Abstract
Colorectal cancer (CRC) is the third-ranked neoplasm in order of incidence and mortality, worldwide, and the second cause of cancer death in industrialized countries. One of the most important environmental risk factors for CRC is a Western-type diet, which is characterized by a low-fiber and high-fat content. Up to 25% of patients with CRC have a family history for CRC, and a fraction of these patients are affected by hereditary syndromes, such as familial adenomatous polyposis, Gardner or Turcot syndromes, or hereditary nonpolyposis colorectal cancer. The onset of CRC is triggered by a well-defined combination of genetic alterations, which form the bases of the adenoma-carcinoma sequence hypothesis and justify the set-up of CRC screening techniques. Several screening and diagnostic tests for CRC are illustrated, including rectosigmoidoscopy, optical colonoscopy (OC), double contrast barium enema (DCBE), and computed tomography colonography (CTC). The strengths and weaknesses of each technique are discussed. Particular attention is paid to CTC, which has evolved from an experimental technique to an accurate and mature diagnostic approach, and gained wide acceptance and clinical validation for CRC screening. This success of CTC is due mainly to its ability to provide cross-sectional analytical images of the entire colon and secondarily detect extracolonic findings, with minimal invasiveness and lower cost than OC, and with greater detail and diagnostic accuracy than DCBE. Moreover, especially with the advent and widespread availability of modern multidetector CT scanners, excellent quality 2D and 3D reconstructions of the large bowel can be obtained routinely with a relatively low radiation dose. Computer-aided detection systems have also been developed to assist radiologists in reading CTC examinations, improving overall diagnostic accuracy and potentially speeding up the clinical workflow of CTC image interpretation.
Colorectal cancer: epidemiology and risk factors
Colorectal cancer (CRC) is the third-ranked neoplasm in order of incidence and mortality worldwide.Citation1 It is more frequent in industrialized countries, where it is the second cause of cancer death and is a social and health care issue of major importance.Citation1,Citation2
In 2009, 146,970 new cases of CRC and 49,920 CRC-related deaths occurred in the United States.Citation3,Citation4 In the European Union, approximately 220,000 new CRC cases per year are estimated to be diagnosed annually.Citation5
The likelihood of developing CRC increases from the second to the ninth decade. CRC is rare before the age of 40 years, and most cases are diagnosed after the age of 50. The incidence of CRC is slightly higher in males than in females, especially for rectal carcinoma. In developing countries CRC has a lower, yet growing incidence.Citation1 Although the etiology of CRC is still unknown, epidemiological and biomolecular studies have unveiled environmental and genetic risk factors that favor the onset of CRC.Citation3
One of the most important environmental risk factors for CRC is a Western-type diet, which is characterized by a low fiber and high fat content. CRC-related mortality is directly correlated with per-person calorie intake, high consumption of meat proteins and fat, and high blood cholesterol levels. Conversely, in African populations, in which consumption of fruit and vegetables is higher than in Western countries, the incidence of CRC is much lower. Moreover, it has been observed that people who have migrated to industrialized countries tend to acquire the same CRC incidence rates of those countries, supporting the hypothesis that geographic differences in CRC incidence are due to dietary habits rather than different genetic patterns.Citation6
Research has shown that up to 25% of patients with CRC have inherited mutations that increase their risk for CRC. A fraction of these patients are affected by well-characterized hereditary syndromes, which can be classified as polyposis and nonpolyposis CRC:
Familial adenomatous polyposis (FAP; also known as familiar polyposis of the colon) is a rare autosomal dominant condition that causes the growth of hundreds or thousands of adenomatous polyps in the large bowel. It is associated with a deletion in the long arm of chromosome 5, which contains the APC oncosuppressor gene. CRC develops in almost all affected patients under 40 years. There is a classic form (in which patients typically harbor between 500 and 2500 colonic adenomas) and an attenuated form of FAP, characterized by a lower number of polyps (typically 30) that are predominantly located in the proximal colon. Affected patients may also have associated extracolonic diseases, such as desmoid tumors and gastroduodenal carcinomas.Citation7
Gardner syndrome is characterized by a combination of intestinal polyposis (identical to classic FAP) and numerous osteomas, epidermal cysts, fibromatosis, as well as thyroid and duodenal carcinomas and dental abnormalities.
