Abstract
In patients with severe emphysema, bronchoscopic lung volume reduction using one-way valves is a promising therapeutic option to improve lung function and quality of life. The goal of this treatment is to achieve a complete lobar atelectasis. In a significant proportion of patients, this atelectasis cannot be achieved due to interlobar collateral ventilation. This collateral ventilation is generated through incomplete lobar fissures. Therefore, only patients with complete fissures and no collateral ventilation can be selected for endobronchial therapy with one-way valves.
Incomplete fissures are very common and exhibit a great variation in anatomy. The reported prevalence is 17%–85% for the right major fissure, 19%–74% for the left major fissure, and 20%–90% for the minor fissure. There are several methods of measuring or predicting the presence of collateral ventilation, with computed tomography (CT)-fissure analysis and the Chartis measurement being the most important. CT-fissure analysis is an indirect method to measure the completeness of fissures as a surrogate for collateral ventilation. The Chartis system is an endobronchial method to directly measure the presence of collateral ventilation. Both methods have unique value, and the combination of both can accurately predict the treatment response to the bronchoscopic placement of endobronchial valves. This review provides an in-depth view of lung fissure and collateral ventilation to help understand its importance in selecting the appropriate patients for new emphysema treatments and thus avoid useless treatment in unsuitable patients.
Introduction
In patients with severe emphysema, lung volume reduction surgery (LVRS) can improve the quality of life, exercise capacity and lung function.Citation1 However, LVRS is a highly invasive therapy and is associated with significant morbidity.Citation1 Therefore, other and less invasive techniques for reduction of lung volume have been studied. An emerging therapy for patients with severe emphysema is bronchoscopic lung volume reduction (BLVR). Several BLVR techniques have been investigated, including one-way endobronchial valves (EBVs) and lung volume reduction coils.Citation2 In patients with severe emphysema, treatment with one-way valves shows promising results.Citation3 The purpose of this technique is to induce atelectasis in the most diseased lobe. Like LVRS, this might relieve symptoms in emphysema patients through a reduction in hyperinflation, with an improved function of diaphragm and chest wall mechanics. Furthermore, increase in elastic recoil pressure leads to increased expiratory airflow and the inhomogeneity of ventilation and perfusion is decreased, leading to an improvement in gas exchange.Citation1,Citation4
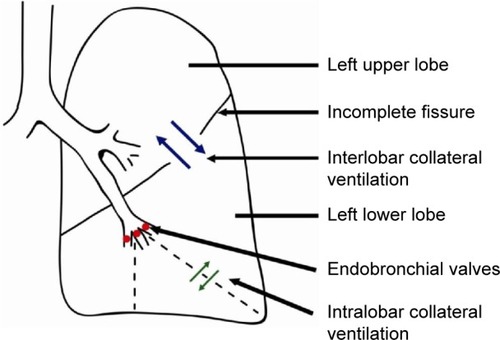
However, to achieve maximal clinical improvement with one-way valve treatment, it is important to achieve complete lobar atelectasis.Citation4–Citation6 The most important factor that may prevent the development of this desired atelectasis after endobronchial one-way valve treatment is interlobar collateral ventilation (CV) through means of an incomplete interlobar fissure.Citation4,Citation6 This led to the development of several methods to measure the CV and investigate the interlobar fissure integrity.
A combination of these measurements, together with endobronchial treatment using one-way valves, leads to new and successful treatment of chronic obstructive pulmonary disease (COPD).Citation3 The use of the proper selection criteria enables customized care specifically applied to the individual patient, thereby showing real personalized medicine.
This review will focus on the prevalence, assessment, and important role of CV and interlobar fissures with respect to BLVR.
Collateral ventilation
CV is defined as “the ventilation of alveolar structures through passages or channels that bypass the normal airways”.Citation7 Intralobar CV (CV within a lobe, segment, or subsegment) was originally described as collateral respiration by Van Allen et alCitation8 in 1931. They observed that after obstruction of the bronchus of an airway, this was not always followed by alveolar collapse.
