Abstract
Background
Little is known regarding the relationship between balance impairments and physical activity in COPD. There has been no study investigating the relationship between balance and objectively measured physical activity. Here we investigated the association between balance and physical activity measured by an activity monitor in elderly COPD patients.
Materials and methods
Twenty-two outpatients with COPD (mean age, 72±7 years; forced expiratory volume in 1 second, 53%±21% predicted) and 13 age-matched healthy control subjects (mean age, 72±6 years) participated in the study. We assessed all 35 subjects’ balance (one-leg standing test [OLST] times, Short Physical Performance Battery total scores, standing balance test scores, 4 m gait speed, and five-times sit-to-stand test [5STST]) and physical activity (daily steps and time spent in moderate-to-vigorous physical activity per day [MV-PA]). Possible confounders were assessed in the COPD group. The between-group differences in balance test scores and physical activity were analyzed. A correlation analysis and multivariate regression analysis were conducted in the COPD group.
Results
The COPD patients exhibited significant reductions in OLST times (P=0.033), Short Physical Performance Battery scores (P=0.013), 4 m gait speed (P<0.001), five-times sit-to-stand times (P=0.002), daily steps (P=0.003), and MV-PA (P=0.022) compared to the controls; the exception was the standing balance test scores. The correlation and multivariate regression analyses revealed significant independent associations between OLST times and daily steps (P<0.001) and between OLST times and MV-PA (P=0.014) in the COPD group after adjusting for possible confounding factors.
Conclusion
Impairments in balance and reductions in physical activity were observed in the COPD group. Deficits in balance are independently associated with physical inactivity.
Introduction
Physical activity levels are significantly decreased in individuals with COPD compared to age-matched healthy controls.Citation1–Citation3 Physical inactivity has adverse effects on lung function, muscle mass, exercise tolerance, and mortality.Citation4–Citation6 Many researchers have investigated the relationships between physical activity and clinical characteristics to reveal the mechanisms in understanding physical inactivity in COPD. For instance, pulmonary function, dyspnea, body composition, extrapulmonary function, and health status have been shown to have significant associations with physical activity in COPD.Citation7,Citation8
The latest systematic review of the determinants of physical activity in COPD indicated that balance might also be associated with physical activity in COPD.Citation8 The association between balance and physical activity in COPD has not yet been established, however. Only two studies examined the relationship between balance and self-reported physical activity in COPD.Citation9,Citation10 In addition, the accuracy of questionnaires that have been used for assessing physical activity is lower than that of objective measurement tools, mainly due to recall bias.Citation11 The relationship between balance and physical activity should thus be investigated by using objective measurement tools for assessing physical activity.
Balance impairments have been commonly recognized in COPD.Citation9,Citation10,Citation12 Beauchamp et al investigated balance impairments in COPD using the Balance Evaluation Systems Test.Citation9 This test evaluates six subcomponents of balance: biomechanical constraints, stability limits/verticality, anticipatory adjustments/transitions, postural responses, sensory orientation, and stability in gait. In the Beauchamp study, COPD patients had deficits in all six subcomponents of the test compared to healthy controls, with marked deficits in biomechanical constraints, anticipatory adjustments/transitions, and stability in gait.Citation9 Although there is insufficient evidence to conclude that there is an association between balance and physical activity, subcomponents of balance such as transitions and gait are essential for independent living and are main components of physical activity.Citation13,Citation14
The aim of our present study, therefore, was to investigate the association between balance impairments and physical activity as measured by an activity monitor in COPD patients. We hypothesized that impaired balance would be associated with physical inactivity in COPD.
Materials and methods
Subjects
Twenty-two stable elderly COPD patients (all male; age 71.6±6.9 years) who had undergone pulmonary rehabilitation in our hospital were enrolled in this study. All 22 patients met the following criteria: diagnosis of COPD according to international guidelinesCitation15 and provided written informed consent. The exclusion criteria were as follows: an inability to communicate, use of medication(s) that may increase the risk of falls, and neurologic or musculoskeletal conditions that limit mobility. Thirteen healthy age-matched male control subjects with normal spirometry (forced expiratory volume in 1 second [FEV1] ≥80% predicted, FEV1/forced vital capacity ≥0.7) were recruited at a local community center. The exclusion criterion was absence of any health problems that may have impaired mobility or postural control.