Turcot syndrome is a rare condition characterized by the association of FAP and tumors of the central nervous system (such as medulloblastoma and glyoblastoma).Citation3
Hereditary nonpolyposis colorectal cancer (HNPCC, also known as Lynch syndrome) is an autosomal dominant hereditary syndrome, associated with germline mutations in genes that result from DNA repair mechanisms (in particular, MSH2, MLH1, PMS, and PMS2), causing microsatellite DNA instability and impaired mismatch repair, and leading to higher risk of cancer development. A strong association exists between HNPCC and endometrial and ovarian carcinomas.Citation8 In HNPCC patients, CRC typically occurs earlier than 50 years, that is, 10 to 15 years before the average onset of CRC in the overall population. Moreover, CRC in HNPCC patients is more frequently located in the cecum or ascending colon.Citation3,Citation9
Chronic inflammatory bowel diseases: CRC is more frequent in patients with ulcerative colitis, in whom the absolute risk of cancer increases at a rate of 0.5% to 1% per year after 10 years of disease, with cancer development typically in 8% to 30% of cases after 25 years from disease onset. Conversely, in patients with Crohn disease, CRC risk is not significantly higher than in the normal population.Citation3
Other conditions that increase the risk for CRC development are:
Bacteremia related to Streptococcus bovis: for unknown reasons, individuals with a history of S. bovis-induced endocarditis or sepsis have a higher incidence of CRC.Citation3
Tobacco smoke, which is associated with the development of colorectal adenomas: several studies have investigated the potential procarcinogenetic role of tobacco smoke, and a dose–response relationship with CRC has been found for cigarette pack-years, smoking duration, smoking intensity, smoking history in the distant past, and younger age at initiation of smoking.Citation10,Citation11
Alcohol abuse: epidemiological studies have demonstrated that another risk for CRC could arise from the malnutrition caused by lower folate concentrations in heavy alcohol drinkers. However, recent studies have yielded inconsistent findings concerning such a relationship.Citation12–Citation14 Despite multiple risk factors increasing the likelihood of
CRC development, the majority of individuals (about 75%) who develop CRC do not have specific risk factors.Citation15 Therefore, the population should be stratified into averagerisk individuals (age greater than 50 years, no personal risk factors, no familial history of CRC) and those with moderate (first-degree relative with a history of adenoma or carcinoma, or personal history of large adenoma or carcinoma) and high CRC risk (inflammatory bowel disease or a family history of an inherited CRC syndrome).Citation16,Citation17
The adenoma-carcinoma sequence
The onset of CRC is triggered by a well-defined sequence of genetic alterations that form the etiological basis of the adenoma-carcinoma sequence hypothesis and justify the rationale for CRC screening. The validity of this hypothesis is supported by several findings:Citation18
Populations with a high incidence of adenomas have a high prevalence of CRC, and vice versa.
There is a substantial overlap between the topographic distribution of colorectal adenomas and that of CRC.
The incidence peak of adenomatous polyps precedes that of CRC by some years.
The risk of CRC is directly proportional to the number of adenomas, and the substantial certainty of developing CRC in patients with FAP is an extreme case of this rule.
Follow-up programs to detect adenomas with resection of suspected lesions are associated with a reduced incidence of CRC.
Polyps with the highest risk of neoplastic degeneration are those characterized by a large size (≥20 mm), villous histology, and sessile morphology. The time of progression from adenomatous polyp to carcinoma is about 10 years.
More than 90% of CRCs arise from adenomatous polyps,Citation19 while a small fraction of CRCs develop without evidence of an adenomatous precursor, suggesting that some lesions may undergo malignant transformation without passing through a polypoid intermediate phase. Evidence exists that polypectomy can reduce CRC incidence and mortality by interrupting the adenoma-carcinoma sequence in the colonic segment where the polyp has been excised.Citation20
From a genetic point of view, carcinogenesis is characterized by the accumulation of multiple, consecutive mutations in oncogenes and oncosuppressor genes, starting the adenoma-carcinoma sequence at the level of the colonic mucosa. The genes most often involved (and the most widely known) are APC, k-RAS, p53, SMAD2, and SMAD4. Moreover, mutations involved in DNA mismatch repair may account for the development of 10% to 15% of sporadic CRC and HNPCC.Citation18
CRC screening
The knowledge of the biological mechanisms underlying CRC carcinogenesis and their slow progression over time make this neoplasm ideal for the development of screening programs. Furthermore, CRC is a common disease with a high mortality rate, for which risk conditions can be detected and treatment is more effective in early than in advanced disease stages.