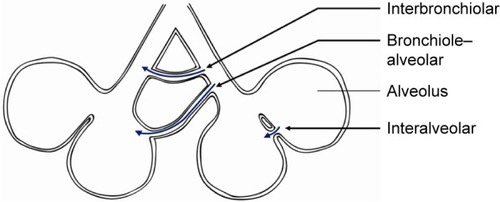
There are three main candidate pathways for CV to take place (). The first described pathway consists of interalveolar communication of air through the pores of Kohn. These are situated in alveolar walls and should permit the passage of fluid and possibly air. However, these pores are small (<5 μm) and very high pressures (estimated 19.2 kPa or 196 cmH2O) would be necessary for air transport.Citation9–Citation13 A second possible pathway is through accessory communication channels (30 μm in diameter) between distal bronchioles and alveoli, as described by Lambert.Citation14 The third pathway is described by MartinCitation13 and contains accessory respiratory bronchioles between the bronchioles of adjacent lung segments. This pathway is probably the most important pathway, due to the larger size (80–150 μm) of bronchioles and thus having a lower resistance when compared to the other pathways.Citation12,Citation13,Citation15
Figure 1 Three different pathways for collateral ventilation.
In healthy persons, CV does not seem to play an important role, as the resistance of the collateral channels is much higher than the regular airways and large pressure gradients are needed for CV.Citation12,Citation16 It is not exactly known why collateral channels exist or why they develop, but they all seem to originate after birth of both animals and humans.Citation13
Despite their origin, CV may assume importance in several diseases. The prevention of atelectasis after obstruction is probably the most important function of CV. For example, obstruction of airways by mucus impaction, tumor or foreign bodies does not always lead to atelectasis due to CV.Citation8 Furthermore, in patients with emphysema, with increased airway resistance, collateral resistance is lower. This was demonstrated by Hogg et al,Citation18 who measured the resistance of CV in excised normal and emphysematous lungs. In normal lungs, the resistance of collateral channels was 260–330 cmH2O (25–324 kPa), whereas this was 5–20 cmH2O (0.5–2.0 kPa) in emphysematous lungs. Therefore, air flows much more easily through collateral channels in an emphysematous than a normal lung.Citation17 Although CV was first only described as intralobar ventilation, there is also interlobar (ie, between the lobes) CV across the fissures. Probably due to the same mechanism, the flow between different lobes is higher in patients with emphysema.Citation15,Citation17,Citation18
Obviously, interlobar CV is an important factor in BLVR therapy, as this therapy is based on complete atelectasis of a lobe (). Most probably, incomplete fissures are responsible for the interlobar collateral flow, although not found in every study regarding this subject.Citation6,Citation15 There is no literature regarding the mechanism of CV in incomplete fissures between lobes. It can be assumed that the mechanism of collateral flow between lobes is the same as within a lobe.
Figure 2 Collateral ventilation.
Embryology
Incomplete fissures are present in the majority of people and arise during fetal development. Approximately 4 weeks after conception, the airways start to develop. The respiratory diverticulum branches into two lung buds, which divide in the lobar bronchi.Citation19
During the development of the fetus, the coelomic cavity is divided into the pericardium, the pleural cavities, and the peritoneal cavity. The pleural cavity is lined by a mesothelial membrane, the pleura. As the lung buds grow into the left and right pleural cavities, they are still covered by this membrane. The membrane covering the pleural cavity becomes the parietal pleura, whereas the mesothelial covering of the lung buds becomes the visceral pleura. As early as the visceral pleura has formed (after around 7 weeks), invaginations of the pleura start to separate the lobar bronchi. This gives rise to the lobar fissure and formation of the lung lobes. However, lobes can be fused if the pleura does not cover the complete lobe, leading to “incomplete fissures”. As the pleura invaginates from lateral, it can be assumed that the fissures are incomplete near the hilus most often, as will be described later.Citation19,Citation20
Anatomy and prevalence of incomplete fissures
