Abstract
Background
The chronic bronchitis (CB) phenotype has been associated with poor quality of life and an increased risk of disease in patients with COPD. However, little information exists regarding the relationship between the CB phenotype and the COPD assessment test (CAT) score. The goal of this study was to reveal the different pattern of CAT scores between CB and non-CB patients. Moreover, we aimed to investigate whether the CB phenotype is an independently associated factor for more symptom and high-risk groups.
Methods
Data were obtained from the Korea COPD Subgroup Study cohort recruited from 46 centers in South Korea since April 2012. CB patients were defined as having a chronic cough and sputum for 3 months per year, for a period of 2 consecutive years. We investigated the pattern of CAT and subquestionnaire scores between CB and non-CB patients. We also analyzed the proportion of CB phenotypes in each Global initiative for chronic Obstructive Lung Disease (GOLD) stage. Finally, we performed a logistic regression analysis to identify whether the CB phenotype was an independently associated factor for more symptom and high-risk groups.
Results
Of the 1,106 study patients, 11.5% of patients were found to have a CB phenotype. CB phenotypes were most common in GOLD III (GOLD 2006) and GOLD D (GOLD 2015) stages. CAT scores were significantly higher in CB patients not only in terms of the total score but also for each subquestionnaire. Logistic regression revealed that the CB phenotype is an independently associated factor for more symptom and high-risk groups.
Conclusion
The present study revealed that CB patients have higher CAT scores and subquestionnaire results compared to non-CB patients. Additionally, we demonstrated that the CB phenotype is an independently associated factor for both more symptom and high-risk groups.
Introduction
COPD is a major public health problem and is expected to be the third leading cause of death, and fifth leading disease burden, by 2020.Citation1 In addition, its economic burden is reported to be substantial, especially when combined with chronic bronchitis (CB).Citation2–Citation4
Recent studies suggest that COPD is a heterogeneous disease and that different phenotypes may be associated with disease severity, quality of life, and mortality.Citation5–Citation8 CB is a common phenotype of COPD that has traditionally been considered to be on the opposite side of the disease spectrum to the emphysema phenotype. However, most individuals have both characteristics.Citation2,Citation9 The definition of CB has been variable but it is classically defined as the presence of a chronic cough and sputum for 3 months per year over a duration of 2 consecutive years.Citation10,Citation11 The prevalence of CB is reported to range from 14% to 74% of all patients with COPD, probably due to the varying definitions of CB, different study populations, and different study designs.Citation6,Citation9,Citation10
Previous investigations have revealed that the CB phenotype is associated with poor quality of life, as well as increased disease severity and risk of exacerbation in COPD patients.Citation6,Citation12–Citation18 However, there has been a limited amount of data available regarding the relationship between the CB phenotype and the COPD assessment test (CAT) score. Since the CAT score was validated in 2009, its usefulness in evaluating symptoms and quality of life has been emphasized.Citation19,Citation20 It is now an important factor in the new Global initiative for chronic Obstructive Lung Disease (GOLD) staging system as revised in 2015.Citation21 Moreover, numerous studies have observed that the CAT score is associated with COPD severity and risk of exacerbation.Citation22–Citation25
In the GOLD 2015 staging system, COPD patients with a high CAT score (≥10) or high modified Medical Research Council (mMRC) score (≥2) are categorized into the more symptom group, and patients with a post-bronchodilator (BD) forced expiratory volume in 1 second (FEV1) <50%, or history of exacerbation ≥2/year, or admission to hospital ≥1/year are categorized into the high-risk group. The proportion of CB phenotypes in each GOLD stage has not yet been fully clarified.
The purpose of the present study was to identify the different characteristics between CB and non-CB COPD patients to determine whether the pattern of CAT scores varies between them. Moreover, we aimed to investigate whether the CB phenotype is an independently associated factor for the more symptom group (CAT score ≥10). Additionally, we aimed to evaluate whether the CB phenotype is an independently associated factor for the high-risk group.