To be effective, screening needs to be performed on a large population, and be sensitive, specific, and well tolerated by patients. Furthermore, it is essential that effective treatment is available once diagnosis has been made. Finally, screening must be cost-effective.
Based on the guidelines of the American Cancer Society, techniques currently available for the secondary prevention of CRC (after the age of 50 years) can be classified as follows:Citation21
Tests for detecting polyps and CRC:
Flexible rectosigmoidoscopy (every 5 years);
Optical colonoscopy (OC, every 10 years);
Double contrast barium enema (DCBE, every 5 years);
Computerized tomography colonography (CTC, also known as virtual colonoscopy: every 5 years).
Tests that can make CRC diagnosis:
Fecal occult blood test (FOBT, every year);
Fecal immunochemical tests (every year);
Fecal DNA test (uncertain time interval).
It must be pointed out that individuals with an increased/ high CRC risk should undergo screening with a higher frequency based on their individual risk level. In Western countries, the most common and readily available tests for CRC screening are rectosigmoidoscopy, OC, and FOBT. Since the introduction of FOBT as a CRC screening test in the 1990s, CRC mortality has been reduced; however, the main limitations of FOBT are a low specificity and a low sensitivity in polyp detection (about 10%), as polyps seldom manifest with bleeding, although, for this same reason, immunochemical FOBT has a sensitivity and specificity of 95% for detection of advanced colonic neoplasms.Citation22
Therefore, it is necessary to shift attention earlier along the adenoma-carcinoma sequence: this task is accomplished by rectosigmoidoscopy and colonoscopy (either optical or virtual).
The rationale for rectosigmoidoscopy (also referred to as left colonoscopy if exploration is extended to the descending colon) is related to different frequency of CRC in the various colonic segments; in fact, CRC arises in the rectum and sigmoid colon in 55% of cases, in the descending colon in 6% of cases, in the transverse colon in 11% of cases, and in the cecum/ascending colon in 22% of cases.Citation18 One limitation of this technique is, of course, the lack of evaluation of the entire colorectum, that may cause a considerable number of colonic adenomas and carcinomas to be missed. In recent years proximal colon CRC has been reported more frequently, especially in elderly individuals and females. For those reasons, whenever a colonic mass, or an advanced adenoma, or 3 or more adenomas, or a villous polyp larger than 10 mm is found, rectosigmoidoscopy must be followed by complete colonoscopy.Citation17
OC is today regarded as the gold standard technique for CRC screening, because it allows lesion detection with both high sensitivity (90%–100% also for small size polyps, ie, less than 6 mm diameter) and high specificity. Moreover, like recto-sigmoidoscopy, OC is not only useful for diagnostic purposes, but has also a therapeutic role as it enables polypectomy by means of a diathermic loop or hot bioptic pliers (hot biopsy), allowing lesion removal and biopsy. It is usually performed with light sedation or conscious sedation and therefore requires an in-patient setting. However, the main limitation of OC is patient intolerance, which can be due either to anatomical conditions (for example, presence of short colonic mesentery or a redundant colon may cause pain with progression of the OC probe), adhesions, or neoplastic and/or fibrotic narrowing/ obstruction of the bowel lumen. Other limitations of OC are the need for a cathartic preparation for optimal bowel cleansing, its high cost, and invasiveness. Moreover, some lesions of the colonic mucosa can be missed even by OC.Citation23 Finally, OC is not completely free from potentially life-threatening complications, such as bowel perforation or bleeding.
DCBE is often used whenever conventional endoscopy fails to explore the entire colon, eg, because of patient intolerance and/or inability to reach the cecum on OC (which is not uncommon in cases of redundant bowel) (). Compared with OC, DCBE has the advantage of less invasiveness, and therefore it is usually better tolerated by patients. However, the visualization of colonic lesions is heavily dependent on the radiographic projections made to obtain DCBE images, and therefore polyps (especially smaller ones) could be masked by extracolonic structures or even by nearby colonic segments or folds, thus causing DCBE to be less sensitive than OC. Sensitivities as low as 42% for 10 mm polyps have been reported.Citation24,Citation25
Figure 1 Large carcinoma of the cecum (red circle) as displayed on double contrast barium enema image. Lesion presence is inferred indirectly as a filling defect of the cecal lumen with irregular mucosal lining.
