-year survival rate that falls to approximately 15% by 5 years.Citation1,Citation2 Fortunately, several promising new agents have been US Food and Drug Administration (FDA)-approved for treatment of advanced or metastatic melanoma in recent years, or are late in clinical development. Among these new options is ipilimumab, a fully human monoclonal antibody that blocks cytotoxic T-lymphocyte antigen 4 (CTLA-4) to augment antitumor T-cell responses.Citation3,Citation4 Ipilimumab is FDA-approved in treatment-naïve and previously treated diseaseCitation5 on the basis of improved overall survival (OS) in two Phase III studies. Importantly, 19%–36% of ipilimumab-treated patients were still alive 4 years after study enrollment. These data suggest that ipilimumab provides an unprecedented extension of life in some patients who until recently had few effective options with manageable safety profiles.Citation2,Citation4,Citation6
Associated with ipilimumab are a set of treatment-related adverse events that are commonly referred to as immune-related adverse events (irAEs) because they are most likely tied to the agent’s immune-related mechanism of action.Citation3,Citation4,Citation7–Citation9 In clinical trials, most of these irAEs were mild to moderate, and most were reversible using a set of treatment guidelines that were developed for ipilimumab based on clinical experience with the drug. These guidelines emphasize vigilant follow-up and early use of corticosteroids when appropriate. Rarely, however, some irAEs can be severe, life-threatening, or irreversible despite immunosuppressive therapy.Citation4,Citation7
Nurses are often the first and most frequent point of contact for patients undergoing cancer treatment. It is therefore crucial that the full clinical management team, particularly nurses, is armed with all necessary information regarding management of patients, side effects, methods of infusion, and other critical aspects related to treatment. Since ipilimumab is a relatively novel treatment with a clinical profile that differs in some respects from those of traditional melanoma therapies, such as cytotoxics, education of this nature related to ipilimumab is particularly important and timely to provide to nurses. Therefore, the purpose of this review is to convey collective learning from ipilimumab clinical trials, case studies, and our own clinical experience to address commonly asked questions related to ipilimumab therapy. These questions include understanding the mechanism of action, efficacy, patient evaluation and follow-up, toxicity management, and patterns of response. We often hear questions on these topics from fellow nurses, but they may also originate from patients and caregivers.
Frequently asked questions: mechanism of action
The mechanism of action of ipilimumab differs from those of traditional chemotherapy or small-molecule inhibitors, which means that response kinetics may differ as well.Citation10,Citation11 Activation of the immune system begins when a T-cell receptor recognizes and binds a foreign compound, or antigen, that is presented on the surface of an antigen-presenting cell. This recognition generates an activation signal to the T-cell. To reinforce this initial activation signal, a costimulatory signal is then provided from the antigen-presenting cell (via the B7 family of molecules) to the T-cell (via the CD28 receptor). Conversely, to keep the activation signal in check and prevent overstimulation, the T-cell expresses a second receptor, CTLA-4, which also binds B7, but results in inhibition of the T-cell. The balance of these stimulatory and inhibitory signals determines whether the T-cell. is activated in response to the antigen or fails to respond (anergy) (). Preclinical and clinical research have revealed that in many types of cancers, tumors evade elimination by the immune system by tipping the balance toward anergy using a variety of mechanisms, some of which directly involve CTLA-4.Citation12 Thus, monoclonal antibodies that bind CTLA-4 were developed as anticancer therapies under the theory that through blockade of the CTLA-4-mediated inhibitory signal, the activity of T-cells may be activated against tumor antigens and their activity harnessed for treatment of cancer.Citation3,Citation13
Figure 1 (A and B) Role of CTLA-4 in T-cell responses and the impact of CTLA-4 blockade with ipilimumab. Ipilimumab mechanism of action (A) and “brake and pedal” analogy (B) as used to explain the mechanism to patients and caregivers.
Abbreviations: CTLA, cytotoxic T-lymphocyte antigen; APC, antigen-presenting cell; MHC, major histocompatibility complex; TCR, T-cell receptor.