Study design
A cross-sectional study design was used. All subjects underwent clinical balance tests and physical activity measures. The COPD patients also performed physical function tests and completed two questionnaires. Pulmonary function data were retrieved from the clinical records of the COPD patients and obtained through simple spirometry in the healthy controls. The pulmonary function data of the COPD patients were measured within the 3 months before the balance tests and physical activity measures. This study was reviewed and approved by the Ethics Committee of Akita City Hospital, 2015 (accepted No 19), and carried out in conformity with the Declaration of Helsinki.
Clinical balance measures
The clinical balance tests included the one-leg standing test (OLST) and the Short Physical Performance Battery (SPPB) to assess four subcomponents of balance: anticipatory adjustments, standing balance, gait stability, and transitions.
The OLST was performed as described by Michikawa et al.Citation16 The length of time that each subject was able to stand on one leg without any support was measured. The test was over after 60 seconds had elapsed, when the stance foot shifted, or when the lifted foot was replaced on a board supplied for the test. The subjects were given two trials unless they were able to complete 60 seconds on the first trial. The better of the two trial times was recorded.
The SPPB consists of three components: a standing balance test, a 4 m gait test (4MGT), and a five-times sit-to-stand test (5STST).Citation17 The standing balance test evaluates the subject’s ability to stand with the feet together side-by-side, in a semi-tandem position and in a tandem position for 10 seconds. The 4MGT evaluates the time that a subject requires to walk 4 m. The 5STST evaluates the time that a subject requires to rise from a chair and return to the seated position five times.Citation17 The three SPPB components were scored from 0 to 4, with higher scores indicating better balance. The SPPB total scores, standing balance test scores, 4 m gait speed (4MGS), and 5STST times were calculated. The reliability of the 4MGT and 5STST is well established in patients with COPD.Citation18
In the present study, we used the OLST times as the measure of anticipatory adjustment,Citation9 the standing balance test scores as the measure of standing balance,Citation17 the 4MGS results as the measure of gait stability,Citation9,Citation19 and the 5STST times as the measure of transitions.Citation9,Citation20 The SPPB total scores were also used to assess the subjects’ balance abilities.
Objectively measured physical activity
Each subject’s physical activity was objectively measured using an accelerometer-based activity monitor (Lifecorder™, Suzuken Co., Nagoya, Japan). The activity monitor (625 × 465 × 260 mm3, 40 g) was firmly attached to a belt or the waistband of the subject’s clothing on at least five weekdays.Citation11 The number of the subject’s average daily steps and the time spent in moderate-to-vigorous physical activity per day (MV-PA) were calculated as their physical activity levels. Daily steps may include activities such as slow and fast walking, household work, or shopping.Citation13,Citation14 Moderate-to-vigorous physical activity, equivalent to >3.0 metabolic equivalents, may include activities such as brisk walking, climbing stairs, aerobics, or heavy household work.Citation21 The validity and reliability of this method have been demonstrated.Citation22,Citation23
Possible confounders
We measured pulmonary function, nutritional status, dyspnea, quadriceps muscle strength, functional exercise capacity, and health-related quality of life as possible confounders in the COPD group. These confounders may affect balance and physical activity in COPD.Citation8,Citation12
For the pulmonary function, FEV1 was measured using a spirometer (Chestgraph Jr. HI-101, Chest M.I., Inc., Tokyo, Japan) by well-trained physical therapists following the American Thoracic Society (ATS) guideline.Citation24 The %FEV1 was calculated following the ATS guidelineCitation24 and the reference values provided by Berglund et al.Citation25 The subjects’ body mass index was measured as an indicator of their nutritional status. The degree of dyspnea was measured using the modified Medical Research Council (MMRC) dyspnea scale.Citation26
For the quadriceps muscle strength data, we measured each subject’s quadriceps isometric maximum voluntary contraction normalized to body weight (QMVC) using the Hydromusculator GT-160 (OG Giken Co., Okayama, Tokyo) in accordance with the method described by Sugawara et al.Citation27 The 6-minute walk distance (6MWD) was measured as an indicator of functional exercise capacity. The subjects performed the 6MWD test according to the European Respiratory Society/ATS technical standard.Citation28 For the health-related quality of life data, each subject completed the COPD assessment test, and the total score was calculated.Citation29
Statistical analysis
The statistical analyses were performed using SPSS software (Version 22.0 for Windows; IBM Corporation, Armonk, NY, USA). The assumption of normality was assessed by graphical and statistical methods. For all analyses, a P-value <0.05 was considered significant. Independent samples t-tests were used to determine between-group differences for continuous variables, and the Mann–Whitney U-test was used for nonparametric and discrete variables. Pearson correlations and Spearman rank correlations were used to determine the relationships among balance, physical activity, and the possible confounders in the COPD patients.