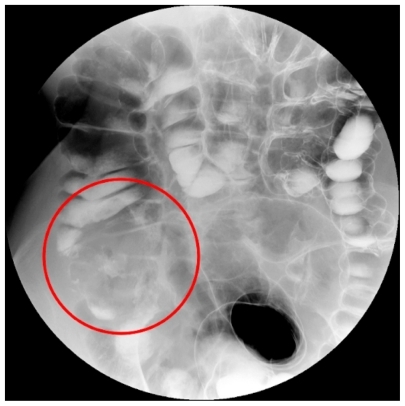
Another important issue of DCBE is radiation dose, that can be quite unpredictable due to the different radiographic technique used for each patient (for example, particular anatomic conditions and/or the different time and maneuvers needed to ensure arrival of barium and air up to the cecum), but it tends to be quite high, in the order of 5 to 8 mSv. Larger patients usually require even higher radiation doses to be imaged, owing to their higher X-ray absorption. This may be a serious limitation in case of younger patients and especially for young females, who are more sensitive to ionizing radiation due to the presence of gonads inside the abdomen.
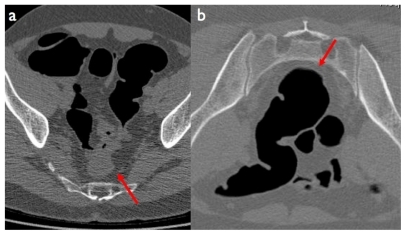
CTC has recently been included by the American Cancer Society among tests recommended for CRC screening, and has evolved from an experimental technique to a universally recognized, accurate, and validated diagnostic tool that is gaining widespread use and acceptance among both patients and referring clinicians. It is based on the acquisition of spiral CT data of the abdomen after adequate distention of the colon through rectal insufflation of gas (either air or carbon dioxide).Citation26–Citation28 The CT acquisition is usually performed twice: once in the supine position and then in the prone position (or vice versa): this is to optimize distention of the various colonic segments depending on gravitational compression by the surrounding abdominal structures, as well as to distinguish polyps (that are fixed to the bowel walls) from fluid and/or fecal residues (that tend to fall down due to gravitation) ().
Figure 2 Computed tomography colonography image in the supine a) and prone b) position. In the supine position the collapsed sigmoid colon may mimic cancer, while on the prone position the bowel walls and the lumen are shown to be normal (red arrows).
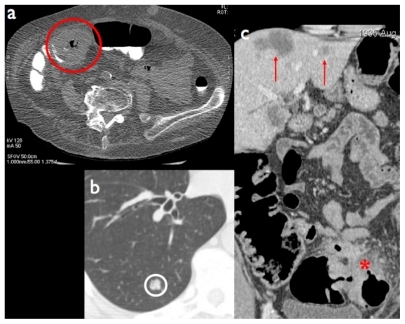
As CTC image formation is based on the X-ray attenuation of low-density, high-intrinsic-contrast objects (such as the air contained in the colonic lumen vs the large bowel walls, acting as an interface between intraluminal air and the extraluminal compartment), low X-ray energy is sufficient to achieve diagnostic CTC images, resulting in a low radiation dose. In other terms, if CTC is aimed at the sole examination of the colon (eg, for CRC screening purposes), use of low-radiation-dose CT acquisition protocols is warranted. Conversely, regular-dose CT protocols will be used if CTC is part of a CT examination in which all abdominal organs are to be investigated. This is the case, for example, of CTC in patients with known CRC and incomplete OC, in whom CT plays a role both for complete assessment of the colonic lumen and for oncological staging (including search of lymph node and liver metastases, or peritoneal carcinomatosis) ().
Figure 3 a) Annular stenosing cancer of the right colonic flexure (red circle) using a low dose computed tomography colonography (CTC) protocol. b, c) CTC can be performed with a regular dose protocol for detection of extracolonic disease, such as lung (b: white circle) and liver metastases (c: red arrows) in a patient with locally advanced colorectal cancer (red asterisk).