The anatomy of fissures and the completeness of fissures have been studied postmortem and by computed tomography (CT). Both the orientation (eg, medially, laterally) and the configuration (eg, concave, convex) exhibits a large variation. A large study performed by Aziz et al,Citation21 described the anatomy of the pulmonary fissures using high-resolution computed tomography (HRCT) in 662 patients without significant pulmonary disease. For the left major fissure, they found that, in most cases, the fissure faces medial in the superior zone (66%), lateral in the suprahilar zone (64%), and medial in the infrahilar and inferior zones (54% and 68%, respectively).Citation21 For the right major fissure, the fissure faces medial in the superior zone (85%) and lateral in the lower zones (63% suprahilar, 55% infrahilar, and 62% inferior). Other studies partly describe the same orientations, but individual variation is large.Citation22–Citation25
There is also some variation in the reported degree of incompleteness of the fissures. Some studies were based on autopsies. For example, Raasch et alCitation22 studied 100 lung specimens and found that incomplete fissures are very common. The incidence ranged from 70% in the right major fissure (mainly superior), 46% across the left major fissure (mainly inferior) and 94% across the minor fissure.Citation22 Other cadaver studies reported incomplete horizontal fissures in 47%–63% of cases, 36%–47% incomplete left oblique fissures, and 37%–39% incomplete right oblique fissures.Citation26,Citation27
Studies using CT also mention variable numbers, which are summarized in . The reported prevalence of incomplete fissures is 17%–85% for the right major fissure, 19%–74% for the left major fissure, and 20%–90% for the minor fissure.Citation21,Citation23,Citation28–Citation35 These studies consistently find that compared to the other fissures, the minor fissure is incomplete most frequently. Furthermore fissures are incomplete near the hilus most frequently.Citation21,Citation30 The reported prevalence exhibits a great variation. Possible explanations are the varying patient groups with and without pulmonary disease, different kind of reviewers, various or no criteria to define an incomplete fissure, or used CT-scan technique (eg, slide thickness).Citation34
Table 1 Prevalence of incomplete fissures
Measurement of CV
There are several methods to assess the presence of CV. Invasive measurement methods include nuclear techniques and an endobronchial pulmonary assessment system. Noninvasive methods for measurement of CV include imaging techniques such as hyperpolarized gas magnetic resonance imaging (MRI) or CT-fissure analysis, which is an indirect method.
Endobronchial CV assessment
Although several endobronchial methods has been described for endobronchial assessment of CV, the study of Aljuri and FreitagCitation36 in 2009 was the first to present a method assessing collateral flow to predict the clinical response to BLVR treatment. Based on this technique the Chartis System® (PulmonX Inc., Redwood City, CA, USA) was developed, and is described and used in several articles regarding this topic.Citation3,Citation37–Citation41
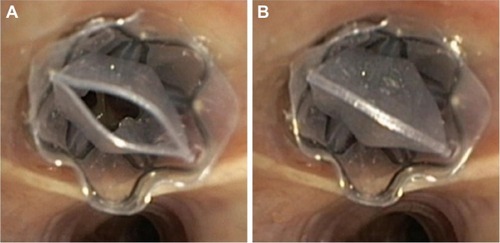
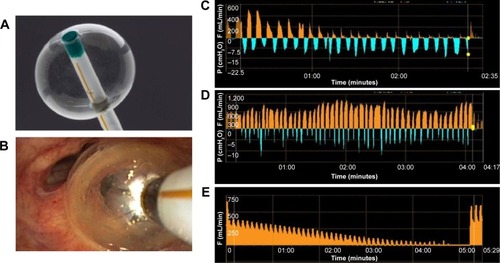
The Chartis system consists of a catheter with a balloon component at the distal tip (). After inflation of the balloon, the airway is blocked and air from the targeted segment or lobe can flow only through the catheter (). The air is directed to the Chartis console, which can assess both expiratory air flow, pressure, and resistance (). If expiratory airflow persists after occlusion of a lobe, this indicates the presence of collateral airflow. In contrast, if there is no more flow, this indicates high collateral resistance and thus no collateral airflow, indicating a suitable candidate for BLVR therapy ( and ).Citation42
Figure 3 Chartis measurement.
Abbreviations: F, flow; P, pressure.
Gompelmann et alCitation43 performed the first clinical trial with the Chartis system in 2010 and showed that in 90% of the patients, the measurements correctly predicted if atelectasis on chest radiograph would occur. A subsequent larger study by Herth et alCitation44 investigated the diagnostic value of the Chartis pulmonary assessment system to predict treatment response, defined as a target lung volume reduction (TLVR) of 350 mL or more. There was a total accuracy of 75%, with a positive predictive value of 71% and negative predictive value of 83%.