Methods
Study design, study population, and data collection
In this study, we used data from the KOCOSS cohort to investigate the difference in clinical outcomes between CB and non-CB patients. KOCOSS is an ongoing multicenter COPD cohort study that recruited participants from 47 centers in South Korea since April 2012. Approximately 1,565 patients were enrolled as of December 28, 2015. The inclusion criteria were Korean patients aged more than 40 years old and a ratio of FEV1 to forced vital capacity (FVC) of <0.7. Written informed consent was obtained from all the study patients. Ethics approval for this study was obtained from the ethics committee at each center. The names of ethics committees are as follows: Gacheon University Gil Medical Center, Hallym University Kangnam Sacred Heart Hospital, Gangnam Severance Hospital, Kyung Hee University Hospital at Gangdong, Hallym University Kang-dong Sacred Heart Hospital, Kangbuk Samsung Hospital, Kangwon National University Hospital, Konkuk University Hospital, Konkuk University Chungju Hospital, Kyungpook National University Hospital, Gyeongsang National University Hospital, Korea University Guro Hospital, Korea University Anam Hospital, Seoul Eulji Hospital, Dongguk University Gyeongju Hospital, Dongguk University Ilsan Hospital, Keimyung University Dongsan Medical Center, Dong-A University Hospital, Hallym University Dongtan Sacred Heart Hospital, Pusan National University Hospital, Inje University Busan Paik Hospital, The Catholic University of Korea Bucheon St. Mary’s Hospital, Soonchunhyang University Hospital Bucheon, Seoul National University Bundang Hospital, Bundang CHA Hospital, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Samsung Medical Center, Soonchunhyang University Hospital Seoul, The Catholic University of Korea Seoul St. Mary’s Hospital, The Catholic University of Korea St. Paul’s Hospital, The Catholic University of Korea St. Vincent’s Hospital, Severance Hospital, Asan Medical Center, Ajou University Hospital, The Catholic University of Korea Yeouido St. Mary’s Hospital, The Catholic University of Korea Uijeongbu St. Mary’s Hospital, Yeungnam University Medical Center, Ulsan University Hospital, Wonkwang University Sanbon Hospital, Wonju Severance Christian Hospital, Ewha Womans University Mokding Hospital, Incheon St. Mary’s Hospital, Inha University Hospital, Chonnam National University Hospital, Chonbuk National University Hospital, Jeju National University Hospital, Soonchunhyang University Hospital Cheonan, Hallym University Chuncheon Sacred Heart Hospital, Hallym University Sacred Heart Hospital, and Hanyang University Guri Hospital. We also received approval from each center to use their subjects’ clinical records for the study while maintaining the confidentiality of the data.
CB was defined using the following self-administered questionnaires: 1) Do you experience a cough most days, for at least 3 months per year? 2) Have you had a cough for more than 2 consecutive years? 3) Do you produce sputum most days, for at least 3 months per year? 4) Have you had sputum for more than 2 consecutive years? If the patient answered “yes” to all questions, then the subject was classified as having CB.Citation14 Patients who answered “I don’t know” or did not answer a question were excluded from the study.
Clinical characterizations
We evaluated the difference in symptoms and COPD-related health status between CB and non-CB patients using the mMRC scale (range 0–4, higher scores mean worse), St George’s Respiratory Questionnaire for COPD patients (SGRQ-C, range 0–100, higher scores mean worse), and CAT score (range 0–40, higher scores mean worse). Specifically, we focused on the CAT score, which is the sum of eight items related to cough (CAT1), phlegm (CAT2), chest tightness (CAT3), breathlessness (CAT4), activity limitations (CAT5), confidence in leaving home (CAT6), sleep (CAT7), and energy (CAT8). The total CAT score was reviewed in addition to each item score to evaluate the symptoms and health status rather than just cough and sputum. The differences in age, sex, and pulmonary function test results of the two phenotypes were also analyzed. FEV1, CAT score, exacerbation, history of admission, and emergency room visitation were used to categorize patients into GOLD 2006 and revised GOLD 2015 stages to evaluate the prevalence of the CB phenotype in each stage.Citation1,Citation21 We defined patients with a CAT score ≥10 as the more symptom group and those with a CAT score <10 as the less symptom group. We also classified patients with a post-BD FEV1 <50%, or a history of exacerbation ≥2/year, or admission to hospital ≥1/year as the high-risk group and those without as the low-risk group.
Statistical analysis
Statistical analysis was performed with the SAS 9.3 (SAS Institute Inc., Cary, NC, USA) software. Quantitative variables are shown as mean ± standard deviation, and categorical variables are reported as numbers and percentages. The total number in each analysis set is provided in . Categorical variables (eg, sex, current smoker, and CAT score ≥10) were compared between groups using a chi-square test. Continuous variables (eg, age, body mass index, mMRC, SGRQ-C, CAT, post-BD FVC, post-BD FEV1, and post-BD FEV1/FVC ratio) were assessed using a Student’s t-test. Differences in categorical variables (eg, the GOLD stage) were assessed using a Mantel–Haenszel test. A P-value of less than 0.05 was considered to be statistically significant.