For adequate colonic distention to be achieved, air or carbon dioxide is usually delivered into the patient’s colon by means of a thin rectal catheter prior to CTC data acquisition. Insufflation can be accomplished either manually or via automatic insufflators, which allow a continuous and reliable measurement of the volume and pressure of insufflated gas. Air has the advantage of no cost and ease of administration, but is less tolerated because it is not absorbed by the colonic mucosa. Conversely, carbon dioxide is more comfortable (after completion of the exam) as it is gradually absorbed by the colonic walls, although larger volumes must be supplied compared with air. In practical terms, administration of 1 to 1.5 L of air or 3 to 4 L of carbon dioxide is usually sufficient.Citation28
Colonic distention is also favored by parenteral administration of spasmolytic agents, such as glucagon or hyoscine-N-butyl bromide, which inhibit peristalsis and reduce the tone of the parietal musculature. Such agents are usually administered intramuscularly or intravenously prior to insufflation. Contraindications to hyoscine-N-butyl bromide are glaucoma, prostatic hypertrophy, and heart rhythm disorders, whereas glucagon is contraindicated in patients with elevated blood glucose levels. In the United States (where glucagon is the only option) most experts have stopped using it.
By orally administering positive contrast material into the large bowel (barium or iodine), fecal and fluid tagging can be performed, helping to distinguish fecal/fluid residues from parietal polyps. Tagged residual fluid can then be electronically removed from CTC images by means of dedicated software. Research is in progress on subtracting solid tagged stool in patients who undergo no cathartic cleansing.
In contrast with the 2D plain film nature of DCBE images, one great advantage of CTC is the fact that cross-sectional images of the abdomen are acquired, which allows analysis of the various anatomical structures (eg, colonic lumen and content vs bowel walls, and the several components of the extracolonic compartment) without the superimposition of the surrounding organs and tissues. CTC also allows quantitative measurements of CT densities (such as that of fat for the normal ileo-cecal valve, or parenchymal density for extracolonic tissues or colonic lesions), as well as quantitative geometrical information, such as distances, areas, or volumes, without the projective distortion that is typical of radiographic images.
In order to achieve a high spatial resolution (which is essential for detection of small polyps and for high quality 2D and 3D image processing), thin slice acquisition is mandatory for CTC examinations. With current multidetector row CT equipment (64 detector rows and beyond), the entire abdomen can be scanned in less than 10 seconds with submillimeter slice thickness, resulting in voxel isotropy and elimination/ minimization of motion artifacts.Citation29
2D and 3D image reconstructions are necessary components of any CTC exam, as they allow assessment of large bowel anatomy on multiple planes (either orthogonal [axial, coronal, and sagittal planes], or user-defined oblique planes), as well as provision of a volumetric depiction of normal anatomy and polyp morphology. 3D reconstructions enable accurate quantification of polyp volume, which can be helpful in a follow-up to assess growth of the polyp. With modern multidetector CT equipment and powerful image processing workstations, 2D (multiplanar reformation) and 3D reconstructions (volume rendering) are now part of the routine diagnostic workflow of CTC. It is also possible to perform volume rendering from an intraluminal perspective, thus simulating endoscopic navigation (virtual endoscopy), or to ‘open up’ the colon and generate dissected views (virtual dissection) ( and ).
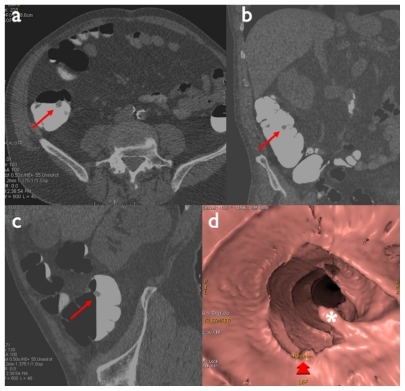
Figure 4 Sessile polyp of the ascending colon: a) native axial image (red arrow), b) virtual endoscopic view (red asterisk).
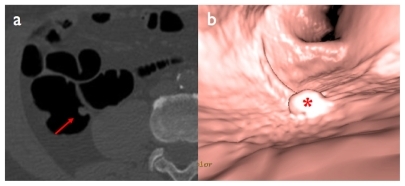
Figure 5 Pedunculated polyp of the ascending colon: a) native axial image, b) coronal reformation, c) sagittal reformation (red arrows), d) virtual endoscopic view (white asterisk).