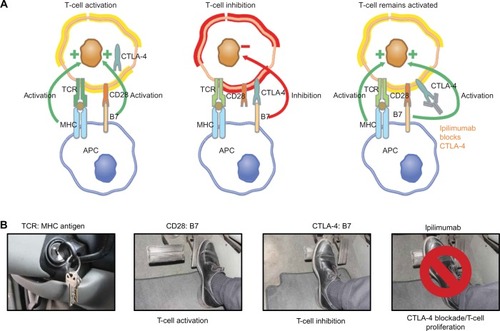
We are often asked by other nurses how to explain these challenging immunologic concepts and the mechanism of action of ipilimumab in terms that patients understand. One analogy we employ is to equate the patient’s immune system to a car ().Citation14 Depressing the accelerator (equivalent to activating T-cells) is necessary for the car to move forward (equivalent to a productive immune response against the tumor). Further, just as a car has a brake to keep forward motion in check, the body’s natural response to immune activation is to keep the immune response in check via engagement of inhibitory pathways, such as CTLA-4. Ipilimumab, by binding CTLA-4, is functioning to “lift the foot off the brake” so that the car can continue forward, ie, so that a T-cell-mediated antitumor immune response can continue. We find that through this analogy, patients can more easily understand the drug’s mechanism of action and are often encouraged by the idea that their own body is fighting their cancer. In addition, a basic understanding of how ipilimumab modulates the immune response makes it easier for patients to understand the risks of adverse events associated with ipilimumab, and it sets patient expectations about how rapidly they may respond to treatment. These topics are covered below.
Frequently asked questions: dose and schedule
Dose adjustments
The approved dose of ipilimumab is 3 mg/kg, administered intravenously over 90 minutes every 3 weeks for a total of four doses.Citation5 Fellow nurses or other health care professionals frequently inquire whether dose adjustments can be made. Of note, a higher dose of ipilimumab (10 mg/kg) is currently being studied, but is not FDA approved; in addition, lower doses have been evaluated as well. A Phase II dose-ranging study did show that the frequency of irAEs of any grade did increase with ipilimumab dose, and a Phase III study comparing the 3 mg/kg with the 10 mg/kg dose is ongoing.Citation15,Citation16 At present, therefore, use at doses other than 3 mg/kg is not recommended outside a clinical trial setting. To mitigate irAEs, the US prescribing information Risk Evaluation and Mitigation Strategy (REMS) for ipilimumab provides suggestions for when to withhold () or permanently discontinue () ipilimumab therapy based on the occurrence and severity of irAEs.Citation9 Furthermore, no dose adjustment of ipilimumab is required or routinely made for body weight after administration on a mg/kg basis,Citation5 and actual (not ideal) weight is used. The exception, as aligned with most institutional guidelines for cancer therapies, is made when a patient experiences a significant weight gain or loss during the course of therapy, and the dose may be recalculated in those instances.
Table 1 When to withhold ipilimumab
Table 2 When to permanently discontinue ipilimumab
Retreatment
As per the approved indication, ipilimumab is given as four doses every 3 weeks; this 12-week time frame was termed the “induction phase” in clinical studies. Following the induction phase, retreatment for qualifying patients was included in the protocols of some ipilimumab Phase II and III clinical trials. The rationale for these inclusions was based on the agent’s immune-related mechanism of action, ie, patients who initially experienced clinical benefit might experience reactivation of the immune system in response to further therapy, which may result in recognition and response to residual tumor cells.Citation17 In the Phase III registration trial, a subanalysis of patients that received retreatment upon disease progression showed ipilimumab further augmented durable objective responses and/or stable disease.Citation18 The safety profile observed during retreatment was similar to that observed during the induction phase of treatment.
The FDA does not contraindicate retreatment in the approved prescribing information, and the National Comprehensive Cancer Network guidelines state that retreatment with ipilimumab may be considered for patients who experienced no significant systemic toxicity during prior ipilimumab therapy, and who relapse after initial clinical response or progress after stable disease for more than 3 months.Citation19 Ongoing research will help to further optimize the schedule for ipilimumab administration.Citation20
Frequently asked questions: efficacy and response
An extensive clinical trial program has established ipilimumab efficacy in a percentage of patients with advanced or metastatic melanoma. The registration trial design included an active control group (n=136) of patients who received gp100 vaccine alone and compared it to those randomized to receive ipilimumab alone (n=137) or ipilimumab combined with the active control gp100 (n=403).Citation4 Median OS was significantly greater with the ipilimumab-gp100 combination compared with gp100 alone (10.0 months versus [vs] 6.4 months, hazard ratio [HR] 0.68; P<0.001). Median OS was also significantly greater with ipilimumab alone compared with gp100 alone (10.1 months vs 6.4 months, HR 0.66; P=0.003). One and 2-year OS rates for ipilimumab alone were 45.6% and 23.5%, respectively; for gp100 alone, 25.3% and 13.7%, respectively; and for ipilimumab plus gp100, 43.6% and 21.6%, respectively.Citation4
In a second Phase III study at the higher dose (10 mg/kg), OS was significantly longer for ipilimumab in combination with dacarbazine compared with dacarbazine plus placebo (11.2 months vs 9.1, respectively).Citation7 OS rates at 1, 2, and 3 years were also significantly higher for ipilimumab plus dacarbazine: these rates were 47.3%, 28.5%, and 20.8%, respectively; for placebo plus dacarbazine, they were 36.3%, 17.9%, and 12.2%, respectively. Survival data is also now available for 5 years postenrollment for patients in the Phase II studies of ipilimumab in advanced melanoma, further supporting the idea that ipilimumab provides a substantial long-term OS potential for some patients.Citation21 To reiterate, ipilimumab has been evaluated at 10 mg/kg and in combination with a variety of chemotherapies, targeted agents, and other immunotherapies, but those doses and schedules have not yet been approved by the FDA for use in patients.