For optimal result and reliability of the Chartis measurement, there are several technical aspects to consider. For example, coughing or multiple mucus plugs might impede the measurement. Furthermore, especially in the lower lobes, the balloon might occlude a segment – for example, B6 in the left lower lobe – so that this segment may not get included in the assessment of CV. Moreover, dynamic airway collapse can occur, which might show as an abrupt or gradual ending of flow, with <100 mL of total exhaled air. To maximize the chance of a successful and reliable measurement, adequate training is required.Citation37,Citation43,Citation45,Citation46 Furthermore, the measurement can be performed under general anesthesia with positive pressure support (), or high frequency jet ventilation. This creates optimal circumstances for a reliable and fast measurement, and if there is no CV, the treatment with EBVs can take place immediately, in the same treatment session ().
Nuclear techniques
Nuclear imaging can be used to demonstrate the presence of CV. For example, Morrell et alCitation47 measured CV using a balloon-tipped catheter, to occlude segmental bronchi. Subjects then breathed heliox (79% helium, 21% oxygen) and the rise of helium concentration in the occluded segment was used as index for CV. Another study used 133Xenon ventilation scintigraphy to prove the presence of interlobar CV, by selectively intubating a lobe and measuring the ventilation of the adjacent lobes.Citation48
Currently, there are no studies that compared the use of nuclear technique with Chartis measurement or CT-fissure analysis, nor their predictive values for developing atelectasis after endobronchial lung volume reduction therapy.
Another, noninvasive, method to measure CV was described by Marshall et al.Citation49 In this study, a mix of hyperpolarized 3He and N2 was inhaled and Citation3He images were acquired using MRI during a single breath-hold. They found ventilation defects with delayed filling in a large proportion of patients. Although this delayed filling may be due to several causes (eg, CV, air trapping), the specific pattern of Citation3He filling from the edges of the ventilation defect toward the centre was believed to be due to CV.
However, although CV may be demonstrated by this method, it demonstrates intersegmental and not the interlobar CV. Therefore, this method is probably less valuable to predict the response to BLVR. Moreover, its availability is probably less than the other measurement methods in most clinics.
CT-fissure analysis
A surrogate- or indirect method for the measurement of CV is by using CT-fissure analysis. CT can be used to assess the completeness of the fissure. If a fissure is complete, there is most probably no CV. However, if the fissures are incomplete and lobes are fused, CV is very likely. Usually, the analysis is done by experienced radiologists or pulmonologists, but it is prone for interobserver variability.
Several authors described the following diagnostic criteria for incomplete interlobar fissures: “1) neither a clear avascular zone nor interlobar line is observed; 2) vascular images in adjacent lobes cross over the interlobar region; 3) pulmonary blood vessels, particularly the pulmonary vein, penetrate the interlobar region; 4) the pulmonary vein is observed in the interlobar region and is related to the vascular images in adjacent lobes.”Citation30,Citation32,Citation34 Other authors did not use predefined diagnostic criteria, but they used consensus or experts opinion to describe the fissures as complete or incomplete.Citation23,Citation28,Citation29,Citation31,Citation33
Interobserver agreement was investigated by several authors. Koenigkam-Santos et alCitation29 described a high agreement between experienced radiologists for the major fissures, but rather moderate for the minor fissure (k=0.53). Cronin et alCitation31 described a greater than 90% agreement for all fissures, Guan et alCitation34 found a fair to nearly perfect agreement between two radiologists (k=0.593–0.652) and Koenigkam-Santos et alCitation28 described a reported interobserver agreement of 0.70–0.76. This is the case with experienced radiologists or pneumologists; inexperienced readers most probably have lower agreement.
Several studies compared the results of CT to intraoperative assessment of the fissures. For example, Diso et alCitation38 evaluated a group of 21 patients undergoing surgery for lung cancer and then compared the intraoperative assessment of fissures to both the Chartis and HRCT measurement of fissures. Compared to inspection at surgery, Chartis measurement had an accuracy of 71% and HRCT of 76%; there was no significant difference between them. Kent et alCitation50 compared CT to intraoperative assessment and also found high predictive values for both major fissures but a positive predictive value of only 33% for the minor fissure.
More recently, automatic methods have been developed to quantify the completeness of the fissures. Several of these methods have been described and used in literature, and the results are comparable to the interpretation of radiologists.Citation35,Citation37,Citation51
Studies comparing CT-fissure analysis and Chartis
The response of valve-based lung volume reduction can be predicted by both the Chartis measurement and the CT-fissure analysis. An interesting question is which of these methods is better to predict this response, or if a combination of both is better.