Table 1 Baseline characteristics of the COPD patients (n=1,106)
A multiple logistic regression analysis was performed to assess the independent association of CB phenotype, sex, age, current smoking, and post-BD FEV1 on the more symptom group. To assess the independently associated factors for the high-risk group, we used a similar logistic regression model, excluding the post-BD FEV1 variable as it is a discriminating factor for both the high- and low-risk groups. Results from the logistic regression are presented with odds ratios (ORs) and 95% confidence intervals (CIs). A Hosmer–Lemeshow test was conducted to assess the goodness of fit for the logistic regression models.
Results
Baseline characteristics and prevalence of CB
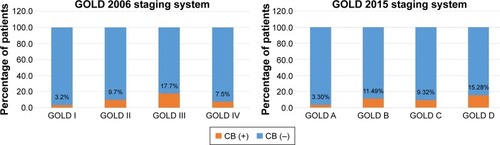
A total of 1,148 patients were enrolled in the KOCOSS cohort study from April 2012 to May 2015. Forty patients were excluded due to missing data and two patients were excluded due to errors in the data set. Therefore, data analysis was performed on 1,106 subjects. The baseline characteristics of the study population are shown in . The prevalence of CB was reported to be 11.5% (n=127). Male sex was predominant in both the non-CB and CB groups, and no significant difference was identified. The subjects in the non-CB group were significantly older than those in the CB group. The CB group had higher mMRC, SGRQ-C, and CAT scores, which indicated more symptoms and poorer COPD-related health status. The post-BD FEV1 and FEV1/FVC ratio were lower in the CB group and showed statistical significance. Among these statistically significant different variables, age difference was not clinically significant because it was minimal. Other variables (current smoker, mMRC, SGRQ-C, CAT, and lung function) showed clinically significant differences between the two groups. The percentage of CB according to GOLD stage is shown in . With the GOLD 2006 staging system, CB was most common in GOLD III (17.7%) and least common in GOLD I (3.2%), with a P-value of 0.0309. With the GOLD 2015 staging system, the CB phenotype was most common in GOLD D (15.28%) and least common in GOLD A (3.30%), with a P-value of 0.0001.
Comparison of CAT scores between CB and non-CB patients
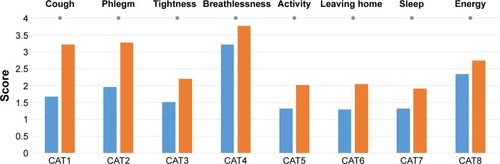
We compared the CAT scores between CB and non-CB patients. The total CAT score was statistically higher in the CB patients (14.6±7.3 vs 21.2±8.2, P<0.01). We also analyzed all eight items that comprise the CAT score questionnaire, and the scores of all items were found to be statistically higher in the CB patients (). The CAT1 and CAT2 scores showed the greatest difference between CB and non-CB patients, and the CAT8 score showed the least ().
Table 2 Comparison of each of the eight items of CAT scores between CB and non-CB patients
Comparison between the more and less symptom groups
The logistic regression for the more symptom group is shown in . The CB phenotype and post-BD FEV1 (% predicted) were identified as independently associated factors for the more symptom group. The OR for the CB phenotype was 2.12 (95% CI, 1.19–3.77).
Table 3 Multiple logistic regression analysis of the associated factors for the more symptom group
Comparison between the high- and low-risk groups
The logistic regression for the high-risk group is shown in . In the regression model, the CB phenotype was shown to be an independently associated factor for the high-risk group. The OR for the CB phenotype was 1.55 (95% CI, 1.03–2.34).
Table 4 Multiple logistic regression analysis of associated factors for the high-risk group
Discussion
In this large, multicenter, observational study, we found that the CAT score was higher in the CB group than in the non-CB group, even in terms of the subquestionnaires. It is evident from the definition that the CB group had more coughs (CAT1) and sputum (CAT2). However, the fact that other items (CAT3–8) were also significantly higher in the CB group is of note. This indicates that CB patients have more symptoms, such as breathlessness and chest tightness, and a poor COPD-related health status, such as confidence in leaving home, activity limitations, sleep, and energy levels. High CAT score in the CB group may be partially because of CB definition. However, despite that, SGRQ-C, lung function, and mMRC were also significantly different between CB and non-CB groups. This result suggests that high CAT score in the CB group represents high disease burden and poor quality of life in these patients.