We performed forced-entry multivariate linear regression analyses to determine the variables independently associated with balance and physical activity in the COPD patients. Analysis 1 was performed to determine the variables influencing balance. One balance measure with the strongest correlations to daily steps and MV-PA was used as the dependent variable. One physical activity outcome with the strongest correlations to the balance measure and possible confounders that correlated with the balance measure were used as predictors.
Analysis 2 was performed to explore the variables influencing daily steps. The number of daily steps was used as the dependent variable. One balance measure with the strongest correlation to daily steps and possible confounders that correlated to daily steps were used as the predictors.
Analysis 3 was performed to detect the variables influencing the MV-PA times. The MV-PA time was used as the dependent variable. One balance measure with the strongest correlation to MV-PA and possible confounders that correlated to MV-PA were used as the predictors.
The three analyses were divided into two steps to adjust for age and possible confounders, in accordance with the method described by Beauchamp et al.Citation9 In the first step, age was included in the models independently of statistical significance. In the second step, the respective predictors were included in the models.
Power analysis was performed with the program G*PowerCitation30 for a multivariate regression with five predictors, an α of 0.05, and power of 0.80. A sample size of 43 was required to detect a large (Cohen f2=0.35) effect size.
Results
The subjects’ characteristics are given in . The COPD group showed significant expiratory airflow limitation consistent with GOLD (Global Initiative for Chronic Obstructive Pulmonary Disease) stage II disease.Citation15 The healthy control subjects had normal spirometry data and were well matched for age and body composition.
Table 1 Characteristics of the COPD patients and healthy controls
The results from the clinical balance tests, the physical activity measures, and the possible confounders are shown in . The COPD patients demonstrated significantly shorter OLST times (P=0.033), lower SPPB total scores (P=0.013), slower 4MGS (P<0.001), and slower 5STST times (P=0.002). In contrast, there were no significant differences between the COPD and control groups in the standing balance test scores. Significantly reduced number of daily steps (P=0.003) and MV-PA times (P=0.014) were evident in the COPD patients compared to the controls.
Table 2 Balance, physical activity, and the possible confounders in COPD patients and controls
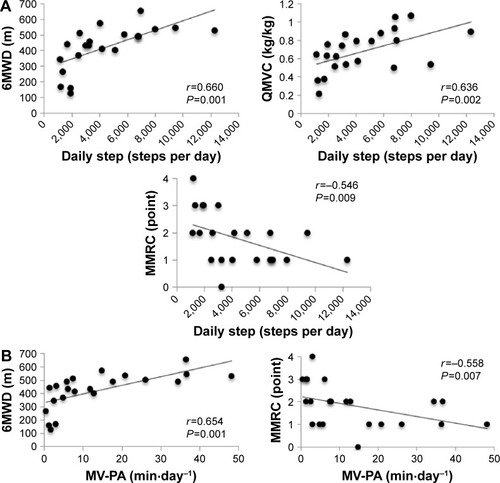
The results of the correlation analysis are given in . The OLST times were significantly associated with daily steps (P<0.001), MV-PA (P=0.001), QMVC (P=0.017), and 6MWD (P=0.016) (). The SPPB total scores were significantly associated with daily steps (P=0.006), MV-PA (P=0.002), MMRC (P=0.020), and 6MWD (P=0.004) (). The 4MGS was significantly correlated with MMRC (P=0.015), QMVC (P=0.010), and 6MWD (P=0.001). Daily steps were also significantly associated with 6MWD (P=0.001), MMRC (P=0.002), and QMVC (P=0.009) (). MV-PA was also significantly associated with 6MWD (P=0.001) and MMRC (P=0.007) ().