Immunotherapies such as ipilimumab stimulate the patient’s immune system to mount an endogenous antitumor immune response ().Citation22 Because of this immune-related mechanism of action, responses to ipilimumab may differ from patient to patient, and it often takes a certain amount of time for patients to mount a response.Citation23 Among patients with metastatic melanoma who experienced a positive outcome from ipilimumab therapy in clinical trials, four patterns of response were observed ().Citation23 As with chemotherapy, patients treated with immunotherapy may demonstrate an immediate reduction in baseline lesions without the presence of new lesions. Ipilimumab has also yielded durable stable disease in some patients, and in some cases it has been followed by a slow, steady decline in total tumor burden. Some ipilimumab-treated patients may present with new lesions concurrently with a decrease in overall burden of disease. Occasionally, a positive tumor response has been observed in patients following an initial increase in total tumor burden.Citation23,Citation24 The idea that a tumor response is possible despite the appearance of new lesions or an initial increase in tumor burden may be unfamiliar to the medical team, and may be of concern to patients, if they are not prepared for this possibility.
Figure 2 (A–D) established patterns of response to ipilimumab.
Notes: Four patterns of response to ipilimumab include (A) response in baseline lesions, (B) stable disease, (C) response after initial increase in total tumor volume, and (D) reduction in total tumor burden after the appearance of new lesions. All four patterns of response have been observed in patients who have been treated with ipilimumab, and all have been associated with positive outcomes in patients. N, tumor burden of new lesions (C and D). (D) top line, total tumor burden; middle line, tumor burden of baseline lesions; bottom line, tumor burden of new lesions. Triangles, ipilimumab dosing time points; dashed lines, thresholds for response or PD/irPD. Reprinted from Clin Cancer Res, 2009, 15(23), 7412-7420, Wolchok JD, Hoos A, O’Day S, et al, Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria,Citation23 with permission from the American Association for Cancer Research (AACR). Copyright © 2009.
Abbreviations: irPD, immune-related progressive disease; SPD, sum of the product of perpendicular diameters.
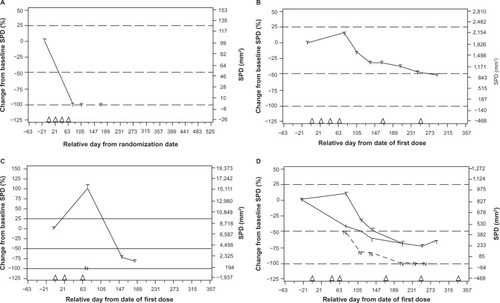
We are frequently asked by our nurse colleagues how to determine the difference between delayed response and progression. In our practices, we base therapeutic decisions on multiple factors that include patient labs and overall clinical performance. If following ipilimumab therapy, a patient exhibits radiographic progression but their labs are stable, especially lactate dehydrogenase (LDH), and they generally feel well or feel they are improving, we may hold to monitor for delayed response with another scan in about 4–6 weeks. Conversely, if the labs are worsening, LDH is increasing, and the patient is physically deteriorating, then nurses note the suspected progression, deferring to the treating physician to make further therapeutic decisions.