Currently, there are few studies that compare these methods. Schuhmann et alCitation37 retrospectively compared CT to the Chartis measurement in a testing dataset of 33 patients. A positive response to treatment was defined as a target lung volume reduction of 350 mL or more. The authors concluded that CT-fissure was comparable with Chartis (P=0.55) with an accuracy of 75.8% for Chartis and 78.8% for CT. In this study, both methods disagreed in eleven of 33 subjects, and the proportion of misclassifications was equal between both methods. However, in 22 of 33 subjects, the methods agreed and the classification into responders and nonresponders was 90.9% correct. There were no false negatives in subjects classified as nonresponders by both Chartis and CT. Two patients were misclassified as responders, but that was due to valve procedural errors in these patients. These results suggest that an agreement of both methods leads to few false positives or false negatives. Comparison of the combination of predictors (Chartis and CT) and CT alone resulted in a small but nonsignificant difference in favor of the combined model.
Gompelmann et alCitation40 also retrospectively compared Chartis to CT-fissure analysis in 69 patients. A complete fissure was defined as >90% of the fissure present. A positive result was defined as a TLVR ≥350 mL. The Chartis method had an accuracy of 74% and the HRCT fissure analysis an accuracy of 77%, meaning both methods are similar in classifying patients to respond or not respond to EBV treatment. Both methods were concordant in two-thirds of the patients, similar to the study of Schuhmann et al.Citation37
Reymond et alCitation41 compared CT-fissure analysis to the Chartis measurement in patients with severe emphysema, but not its clinical response. This retrospective study showed an agreement of 73% between the two methods, with a high sensitivity and few false negatives.
Although these studies were retrospective and originally not designed to compare these methods, Chartis and CT-fissure analysis appear to be equivalent to correctly predict a positive or negative response to EBV. New and preferably prospective studies are required to confirm this conclusion and see if the combination of the two methods is superior to a single method to successfully predict the response to EBV.
Clinical application
Another question is whether the measurement of CV is indeed correlated with the results of BLVR.
The first study to correlate the success of EBV placement to CV was the Endobronchial Valve for Emphysema Palliation Trial (VENT), performed by Sciurba et al.Citation6 They randomly assigned patients to endobronchial therapy, independent of CV or fissure completeness. Although there was a significant improvement of pulmonary function in the treated patients, in a post hoc analysis, they found an enhanced effect of therapy in patients with complete fissures (>90% complete) on CT.
A European multicenter study by Herth et al,Citation44 designed as a validation study for the Chartis system, compared patients with and without CV. There were significantly more responders in the group without CV (target lung volume reduction of 350 mL or more). The TLVR was 743 versus 99 mL in favor of the group without CV. Furthermore, the FEV1 increased (mean percentage 16 vs 1). The 6 minutes walking distance and St George’s Respiratory Questionnaire were in favor of the collateral negative group, but not significant.
An important study is the BeLieVeR-HIFi study by Davey et al;Citation39 a double-blind sham controlled trial. Patients were selected for bronchoscopic intervention based on the CT-scan alone. They found an increase of the FEV1 of 8.77%. However, the CV was also measured to compare these two methods. Of the 25 patients with intact fissures and who received EBVs, CV was found in four patients. These patients had no benefit or less benefit from the treatment.
More recently, Klooster et alCitation3 published the STELVIO-trial, a randomized controlled trial to compare endobronchial-valve treatment with standard medical care. Only patients with a complete or near complete fissure were included. CV was measured using the Chartis measurement system. Patients without interlobar CV were randomized to treatment with EBVs or as control. The EBV group improved significantly in FEV1 (+22.7%), FVC (+442 mL), and 6 minutes walking distance (+106 m).Citation3
An important question is whether the HRCT and Chartis measurement should always be used to determine if a patient is a potential responder to EBVs. Schuhmann et alCitation37 reported a high response rate of almost 65% if the fissure was >90% complete. Nevertheless, this means there is a significant proportion of patients without response. This is also shown by the studies by Klooster et alCitation3 and Davey et al.Citation39 As shown in both studies, an HRCT with complete or near complete fissure (>90%) does not predict the presence of CV in all patients. Therefore, the Chartis measurement is probably useful in all patients who are eligible for treatment with EBVs based on fissure completeness.