Table 3 Correlations among balance, physical activity, and the possible confounders in the COPD patients (n=22)
Figure 1 Correlations between balance measures and physical activity and possible confounders in subjects with COPD (n=22).
Notes: (A) OLST and (B) SPPB.
Abbreviations: OLST, one-legged standing test; SPPB, Short Physical Performance Battery; MV-PA, time spent in moderate-to-vigorous physical activity per day; 6MWD, 6-minute walk distance; QMVC, quadriceps isometric maximum voluntary contraction normalized to body weight; MMRC, modified Medical Research Council.
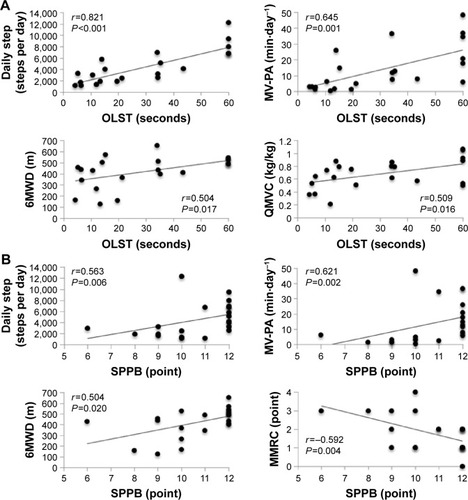
Figure 2 Correlations between physical activity and possible confounders in subjects with COPD (n=22).
Abbreviations: MV-PA, time spent in moderate-to-vigorous physical activity per day; 6MWD, 6-minute walk distance; QMVC, quadriceps isometric maximum voluntary contraction normalized to body weight; MMRC, modified Medical Research Council.
In the three multivariate regression analyses, the OLST times were used as an indicator of balance because the OLST times had the strongest correlations to the daily steps and MV-PA among the five balance measures. provides the results of Analysis 1 using the OLST times as the dependent variable and daily steps, QMVC, and 6MWD as predictors. Analysis 1 including age, daily steps, 6MWD, and QMVC explained 70% of the variance in the OLST times (P<0.001). Only daily steps were independently associated with OLST times (P<0.001).
Table 4 The independent variables influencing the OLST times in the COPD patients (n=22)
summarizes the results of Analysis 2 using daily steps as the dependent variable and the OLST times, MMRC, QMVC, and 6MWD as predictors. Analysis 2 including age, the OLST times, 6MWD, QMVC, and MMRC explained 73% of the variance in the daily steps (P<0.001). Only the OLST times were independently associated with daily steps (P<0.001). shows the results of Analysis 3 using the MV-PA as the dependent variable and the OLST times, 6MWD, and MMRC as predictors. Analysis 3 including age, the OLST times, 6MWD, and MMRC explained 52% of the variance in the MV-PA (P=0.002). The OLST times (P=0.014) and 6MWD (P=0.024) were independently associated with MV-PA.
Table 5 The independent variables influencing daily steps in the COPD
Table 6 The independent variables influencing MV-PA in the COPD patients (n=22)
Discussion
The present study is the first to demonstrate an association between balance and objectively measured physical activity. As noted in the section “Introduction”, only two studies have investigated the relationship between balance and self-reported physical activity in COPD.Citation9,Citation10 Our present work extends these observations by using objective measurement tools for assessing physical activity. Three novel findings regarding COPD patients were obtained in this study. First, their physical activity (daily steps and MV-PA), exercise capacity, lower muscle strength, and dyspnea were associated with their balance. Second, the patients’ balance, exercise capacity, lower muscle strength, and dyspnea were correlated with daily steps, and balance, exercise capacity, and dyspnea were associated with the MV-PA. Third, the COPD patients’ balance was independently associated with the number of their daily steps adjusting for possible confounding variables.
We observed that our COPD patients had deficits in anticipatory adjustments, gait stability, and transitions compared to the healthy controls. Nonsignificant reductions in standing balance were observed in the COPD group. It is difficult to directly compare our results with those of previous studies because the characteristics of the study subjects differed. However, the present differences in OLST times (−16.0 seconds) and SPPB total scores (−1.3 points) exceeded the previously reported differences in both measurements between COPD and healthy controls (−13.4 secondsCitation31 and −1.0 points,Citation32 respectively). Deficits in the 4MGS (−0.49 m⋅s−1) and 5STST times (3.30 seconds) also exceeded the minimal clinically important thresholds previously identified for these measures in COPD (0.11 m⋅s−1;Citation33 and 1.7 seconds,Citation34 respectively). In contrast, our COPD patients showed a nonsignificant difference in the standing balance test scores.