This decision-making process related to ipilimumab differs from the typical experience with chemotherapy or targeted therapy. In fact, because beneficial responses may be atypical, the most appropriate end point to evaluate responses to ipilimumab is still a subject of debate across the oncology community.Citation23,Citation24 At present, however, nurses need to understand the interrelatedness of ipilimumab’s mechanism of action and efficacy, and the symptoms that the patient may experience during therapy, and be prepared to answer questions from patients and caregivers. For example, palpable tumors may become more painful; change in color, warmth, or tenderness; or increase in size during treatment with ipilimumab. When patients report these phenomena, we explain that they could be related to a T-cell-mediated attack on the tumors, and that this will be confirmed through continued monitoring for a subsequent decrease in tumor size or other response. In short, we counsel patients and caregivers on the immunologic mechanism of action of ipilimumab as a method of helping to explain treatment-related changes that patients may experience. Such counsel also appears to relieve some concerns around why immediate tumor regression may not occur in every patient who receives therapy. It also supports the rationale for waiting until week 12 for the initial scan as per guidelines, or possibly longer in some patients, to allow time for the immune system to mount a response to tumors.
Frequently asked questions: identification and management of ipilimumab-associated toxicities
Kinetics of onset and resolution of irAEs
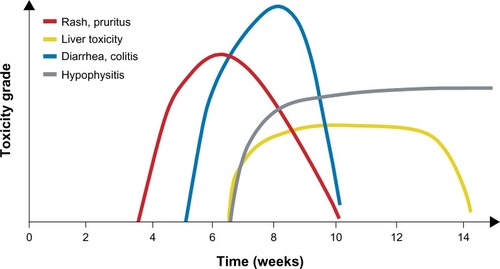
As already noted, most adverse reactions to ipilimumab are inflammatory in nature.Citation4,Citation7–Citation9 The most common irAEs affect the skin (rash, pruritus) or gastrointestinal system (GI; diarrhea, nausea, abdominal pain); endocrine, hepatic, and neurologic events occur with less frequency ().Citation4,Citation7,Citation25 Individual clinical studies and a pooled analysis across the ipilimumab clinical program showed most irAEs were reported during the induction dosing period.Citation3,Citation4,Citation7,Citation25 Median time to onset of irAEs was approximately 5–9 weeks, depending on dose of ipilimumab and organ class affected ().Citation8,Citation26 Skin reactions seem to appear first, on average within the first couple of weeks of treatment, with GI reactions following shortly afterward. Endocrinopathies, in contrast, can occur late in the induction treatment phase or even weeks or months following the last dose of ipilimumab. Despite these observed trends, the clinical team should remain vigilant for irAEs throughout treatment and in the weeks or months afterward, and instruct patients to do the same. As part of this vigilance, nurses should continue to follow-up with patients even after they have completed ipilimumab therapy. Events managed as per the treatment guidelines were generally resolved within 4–8 weeks.Citation25,Citation26
Table 3 Frequency of immune-related adverse events (irAEs) in pooled analysis across Phase I–III ipilimumab clinical trials
Figure 3 Average time to onset of adverse events associated with ipilimumab. Kinetics of appearance of immune-related adverse events by organ class over time.
Note: Reprinted with permission. © 2012 American Society of Clinical Oncology. All rights reserved. Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–2697.Citation8
Education and testing before treatment
The nursing team plays a critical role in continuously educating patients and caregivers to alleviate fears and misconceptions that may arise before, during, and after treatment. As part of this role, nurses should prepare patients for what to expect and what to look for in terms of signs and symptoms of irAEs, and emphasize that early reporting of possible irAEs and prompt intervention increases the likelihood that the patient can continue to receive the drug on schedule, maximizing the chances that he or she will benefit from therapy.
It is recommended that nurses provide a questionnaire for patients to answer prior to each infusion, or verbally cover a series of questions on signs and symptoms (). Information in the responses can be used to identify and help guide discussions between nurses and patient/caregivers. It can often help distinguish between specific irAEs, eg, whether the diarrhea that a patient is experiencing might be associated with a GI or an endocrine toxicity. It can also capture a potential irAE promptly, so that with early intervention, higher-grade adverse events may be prevented.
Figure 4 Common signs and symptoms of immune-related adverse events (irAEs). Signs and symptoms nurses should monitor for at every patient visit to assist in early identification of irAEs associated with ipilimumab. Ipilimumab [US prescribing information]: risk evaluation and mitigation strategy (REMS). Bristol-Myers Squibb. 2012. Available from: http://www.yervoy.com/hcp/rems.aspx. Accessed July 12, 2013.Citation9
Abbreviation: GI, gastrointestinal.