We divided the patients with GOLD 2006 and GOLD 2015 stages into separate groups and analyzed the prevalence of CB in each group. Previous studies have shown that the proportion of the CB phenotype increases with GOLD 2006 stage.Citation6,Citation7,Citation13 However, in this study, the proportion of CB phenotype increased from GOLD I to III but the correlation was reversed in GOLD IV group. Moreover, with the GOLD 2015 staging system, we found that CB was most prevalent in GOLD D (15.28%), followed by GOLD B (11.49%). This may be associated with a high CAT score in the GOLD B and D stages, by definition. A previous study by Han et al as part of the COPDGene study reported similar results, but in this case CB was more prevalent in GOLD B than in GOLD D (35% vs 32.7%).Citation26
In this study, we determined that the CB phenotype was an independent factor for the more symptom group. Recent studies have demonstrated that the CB group has poor symptoms and quality of life, but these studies were mostly based on other clinical indices such as the mMRC or SGRQ scores, unlike our study.Citation7,Citation13,Citation15–Citation18,Citation27 In the ECLIPSE study, Agusti et al reported significant differences in mMRC and SGRQ scores between the CB and non-CB groups in an overall group of COPD patients (P<0.0001), but these did not correspond to each GOLD subgroup.Citation7 However, differences in the SGRQ scale were statistically significant in all GOLD groups. In the COPDGene study, Kim et al found that the CB group was more symptomatic than the non-CB group according to the mMRC (2.55±1.31 vs 2.11±1.41, P<0.0001) and SGRQ (49.9±19.7 vs 36.6±20.0, P<0.0001) scales.Citation17 In the PLATINO study, de Oca et al assessed the CB phenotype using multivariate analysis and concluded that the CB phenotype was associated with additional symptoms such as wheezing (OR 2.40) and dyspnea (OR 2.42), and also with a poor general health status assessed by the Short Form-12 generic quality of life questionnaire (OR 0.60).Citation13
Previous studies have shown that the CB phenotype is associated with a high rate of exacerbation and poor lung function.Citation6,Citation9,Citation15,Citation17 In addition, some reports have indicated that the CB phenotype is associated with frequent hospitalization.Citation28 The results of the present study correspond well with those of earlier studies. We performed multivariate analysis to prove that the CB phenotype is an independent risk factor for the high-risk group.
In this study, CB was prevalent in 11.5% of the COPD patients. This result is relatively lower than previous studies, which reported a prevalence of 14%–74%.Citation6,Citation9 Considering that the prevalence of CB in clinical-based studies was higher (27.3%–74.1%) than population-based studies (14%–30%), our clinical study showed a much lower prevalence than previous studies.Citation6 However, because the definition of CB is variable, our definition using chronic cough and sputum may have lowered the prevalence compared with other studies that defined CB with chronic sputum only.Citation10,Citation13,Citation29,Citation30 Additionally, the definition of CB can be ambiguous when translated into Korean, especially the phrase “2 consecutive years”. Therefore, patients may not have understood the questionnaire, and this may have caused a reduction in the response rate.
Our study has several limitations. First, subjects in the study were recruited from populations who were receiving treatment from the participating hospitals – these hospitals are mainly tertiary medical centers. Therefore, our results may not be consistent with the general population. Second, this was a cross-sectional study. In this study, we did not use follow-up data relating to exacerbation. Although prospective follow-up data for exacerbation exist for this cohort, the data were insufficient because the cohort is relatively new and therefore the follow-up period was short in most cases. When we classified the high- and low-risk groups, we used a simple question relating to the previous history of exacerbation over 1 year. Thus, there is the potential for some recall bias, and the history of exacerbation may have been underestimated in some patients. Third, there is a possibility of misclassification of CB patients in this study given the self-report status and potential misunderstanding of the question in Korean. This may underestimate the prevalence of CB in this cohort. As a result, among real CB patients, only some more symptomatic CB patients may be classified as CB group in this study. This may have affected the result of this study.
Conclusion
In summary, our data support the fact that CB patients have a higher CAT score than non-CB patients. This is consistent not only in terms of the total CAT score but also for each subquestionnaire in addition to those querying cough and sputum. Moreover, this study revealed that the CB phenotype is an independent risk factor for both the more symptom and high-risk groups, as defined by GOLD 2015 guidelines.
Disclosure
The authors report no conflicts of interest in this work.