A possible explanation for these results may be that the ability to maintain a calm standing position is less likely to be impaired in COPD. Kawagoshi et alCitation35 revealed that ambulatory COPD patients had similar values of daily time spent standing compared to age-matched healthy controls,Citation35 which suggests that standing balance may be maintained in ambulatory COPD patients. In the present study, all of the COPD patients were able to walk without any supports, and they might spend enough time standing each day to maintain their standing balance. Thus, there were no significant differences in standing balance test scores between our COPD and control groups.
Our COPD patients showed reduced physical activity. The mean of their daily steps was 4,546 steps per day, which is lower than a step-defined sedentary lifestyle threshold (5,000 steps per day)Citation36 in adults and the mean number of daily steps (5,876 steps per day) that was demonstrated in COPD patients with similar ages and disease severity.Citation37 The present deficits in daily steps compared to the healthy controls (−4,167 steps per day) also exceeded a clinically important difference for daily steps in older adults (2,000 steps per day).Citation38 We also observed that the MV-PA was significantly reduced in our COPD patients. The mean MV-PA in the COPD group was 13.9 min⋅day−1, which is approximately one-half as long as that of the control group (27.4 min⋅day−1). The mean was also lower than a recommended level of physical activity for adultsCitation11 (30 minutes of at least moderate intensity physical activity on 5 or more days every week). These results are consistent with previous studies and emphasize that individuals with COPD have impaired balance and reduced physical activity levels compared to healthy controls.
In our study, physical activity (daily steps and MV-PA), exercise capacity, muscle strength, and dyspnea were associated with balance. Only physical activity had an independent relationship with balance in COPD after adjusting for possible confounding variables. Although a relationship between self-reported physical activity and balance has been shown,Citation9 the present study is the first to explore the relationship between balance and physical activity measured by an activity monitor in this patient population. Our finding of an association between balance and physical activity was consistent with previous results.Citation9 The correlation between balance and physical activity in the present study (OLST – r=0.821; SPPB total – r=0.563) exceeded that in the previous study (r=0.040).Citation9
The relationship between balance and physical activity has been less studied. Some research groups suggest that sedentary older adults have less postural control than their more active peers.Citation39 Kawagoshi et alCitation35 reported that time spent at slow walking (<2 km⋅h−1), fast walking (>2 km⋅h−1), and the frequency of standing up were significantly lower in COPD patients compared to healthy controls.Citation35 That study suggests that COPD patients may have reduced physical activity that requires anticipatory adjustments, gait stability, and transitions such as slow and fast walking, standing up from a chair, and climbing and descending stairs. In the present study, the COPD patients had significant lower numbers of daily steps and shorter MV-PA, and thus we also observed that the physical activity requiring dynamic balance might be also reduced in COPD. Such a sedentary lifestyle may lead to deterioration in balance.
Muscle weakness is a well-established risk factor for impaired balance in individuals in COPD.Citation9 Exercise intolerance may be associated with balance in COPD.Citation40 Dyspnea may also be related to balance in COPD populations.Citation10 In a previous study, “fallers” with COPD had significantly greater reductions in CRQ dyspnea scores compared to non-fallers with COPD.Citation10 Based on our present findings and those of previous studies, reduced physical activity in COPD may contribute to impairments in balance independent of some possible confounding factors, such as loss of muscle strength, exercise intolerance, and dyspnea.
In the present study, balance, exercise capacity, lower muscle strength, and dyspnea were associated with the number of daily steps. Only balance was independently associated with daily steps in the COPD patients after the adjustment for possible confounding variables. Balance, exercise capacity, and dyspnea were also associated with MV-PA. Balance and exercise capacity had independent associations with MV-PA in our COPD group.