![Figure 4 Common signs and symptoms of immune-related adverse events (irAEs). Signs and symptoms nurses should monitor for at every patient visit to assist in early identification of irAEs associated with ipilimumab. Ipilimumab [US prescribing information]: risk evaluation and mitigation strategy (REMS). Bristol-Myers Squibb. 2012. Available from: http://www.yervoy.com/hcp/rems.aspx. Accessed July 12, 2013.Citation9Abbreviation: GI, gastrointestinal.](/cms/asset/a46a3dfb-4d00-4610-adbd-2a67526e9b7c/dcmr_a_52543_f0004_c.jpg)
Important labs at baseline and prior to each infusion include full thyroid-function (thyroid-stimulating hormone, free triiodothyronine and thyroxine, cortisol, adrenocorticotropic hormone, testosterone, luteinizing hormone, follicle-stimulating hormone) and liver-function tests, as recommended by the FDA.Citation5 Additionally, testing for LDH levels may help to discern how patients are trending. In some cases, lipase and amylase may be drawn to rule out pancreatitis. These data will help establish the patient’s baseline and later inform whether an antitumor immune reaction is occurring.
Management during and after treatment
Any clinic administering ipilimumab should have an established management protocol in place to mitigate irAEs, and there is a wealth of information from the ipilimumab REMS and other published clinical trial data to inform the teams.Citation3,Citation4,Citation7,Citation9,Citation25 Most low-grade events can be managed symptomatically, eg, creams and oatmeal baths may alleviate very minor skin reactions, and over-the-counter treatments may help manage low-grade diarrhea but should only be taken under medical supervision. Persistent or higher-grade events require prompt intervention with corticosteroid therapy, which should be started as soon as the patient reports higher-grade or recurrent problems in accordance with established guidelines in the REMS; ipilimumab may need to be withheld or permanently withdrawn in some circumstances ( and ).Citation9 Upon improvement to grade 1 or baseline, corticosteroids should be tapered over at least 1 month. In some patients, referral to an endocrinology or GI specialist may be necessary to assist in the management of certain toxicities. As previously mentioned, nurses have the greatest contact with the patients, and may likely be the ones that recognize the need for referral and inform the physician.
Individualized follow-up and counseling
To ensure adequate and personalized management takes place, our practice has established a system of daily telephone communication between nurses and the patients experiencing irAEs (). We recommend that our colleagues consider implementing similar systems that align with the needs of their individual practices. Such a system should track the daily patient point of contact in a way that is easily accessible to all (eg, a whiteboard). Updated with this information, the team may recommend increased office visits between doses to monitor patients and draw labs for patients who are experiencing an irAE. We also recommend special preparation for patients traveling out of state or the country (). These proactive management steps may prevent escalation of mild irAEs to more serious events that are more likely to require emergency room visits. Furthermore, prompt management and resolution of irAEs may allow patients to continue or resume ipilimumab therapy where appropriate.
Discussion
Ipilimumab has demonstrated the potential to extend life in patients with unresectable or metastatic melanoma, a disease that until recently has had a poor prognosis. Moreover, this agent has shown efficacy in patients with brain metastases and other characteristics associated with poorer prognosis.Citation4 Most patients will experience an irAE during their course of treatment with ipilimumab,Citation25 and these toxicities require decision-making and management activities that may be somewhat unfamiliar to the health care team. Newer, more effective oncology therapies like ipilimumab are becoming more widely used, and to ensure these therapies meet their full therapeutic potential, it is essential that nurses and other members of the management team be thoroughly educated about appropriate use, patterns of response, safety, and administration. This education will in turn give nurses the knowledge and confidence to educate their patients. Not only does this process help relieve patients’ and caregivers’ anxieties but it also facilitates timely identification and prompt treatment of irAEs. It is our hope that the information provided in this review will further these efforts and serve as a foundation for broader nursing education programs at hospitals or clinics.
Acknowledgment
The authors take full responsibility for the content of this publication and confirm that it reflects their viewpoints and medical expertise. The authors wish to acknowledge StemScientific, funded by Bristol-Myers Squibb, for providing writing and editorial support. Neither Bristol-Myers Squibb nor StemScientific influenced the content of the manuscript, nor did the authors receive financial compensation for authoring the manuscript.
Disclosure
The authors have no conflicts of interest to disclose.