However, if the fissure is not (near) complete (<80%–90%), the chance of success is very small, and it is probably not necessary to perform a Chartis measurement.Citation37 The combination of a complete fissure on HRCT and no CV with Chartis measurement should result in an atelectasis of the target lobe. If nevertheless, there is no response, there is most likely a problem at the endobronchial level (forgotten subsegment, misplaced valve, wrong sizing), and a re-bronchoscopy might resolve the problem.Citation6,Citation46
Airway bypass
Although this review mainly focuses on BLVR using EBVs, the concept of CV has also been used in the treatment of emphysema with so called “airway bypasses”.Citation52 With this method, a direct passage between the lung parenchyma and large airways or through the thorax is created, allowing air to exit the lung, and thus bypassing the expiratory flow limitation. CV then should ensure the exit of air and clinical benefit. Two approaches using the airway bypass have been investigated. The transthoracic airway bypass approach has been described by Saad Junior et alCitation53 and Moore et al.Citation54 Saad Junior et alCitation53 performed this procedure in three patients with emphysema. These patients reported improvement of symptoms. However, due to the small number treated, no statistical analysis was performed.Citation53 Moore et alCitation54 first performed an ex vivo study and found an increase in the expiratory flow (169–235 mL; P<0.05) after insertion of an extrapulmonary airway bypass in seven explanted emphysematous lungs. After that, an in vivo study was performed in four patients. There was a reduction of total lung capacity in all patients and an increase of the FEV1 in three patients.Citation54 A larger study (trial ID: ACTRN12610000190000) using the transthoracic airway bypass approach was prematurely stopped due to lack of funding.Citation55
The bronchoscopic approach to create airway bypasses using drug-eluting transbronchial airway stents has been extensively studied in severe emphysema patients. In the “EASE-trial” by Shah et al,Citation56 a multicentre, randomized, full sham bronchoscopy controlled trial, 319 patients were randomized and 212 were assigned to airway bypasses. Although after 1 day the pulmonary function significantly improved, showing a strong signal for proof of concept, there was no sustained long-term effect at 6 months or 1 year due to closure of the created bypasses.Citation56 There are no other larger studies regarding this subject, and currently not much more is known about the optimal patient selection criteria, number of airway bypasses, and optimal target areas.
Conclusion
Endobronchial lung volume reduction is a promising and proven effective therapy for selected patients with emphysema. The absence of interlobar CV is the most discriminating factor for therapeutic success, as this may prevent the intended lobar atelectasis after valve placement. CV is generated through incomplete lobar fissures. Therefore, only patients with complete fissures and no CV can be selected for endobronchial therapy. Both CT-fissure analysis (indirect) and Chartis measurement (direct) appear to successfully predict the presence of CV and thus the success rate of endobronchial therapy. Both methods have specific pros and cons. However, a combination of the two methods most probably provides the highest accuracy and has proven to successfully predict a positive or negative treatment response. This approach enables personalized medicine and provides an excellent treatment strategy in selected patients with the right phenotype of emphysema, whilst it avoids the possibility of unsuitable patients with the wrong phenotype undergoing useless treatment.
Disclosure
TDK reports no conflicts of interest in this work. DJS is receiving consulting fees from PneumRx/BTG, lecture fees from PneumRx/BTG, Boston Scientific, Holaira, Olympus Europe, and Pulmonx, devices for treatments from PneumRx/BTG, Holaira, and Pulmonx, travel support from PneumRx/BTG, Boston Scientific, Holaira, Olympus Europe, and Pulmonx, and grant support from PneumRx/BTG, Boston Scientific, Aeris Therapeutics, Holaira, and Pulmonx, and reports no other conflicts of interest in this work.