These results are consistent with those of previous studies,Citation7,Citation8 and they emphasize that balance impairments lead to reductions in physical activity in COPD patients. Deficits in balance are well-established predictors of activity limitations such as walking around the house and sitting/standing transferring among elderly people.Citation16,Citation41 Shorter OLST times, for example, have been shown to be a marker of activity limitations in elderly people.Citation16 Poor performance in walking and rising from a sitting position has a good predictive value of activity limitations in elderly people.Citation41 Durutürk and TongaCitation42 investigated the relationship between balance and activity limitations assessed by the London Chest Activities of Daily Living (LCADL) scale in COPD.Citation42 The scale consists of four subcomponents of activities of daily livings: self-care activities, household activities, transitional activities, and leisure time activities.Citation43 Deficits in balance were significantly associated with LCADL household activities and leisure time activities.Citation42 COPD patients with balance impairments may thus choose a sedentary lifestyle in order to prevent falls while engaging in activities of daily livings.
Exercise intolerance,Citation8 muscle weakness,Citation7 and dyspneaCitation8 have also been shown to affect physical activity in COPD. In the present study, exercise capacity measured by the 6MWD test had an independent association with MV-PA, but not with daily steps. Additionally, the association of 6WMD with MV-PA (β=0.645) was greater than that with daily steps (β=0.339). These results are consistent with those of a previous study.Citation44 That study investigated the relationships between self-reported physical activity and 6MWD in COPD patients, and the results indicated that time spent in high physical activity per week was more strongly associated with 6MWD than light physical activity.Citation44 These findings suggest that in COPD, exercise capacity may have a greater impact on the intensity of physical activity compared to the total amount of physical activity. The results of the present study and those of the previous studies suggest that balance impairments may also lead to a sedentary lifestyle independently of some confounding factors in COPD.
Here we showed a possible bidirectional association between balance and physical activity in COPD, which indicates that deficits in balance and physical inactivity may affect each other. Beauchamp et alCitation45 reported that a standardized 6-week multidisciplinary pulmonary rehabilitation (PR) program contributed to minor improvements in balance in COPD patients. They suggested that balance training should be included in a PR program to improve balance.Citation45 Our present findings emphasize the importance of balance training in PR for improving not only balance but also the level of physical activity, especially in COPD patients with impaired balance.
Certain limitations must be taken into consideration when interpreting our results. Because the design of this study was cross-sectional, the causality or directionality of the findings is uncertain. Because the sample size was small for a regression analysis with five predictors to achieve sufficient statistical power, larger prospective studies are required to establish the relationship between balance and objectively measured physical activity in COPD.
Conclusion
In summary, we note that our patients with COPD had reduced performance in three subcomponents of balance (anticipatory adjustments, walking stability, and transitions) and lower physical activity (daily steps and MV-PA) compared to age-matched healthy control subjects. There was an independent association between impaired balance and low number of daily steps after adjusting for possible confounding factors. In light of the present and previous findings,Citation9 a balance evaluation should be conducted to assess not only the risk of falling but also the causes of physical inactivity, especially in elderly COPD patients who are at risk of falling and/or have reduced physical activity. There is a clear need for further research to clarify the causal direction between balance and physical activity in people with COPD.
Acknowledgments
The authors acknowledge the members of the Department of Rehabilitation at Akita City Hospital (C Kasai, N Kiyokawa, T Watanabe, Y Ishikawa, and M Oshima) for their assistance in data collection.
Disclosure
The authors report no conflicts of interest in this work.