References
- KornELLiuPYLeeSJChapmanJAMeta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trialsJ Clin Oncol200826452753418235113
- AltekruseSFKosaryCLKrapchoMSEER Cancer Statistics Review, 1975–2007Bethesda, MDNational Cancer Institute2010 Available from: http://seer.cancer.gov/csr/1975_2007Accessed September 22, 2011
- HoosAIbrahimRKormanADevelopment of ipilimumab: contribution to a new paradigm for cancer immunotherapySemin Oncol201037553354621074069
- HodiFSO’DaySJMcDermottDFImproved survival with ipilimumab in patients with metastatic melanomaN Engl J Med2010363871172320525992
- Bristol-Myers SquibbYervoy (ipilimumab) [package insert]Princeton, NJBristol-Myers Squibb2011
- AgarwalaSSCurrent systemic therapy for metastatic melanomaExpert Rev Anticancer Ther20099558759519445576
- RobertCThomasLBondarenkoIIpilimumab plus dacarbazine for previously untreated metastatic melanomaN Engl J Med2011364262517252621639810
- WeberJSKählerKCHauschildAManagement of immune-related adverse events and kinetics of response with ipilimumabJ Clin Oncol201230212691269722614989
- Bristol-Myers SquibbIpilimumab US prescribing information: risk evaluation and mitigation strategy (REMS)2012 Available from: http://www.yervoy.com/hcp/rems.aspxAccessed July 12, 2013
- DunnGPOldLJSchreiberRDThe immunobiology of cancer immunosurveillance and immunoeditingImmunity200421213714815308095
- MeleroIHervas-StubbsSGlennieMPardollDMChenLImmunostimulatory monoclonal antibodies for cancer therapyNat Rev Cancer2007729510617251916
- GrossoJFJure-KunkelMNCTLA-4 blockade in tumor models: an overview of preclinical and translational researchCancer Immun201313523390376
- BoasbergPHamidOO’DaySIpilimumab: unleashing the power of the immune system through CTLA-4 blockadeSemin Oncol201037544044921074058
- LedezmaBAtypical clinical responses to immunotherapy in patients with advanced melanomaPoster presented at: Oncology Nursing Society 36th Annual CongressApril 28–May 1, 2011Boston, MA
- WolchokJDNeynsBLinetteGIpilimumab monotherapy in patients with pretreated, advanced melanoma: a randomised, doubleblind, multicentre, phase 2, dose-ranging studyLancet Oncol201011215516420004617
- Bristol-Myers SquibbPhase 3 trial in subjects with metastatic melanoma comparing 3 mg/kg ipilimumab versus 10 mg/kg ipilimumab Available from: http://clinicaltrials.gov/ct2/show/NCT01515189. NLM identifier: NCT01515189Accessed July 12, 2013
- BhardwajNHarnessing the immune system to treat cancerJ Clin Invest200711751130113617476342
- RobertCSchadendorfDMessinaMHodiFSO’DaySEfficacy and safety of retreatment with ipilimumab in patients with pretreated advanced melanoma who progressed after initially achieving disease controlClin Cancer Res20131982232223923444228
- National Comprehensive Cancer NetworkNCCN guidelines version 2. 2013 updates – melanoma2012 Available from: http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdfAccessed July 12, 2013
- Bristol-Myers SquibbStudy to compare the effect of ipilimumab retreatment with chemotherapy in advanced melanoma Available from: http://www.clinicaltrials.gov/ct2/show/NCT01709162. NLM identifier: NCT01709162Accessed July 12, 2013
- LebbeCWeberJSMaioMFive-year survival rates for patients (pts) with metastatic melanoma (MM) treated with ipilimumab (IPI) in phase II trialsPoster presented at: 37th European Society of Medical Oncology (ESMO) CongressSeptember 28–October 2, 2012Vienna, Austria
- DouganMDranoffGThe immune response to tumorsCurr Protoc Immunol2009 Chapter 20: Unit 20.11
- WolchokJDHoosAO’DaySGuidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteriaClin Cancer Res200915237412742019934295
- HoosAEggermontAMJanetzkiSImproved endpoints for cancer immunotherapy trialsJ Natl Cancer Inst2010102181388139720826737
- IbrahimRABermanDMde PrilVIpilimumab safety profile: summary of findings from completed trials in advanced melanomaJ Clin Oncol201129Suppl8583
- DummerRMaioMHamidOTime to onset and resolution of immune-related adverse events associated with ipilimumab therapy in patients with advanced melanomaPoster presented at: Perspectives in Melanoma XIVSeptember 17–18, 2010Amsterdam, The Netherlands