References
- TiongLUDaviesRGibsonPGLung volume reduction surgery for diffuse emphysemaCochrane Database Syst Rev2006184
- MineshitaMSlebosDBronchoscopic interventions for chronic obstructive pulmonary diseaseRespirology2014191126113725124070
- KloosterKTen HackenNHartmanJKerstjensHvan RikxoortESlebosDEndobronchial valves for emphysema without interlobar collateral ventilationN Engl J Med2015373242325233526650153
- ShahPLHerthFJCurrent status of bronchoscopic lung volume reduction with endobronchial valvesThorax201469328028624008689
- EberhardtRGompelmannDSchuhmannMHeusselCPHerthFJComplete unilateral vs partial bilateral endoscopic lung volume reduction in patients with bilateral lung emphysemaChest2012142490090822459779
- SciurbaFCErnstAHerthFJVENT Study Research GroupA randomized study of endobronchial valves for advanced emphysemaN Engl J Med2010363131233124420860505
- CettiEMooreAGeddesDCollateral ventilationThorax200661537137316648350
- Van AllenCLindskogGRichterHCollateral respiration. Transfer of air collaterally between pulmonary lobulesJ Clin Invest193110355959016693999
- MacklemPAirway obstruction and collateral ventilationPhysiol Rev19715123684364928122
- MenkesHTraystmanRCollateral ventilationAm Rev Respir Dis19771162287309889177
- GompelmannDEberhardtRHerthFCollateral ventilationRespiration201385651552023485627
- DelaunoisLAnatomy and physiology of collateral respiratory pathwaysEur Respir J1989298939042680588
- MartinHRespiratory bronchioles as the pathway for collateral ventilationJ Appl Physiol1966215144314475923213
- LambertMAccessory bronchiolealveolar communicationsJ Pathol Bacteriol195570231131413295905
- HiguchiTReedAOtoTRelation of interlobar collaterals to radiological heterogeneity in severe emphysemaThorax200661540941316467071
- TerryPTraystmanRNewballHBatraGMenkesHCollateral ventilation in manN Engl J Med197829811015618444
- TsaiLHoffmanAMazanMIngenitoEBronchoscopic measurement of collateral ventilation in a sheep model of emphysemaRespiration200774556557117541261
- HoggJMacklemPThurlbeckWThe resistance of collateral channels in excised human lungsJ Clin Invest19694834214315773080
- FishmanAEliasJFishmanJGrippiMSeniorRPackAFishman’s Pulmonary Diseases and Disorders4th edNew YorkMcGraw-Hill Professional2008123127
- LightRWChapter 1. Anatomy of the pleuraPleural Diseases5th edPhiladelphiaLippincott Williams & Wilkins200716
- AzizAAshizawaKNagaokiKHayashiKHigh resolution CT anatomy of the pulmonary fissuresJ Thorac Imaging200419318619115273615
- RaaschBCarskyELaneEO’CallaghanJHeitzmanERadiographic anatomy of the interlobar fissures: a study of 100 specimensAJR Am J Roentgenol19821386104310496979204
- GülsünMAriyürekOCömertRKarabulutNVariability of the pulmonary oblique fissures presented by high-resolution computed tomographySurg Radiol Anat200628329329916463080
- ProtoABallJJComputed tomography of the major and minor fissuresAJR Am J Roentgenol198314034394486600530
- HayashiKAzizAAshizawaKHayashiHNagaokiKOtsujiHRadiographic and CT appearances of the major fissuresRadiographics200121486187411452059
- MeenakshiSManjunathKBalasubramanyamVMorphological variations of the lung fissures and lobesIndian J Chest Dis Allied Sci200446317918215553206
- PrakashBShashirekhaMSumaHKrishnaCSinghGLung morphology: a cadaver study in Indian populationItal J Anat Embryol2010115323524021287979
- Koenigkam-SantosMde PaulaWDOwsijewitschMIncomplete pulmonary fissures evaluated by volumetric thin-section CT: semi-quantitative evaluation for small fissure gaps identification, description of prevalence and severity of fissural defectsEur J Radiol201382122365237024016827
- Koenigkam-SantosMPuderbachMGompelmannDIncomplete fissures in severe emphysematous patients evaluated with MDCT: incidence and interobserver agreement among radiologists and pneumologistsEur J Radiol201281124161416622770581
- HeřmanováZCtvrtlíkFHeřmanMIncomplete and accessory fissures of the lung evaluated by high-resolution computed tomographyEur J Radiol201483359559924377673