In the systematic review by Halligan et al in 2005,Citation30 who assessed 24 studies for a total of 4181 patients, average CTC sensitivity for large polyp (1 cm or above) detection was 93% with an average specificity of 97%. For large and medium size polyps (6 mm or above), average sensitivity and specificity were both 86%, while for polyps of all sizes, sensitivity ranged from 45% to 97% and specificity from 26% to 97%. CTC sensitivity for detection of CRC was 96%.Citation30
In another systematic review of the literature by Mulhall et al,Citation31 results were substantially similar to those by Halligan et al.Citation30 In summary, CTC has been shown to be highly sensitive for CRC detection, as well as highly specific for detection of small size polyps, which makes it suitable as a CRC screening tool.Citation32,Citation33
CTC vs DCBE: radiation dose issues
Few studies have compared the radiation dose of CTC and DCBE.Citation34,Citation35 Hirofuji et alCitation34 measured a DCBE effective dose value of 12.7 mSv (decreasing by 12% when digital radiography equipment was used), while an effective dose of CTC performed with a low-dose protocol was 5.7 mSv. Neri et al managed to perform successful CTC examinations for CRC screening purposes with an even lower radiation dose (2.2 mSv) by using a combination of low exposure settings and tube current modulation.Citation36 The lower radiation dose of CTC compared with DCBE is due to the fact that with CTC, fluoroscopy is not needed and data acquisition is performed in the supine and prone positions only. Single-acquisition protocols are currently discussed (eg, in the pediatric population), which would actually halve radiation exposure.Citation37
According to the radiological risk figures of the International Commission on Radiological Protection,Citation38 an average effective dose value of around 2 mSv for CTC is associated with a theoretical 0.005% risk of lethal radiation-induced malignancy, which further decreases with increasing patient age. Thus, CTC is an important CRC screening tool especially in middle-aged and elderly patients, in whom the potential benefit of early diagnosis of CRC far outweighs the risk associated with radiation exposure.
Computer aided diagnosis
Computer aided diagnosis (CAD) tools are software applications designed for assisting the radiologist in the diagnosis of several conditions; for detection of lung nodules on chest X-ray or CT, breast nodules in screening mammography, and polyps in CT colonography.Citation39–Citation41
As CTC continues to evolve and improve, its use is shifting from highly specialized academic centers to community hospitals and nonacademic radiology practices.Citation42,Citation43 Thus, many radiologists are experiencing pressure from clinical colleagues to offer CTC as part of the routine services provided in their practices.
In parallel, modern multidetector CT scanners can generate data with unprecedented spatial resolution (down to 0.5 mm), with a consequent enormous increase in image number; therefore, manual reading of CTC images may become error-prone due to reader fatigue.Citation44 Moreover, image interpretation is subject to reader bias, and no systematic method has been devised so far for lesion visualization (either 2D or 3D).Citation45
CAD systems have been developed in an attempt to overcome the limitations of software-unassisted reading of CTC datasets. Such applications include dedicated algorithms for automatic detection of geometrical properties of image objects (such as the shape of colonic polyps and their relationship with the bowel wall) and CT density of the normal colon and polyps. The output of those algorithms is used to create a list of ‘candidate’ polyps, which are shown on 2D and 3D reconstructions and native CTC images, and which must be validated or rejected by the user. CAD systems can be used in a first-reader or second-reader paradigm, depending on CAD being switched on before or after examination of CTC datasets by the human reader. Several studies have addressed the issue of evaluating the diagnostic performance of CAD systems for CTC. They are valuable for assisting radiologists in the detection of polyps, especially for detection of small lesions. However, such an increase in sensitivity is usually paralleled by a decrease of specificity, suggesting that results of CAD systems need to be adjusted to maximize overall diagnostic accuracy.Citation39–Citation41
The impact of CAD as second reader on experienced readers has recently been investigated in a multireader, multicase trial.Citation46 Thus, it would be of interest to evaluate the impact of CAD systems on the diagnostic performance of readers without dedicated CTC experience, such as readers working in nonacademic centers, involved in reading a large amount of CTC generated by a screening program. In this respect, Baker et alCitation47 have concluded that application of a CAD system for CTC is advantageous for assisting diagnostic performance of inexperienced readers, with decreased specificity being offset by a higher increase in sensitivity. However, CAD power cannot compensate for reader inexperience, as it has been shown that use of CAD software for CTC image evaluation by inexperienced readers does not significantly increase sensitivity of individual raters, thus stressing the importance of adequate training.Citation48
Conclusion
Colorectal cancer is one of the most frequent malignancies in the Western world, for which genetic and environmental risk factors have been identified and form the basis for disease prevention. Several imaging techniques are currently available that allow detection of colorectal polyps at an early stage of development. Among those, CT colonography is one of the most promising and will likely gain increasing importance for CRC screening purposes.