References
- RabeKFHurdSAnzuetoAGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2007176653255517507545
- AbuDaggaASunSXTanHSolemCTHealthcare utilization and costs among chronic bronchitis patients treated with maintenance medications from a US managed care populationJ Med Econ201316342142923336296
- BlanchetteCMRobertsMHPetersenHDalalAAMapelDWEconomic burden of chronic bronchitis in the United States: a retrospective case–control studyInt J Chron Obstruct Pulmon Dis20116738121311695
- PasqualeMKSunSXSongFHartnettHJStemkowskiSAImpact of exacerbations on health care cost and resource utilization in chronic obstructive pulmonary disease patients with chronic bronchitis from a predominantly Medicare populationInt J Chron Obstruct Pulmon Dis2012775776423152680
- RheeCKPhenotype of asthma-chronic obstructive pulmonary disease overlap syndromeKorean J Intern Med201530444344926161009
- CorhayJLVinckenWSchlesserMBossuytPImschootJChronic bronchitis in COPD patients is associated with increased risk of exacerbations: a cross-sectional multicentre studyInt J Clin Pract201367121294130124246208
- AgustiACalverleyPMCelliBCharacterisation of COPD heterogeneity in the ECLIPSE cohortRespir Res20101112220831787
- AgustiAPhenotypes and disease characterization in chronic obstructive pulmonary disease. Toward the extinction of phenotypes?Ann Am Thoracic Soc201310SupplS125S130
- KimVCrinerGJChronic bronchitis and chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013187322823723204254
- BurgelPRChronic cough and sputum production: a clinical COPD phenotype?Eur Respir J20124014622753831
- ManninoDMChronic obstructive pulmonary disease: definition and epidemiologyRespir Care2003481211851191 discussion 1191–118314651759
- Allen-RameyFCGuptaSDiBonaventuraMDPatient characteristics, treatment patterns, and health outcomes among COPD phenotypesInt J Chron Obstruct Pulmon Dis2012777978723226014
- de OcaMMHalbertRJLopezMVThe chronic bronchitis phenotype in subjects with and without COPD: the PLATINO studyEur Respir J2012401283622282547
- KanervistoMSaarelainenSVasankariTCOPD, chronic bronchitis and capacity for day-to-day activities: negative impact of illness on the health-related quality of lifeChronic Respir Dis201074207215
- KimVCrinerGJThe chronic bronchitis phenotype in chronic obstructive pulmonary disease: features and implicationsCurr Opin Pulm Med201521213314125575367
- KimVDaveyAComellasAPClinical and computed tomographic predictors of chronic bronchitis in COPD: a cross sectional analysis of the COPDGene studyRespir Res2014155224766722
- KimVHanMKVanceGBThe chronic bronchitic phenotype of COPD: an analysis of the COPDGene StudyChest2011140362663321474571
- RamosFLKrahnkeJSKimVClinical issues of mucus accumulation in COPDInt J Chron Obstruct Pulmon Dis2014913915024493923
- BeckerCSchaferJCarvalhoLLVitielloIPda SilvaALCAT correlates positively with respiratory rate and is a significant predictor of the impact of COPD on daily life of patients: a cross sectional studyMultidiscip Respir Med2014914725485107
- JonesPWHardingGBerryPWiklundIChenWHKline LeidyNDevelopment and first validation of the COPD Assessment TestEur Respir J200934364865419720809
- FuJJMcDonaldVMGibsonPGSimpsonJLSystemic inflammation in older adults with asthma – COPD overlap syndromeAllergy Asthma Immunol Res20146431632424991455
- LadeiraIGomesTCastroARibeiroCGuimaraesMTaveiraNThe overall impact of COPD (CAT) and BODE index on COPD male patients: correlation?Rev Port Pneumol2015211111525854130
- LeeSDHuangMSKangJThe COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patientsRespir Med2014108460060824456695
- MackayAJDonaldsonGCPatelARJonesPWHurstJRWedzichaJAUsefulness of the chronic obstructive pulmonary Disease Assessment Test to evaluate severity of COPD exacerbationsAm J Respir Crit Care Med2012185111218122422281834
- VarolYOzacarRBalciGUstaLTaymazZAssessing the effectiveness of the COPD Assessment Test (CAT) to evaluate COPD severity and exacerbation ratesCOPD201411222122524111793
- HanMKMuellerovaHCurran-EverettDGOLD 2011 disease severity classification in COPDGene: a prospective cohort studyLancet Respir Med201311435024321803
- FerreAFuhrmanCZureikMChronic bronchitis in the general population: influence of age, gender and socio-economic conditionsRespir Med2012106346747122197577
- VestboJPrescottELangePAssociation of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study GroupAm J Respir Crit Care Med19961535153015358630597
- PrescottELangePVestboJChronic mucus hypersecretion in COPD and death from pulmonary infectionEur Respir J199588133313387489800
- MiravitllesMGuerreroTMayordomoCSanchez-AgudoLNicolauFSeguJLFactors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study GroupRespir Int Rev Thoracic Dis2000675495501