- CroninPGrossBHKellyAMPatelSKazerooniEACarlosRCNormal and accessory fissures of the lung: evaluation with contiguous volumetric thin-section multidetector CTEur J Radiol2010752e1e819896788
- MahmutMNishitaniHEvaluation of pulmonary lobe variations using multidetector row computed tomographyJ Comput Assist Tomogr200731695696018043363
- OzmenCANazarogluHBayrakAHSenturkSAkayHOEvaluation of interlobar and accessory pulmonary fissures on 64-row MDCTClin Anat201023555255820235172
- GuanCSXuYHanDChenJHWangXLMaDQVolumetric thin-section CT: evaluation of pulmonary interlobar fissuresDiagn Interv Radiol201521646647026359877
- PuJWangZGuSPulmonary fissure integrity and collateral ventilation in COPD patientsPLoS One201495e9663124800803
- AljuriNFreitagLValidation and pilot clinical study of a new bronchoscopic method to measure collateral ventilation before endobronchial lung volume reductionJ Appl Physiol2009106377478319036886
- SchuhmannMRaffyPYinYComputed tomography predictors of response to endobronchial valve lung reduction treatment. Comparison with ChartisAm J Respir Crit Care Med2015191776777425635349
- DisoDAnileMCarilloCCorrelation between collateral ventilation and interlobar lung fissuresRespiration201488431531925170658
- DaveyCZoumotZJordanSBronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trialLancet201538699981066107326116485
- GompelmannDEberhardtRSlebosDJDiagnostic performance comparison of the Chartis System and high-resolution computerized tomography fissure analysis for planning endoscopic lung volume reductionRespirology201419452453024612306
- ReymondEJankowskiAPisonCPrediction of lobar collateral ventilation in 25 patients with severe emphysema by fissure analysis with CTAJR Am J Roentgenol20132014571575
- MantriSMacaraegCShettySTechnical advances: measurement of collateral flow in the lung with a dedicated endobronchial catheter systemJ Bronchology Interv Pulmonol200916214114423168520
- GompelmannDEberhardtRMichaudGErnstAHerthFJPredicting atelectasis by assessment of collateral ventilation prior to endobronchial lung volume reduction: a feasibility studyRespiration201080541942520664194
- HerthFEberhardtRGompelmannDRadiological and clinical outcomes of using Chartis™ to plan endobronchial valve treatmentEur Respir J201341230230822556025
- GesierichWSamitaKBehrJDetermining collateral ventilation during bronchoscopy: unanswered questionsThorax201469328929024343787
- ShahPLHerthFDynamic expiratory airway collapse and evaluation of collateral ventilation with ChartisThorax201469329029124355826
- MorrellNWWignallBKBiggsTSeedWACollateral ventilation and gas exchange in emphysemaAm J Respir Crit Care Med199415036356418087331
- SalanitriJKalffVKellyMHolsworthLWilliamsTSnellG133Xenon ventilation scintigraphy applied to bronchoscopic lung volume reduction techniques for emphysema: relevance of interlobar collateralsIntern Med J20053529710315705138
- MarshallHDeppeMParra-RoblesJDirect visualisation of collateral ventilation in COPD with hyperpolarised gas MRIThorax201267761361722286930
- KentMSRidgeCO’DellDLoPWhyteRGangadharanSPThe accuracy of computed tomography to predict completeness of pulmonary fissures. A prospective studyAnn Am Thorac Soc201512569670025746111
- van RikxoortEGoldinJGGalperin-AizenbergMA method for the automatic quantification of the completeness of pulmonary fissures: evaluation in a database of subjects with severe emphysemaEur Radiol201222230230921984417
- LausbergHFChinoKPattersonGAMeyersBFToeniskoetterPDCooperJDBronchial fenestration improves expiratory flow in emphysematous human lungAnn Thorac Surg200375239339812607646
- Saad JuniorRDorgan NetoVBotterMStirbulovRRivabenJHGonçalvesRTherapeutic application of collateral ventilation with pulmonary drainage in the treatment of diffuse emphysema: report of the first three casesJ Bras Pneumol200935141919219326
- MooreAJCettiEHaj-YahiaSUnilateral extrapulmonary airway bypass in advanced emphysemaAnn Thorax Surg201089899906
- SlebosDKloosterKErasmusMEmphysemaAm J Respir Crit Care Med2012186219722798416
- ShahPSlebosDCardosoPBronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham-controlled, multicentre trialLancet2011378)997100521907863