Disclosure
The authors declare no conflicts of interest.
References
- LongoDLApproach to the patient with cancerFauciASBraunwaldEKasperDLHauserSLLongoDLJamesonJLLoscalzoJHarrison’s Principles of Internal Medicine17th edNew YorkMcGraw-Hill Professional2008
- NeriEGiustiPBattollaLColorectal cancer: role of CT colonography in preoperative evaluation after incomplete colonoscopyRadiology200222361561912034925
- MayerRJGastrointestinal tract cancerFauciASBraunwaldEKasperDLHauserSLLongoDLJamesonJLLoscalzoJHarrison’s Principles of Internal Medicine17th edNew York, NYMcGraw-Hill Professional2008
- JemalASiegelRWardEHaoYXuJThunMJCancer statistics, 2009CA Cancer J Clin20095922524919474385
- FerlayJBrayFSankilaRParkinDMEUCAN: Cancer incidence, mortality and prevalence in the European Union 1997, version 4.0IARC CancerBase No 4LyonIARCPress1999
- MooreHGColorectal cancer: what should patients and families be told to lower the risk of colorectal cancer?Surg Oncol Clin N Am20101969371020883947
- De CamposFGPerezROImperialeARSeidVENahasSCCecconelloIEvaluating causes of death in familial adenomatous polyposisJ Gastrointest Surg2010141943194920676788
- HadleyDWJenkinsJFSteinbergSMPerceptions of cancer risks and predictors of colon and endometrial cancer screening in women undergoing genetic testing for Lynch syndromeJ Clin Oncol20082694895418281669
- PandeMAmosCIOsterwischDRGenetic variation in genes for the xenobiotic-metabolizing enzymes CYP1A1, EPHX1, GSTM1, GSTT1, and GSTP1 and susceptibility to colorectal cancer in Lynch syndromeCancer Epidemiol Biomarkers Prev2008172393240118768509
- PandeMAmosCIEngCFrazierMLInteractions between cigarette smoking and selected polymorphisms in xenobiotic metabolizing enzymes in risk for colorectal cancer: A case-only analysisMol Carcinog20104997498020886582
- GiovannucciEAn updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancerCancer Epidemiol Biomarkers Prev20011072573111440957
- DuWLiWYLuRFangJYFolate and fiber in the prevention of colorectal cancer: between shadows and the lightWorld J Gastroenterol20101692192620180229
- MizoueTTanakaKTsujiIResearch Group for the Development and Evaluation of Cancer Prevention Strategies in JapanAlcohol drinking and colorectal cancer risk: an evaluation based on a systematic review of epidemiological evidence among the Japanese populationJpn J Clin Oncol20063658259716870695
- ParkJYDahmCCKeoghRHAlcohol intake and risk of colorectal cancer: results from the UK Dietary Cohort ConsortiumBr J Cancer201010374775620648013
- MacariMBiniEJCT colonography: where have we been and where are we going?Radiology200523781983316237143
- American College of RadiologyACR Appropriateness Criteria ® Colorectal Cancer Screening http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonGastrointestinalImaging/ColorectalScreeningCancer-Doc4.aspxAccessed Nov 15, 2010
- LevinBLiebermanDAMcFarlandBScreening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of RadiologyCA Cancer J Clin20085813016018322143
- TurnerJRThe gastrointestinal tractKumarVAbbasAKFaustoNAsterJCRobbins and Cotran Pathologic Basis of DiseaseProfessional Edition, 8th edPhiladelphia, PASaundersElsevier2009
- StrykerSJWolffBGCulpCELibbeSDIlstrupDMMacCartyRLNatural history of untreated colonic polypsGastroenterology198793100910133653628
- HewettDGKahiCJRexDKDoes colonoscopy work?J Natl Compr Canc Netw20108677620064290
- SmithRACokkinidesVBrooksDSaslowDBrawleyOWCancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screeningCA Cancer J Clin2010609911920228384
- ZhuMMXuXTNieFTongJLXiaoSDRanZHComparison of immunochemical and guaiac-based fecal occult blood test in screening and surveillance for advanced colorectal neoplasms: a meta-analysisJ Dig Dis20101114816020579218
- SinghHNugentZDemersAABernsteinCNRate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based studyAm J Gastroenterol20101052588259620877348
- WinawerSJStewartETZauberAGA comparison of colonoscopy and double-contrast barium enema for surveillance after polypectomy. National Polyp Study Work GroupN Engl J Med20003421766177210852998
- RockeyDCPaulsonENiedzwieckiDAnalysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparisonLancet200536530531115664225
- VosFMvan GelderRESerlieIWThree-dimensional display modes for CT Colonography: conventional 3D virtual colonoscopy versus unfolded cube projectionRadiology200322887888512954902
- KimSHLeeJMEunHWTwo-versus three-dimensional colon evaluation with recently developed virtual dissection software for CT colonographyRadiology200724485286417709833
- TaylorSALaghiALeferePHalliganSStokerJEuropean Society of Gastrointestinal and Abdominal Radiology (ESGAR): consensus statement on CT colonographyEur Radiol20071757557916967260
- FaggioniLNeriECerriFTuriniFBartolozziCIntegrating image processing in PACSEur J Radiol2009 Jul 18 [Epub ahead of print]
- HalliganSAltmanDGTaylorSACT colonography in the detection of colorectal polyps and cancer: systematic review, meta-analysis, and proposed minimum data set for study level reportingRadiology200523789390416304111
- MulhallBPVeerappanGRJacksonJLMeta-analysis: computed tomographic colonographyAnn Intern Med200514263565015838071
- PickhardtPJChoiJRHwangIComputed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adultsN Engl J Med20033492191220014657426
- MacariMBiniEJJacobsSLClinical significance of missed polyps at CT ColonographyAJR Am J Roentgenol200418312713415208126
- HirofujiYAoyamaTKoyamaSKawauraCFujiiKEvaluation of patient dose for barium enemas and CT colonography in JapanBr J Radiol20098221922719064598
- KemerinkGJBorstlapACWFrantzenSchultzFWZoeteliefJvan EngelshovenJMPatient and occupational dosimetry in double contrast barium enema examinationsBr J Radiol20017442042811388990
- NeriEFaggioniLCerriFCT colonography versus doublecontrast barium enema for screening of colorectal cancer: comparison of radiation burdenAbdom Imaging20103559660119777290
- SugiyamaAOhashiYGomiAColorectal screening with single scan CT colonography in childrenPediatr Surg Int20072398799017665204
- 1990 Recommendations of the International Commission of Radiological ProtectionInternational Commission on Radiological Protection publication no. 60SmithHAnnals of the ICRP 21 (no. 1–3OxfordPergamon Press1991
- YoshidaHDachmanAHCAD techniques, challenges, and controversies in computed tomographic colonographyAbdom Imaging200530264115647868
- TaylorSAHalliganSBurlingDComputer-assisted reader software versus expert reviewers for polyp detection on CT colonographyAm J Roentgenol200618669670216498097
- HalliganSAltmanDGMallettSComputed tomographic colonography: assessment of radiologist performance with and without computer-aided detectionGastroenterology20061311690169917087934
- BurlingDHalliganSAtchleyJCT colonography: interpretative performance in a non-academic environmentClin Radiol20076242442917398266
- ThomasSAtchleyJHigginsonAAudit of the introduction of CT colonography for detection of colorectal carcinoma in a non-academic environment and its implications for the national bowel cancer screening programmeClin Radiol20096414214719103343
- SlaterATaylorSATamEReader error during CT colonography: causes and implications for trainingEur Radiol2006162275228316703308
- PickhardtPJLeeADTaylorAJPrimary 2D versus primary 3D polyp detection at screening CT colonographyAJR Am J Roentgenol20071891451145618029884
- DachmanAHObuchowskiNAHoffmeisterJWEffect of computer-aided detection for CT colonography in a multireader, multicase trialRadiology201025682783520663975
- BakerMEBogoniLObuchowskiNAComputer-aided detection of colorectal polyps: can it improve sensitivity of less-experienced readers? Preliminary findingsRadiology200724514014917885187
- NeriEFaggioniLReggeDCT colonography: role of a second reader CAD paradigm in the initial training of radiologistsEur J Radiol2010 Sep 8 [Epub ahead of print]