References
- WatzHWaschkiBMeyerTMagnussenHPhysical activity in patients with COPDEur Respir J20093326227219010994
- PittaFTroostersTSpruitMAProbstVSDecramerMGosselinkRCharacteristics of physical activities in daily life in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200517197297715665324
- VorrinkSNKortHSTroostersTLammersJWLevel of daily physical activity in individuals with COPD compared with healthy controlsRespir Res2011123321426563
- Garcia-AymerichJLangePSerraISchnohrPAntóJMTime-dependent confounding in the study of the effects of regular physical activity in chronic obstructive pulmonary disease: an application of the marginal structural modelAnn Epidemiol2008181077578318708291
- WaschkiBKirstenAMHolzODisease progression and changes in physical activity in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2015192329530626020495
- VaesAWGarcia-AymerichJMarottJLChanges in physical activity and all-cause mortality in COPDEur Respir J20144451199120925063247
- OsthoffAKTaeymansJKoolJMarcarVvan GestelAJAssociation between peripheral muscle strength and daily physical activity in patients with COPD: a systematic literature review and meta-analysisJ Cardiopulm Rehabil Prev201333635135924142041
- Gimeno-SantosEFreiASteurer-SteyCDeterminants and outcomes of physical activity in patients with COPD: a systematic reviewThorax201469873173924558112
- BeauchampMKSibleyKMLakhaniBImpairments in systems underlying control of balance in COPDChest20121411496150322116798
- RoigMEngJJMacIntyreDLFalls in people with chronic obstructive pulmonary disease: an observational cohort studyRespir Med201010546146920869227
- WatzHPittaFRochesterCLAn official European Respiratory Society statement on physical activity in COPDEur Respir J20144461521153725359358
- PortoEFCastroAASchmidtVGPostural control in chronic obstructive pulmonary disease: a systematic reviewInt J Chron Obstruct Pulmon Dis2015101233123926170652
- CaspersenCJPowellKEChristensonGMPhysical activity, exercise, and physical fitness: definitions and distinctions for health-related researchPublic Health Rep19851001261313920711
- HowleyETType of activity: resistance, aerobic and leisure versus occupational physical activityMed Sci Sports Exerc200133Supp lS364S36911427761
- VestboJHurdSSAgustíAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- MichikawaTNishiwakiYTakebayashiTToyamaYOne-leg standing test for elderly populationJ Orthop Sci20091467568519802686
- GuralnikJMSimonsickEMFerrucciLA short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admissionJ Gerontol1994498594
- BiscaGWMoritaAAHernandesNAProbstVSPittaFSimple lower limb function tests in patients with chronic obstructive pulmonary disease: a systematic reviewArch Phys Med Rehab2015961222212230
- MuehlbauerTGollhoferAGranacherUAssociations between measures of balance and lower-extremity muscle strength/power in healthy individuals across the lifespan: a systematic review and meta-analysisSports Med201545121671169226412212
- WhitneySLWrisleyDMMarchettiGFGeeMARedfernMSFurmanJMClinical measurement of sit-to-stand performance in people with balance disorders: validity of data for the five-times-sit-to-stand testPhys Ther2005851034103516180952
- United States Department of Health and Human Services2008 Physical Activity Guidelines for Americans: Be Active, Healthy, and Happy!Washington, DCUnited States Department of Health and Human Services2008
- SchneiderPLCrouterSELukajicOBassettDRJrAccuracy and reliability of 10 pedometers for measuring steps over a 400-m walkMed Sci Sports Exerc2003351779178414523320
- KumaharaHSchutzYAyabeMThe use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: a validation study against whole-body indirect calorimetryBr J Nutr200491223524314756909
- American Thoracic SocietyStandardization of spirometry – 1987 update. Statement of the American Thoracic SocietyAm Rev Respir Dis1987136128512983674589
- BerglundEBirathGBjureJSpirometric studies in normal subjects. I. Forced expirograms in subjects between 7–70 years of ageActa Med Scand196317318519213970718
- MahlerDWellsCEvaluation of clinical methods for rating dyspneaChest1988935805863342669
- SugawaraKTakahashiHKashiwaguraTEffect of anti-inflammatory supplementation with whey peptide and exercise therapy in patients with COPDRespir Med20121061526153422857881
- HollandAESpruitMATroostersTAn official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory diseaseEur Respir J2014441428144625359355
- JonesPWHardingGBerryPWiklundIChenWHKline LeidyNDevelopment and first validation of the COPD Assessment TestEur Respir J200934364865419720809
- FaulFErdfelderEBuchnerALangAGStatistical power analyses using G*Power 3.1: tests for correlation and regression analysesBehav Res Methods20094141149116019897823
- CrişanAFOanceaCTimarBFira-MladinescuOTudoracheVBalance impairment in patients with COPDPLoS One2015103e012057325768731
- EisnerMDBlancPDYelinEHCOPD as a systemic disease: impact on physical functional limitationsAm J Med2008121978979618724969
- KonSSCanavanJLNolanCMThe 4-metre gait speed in COPD: responsiveness and minimal clinically important differenceEur Respir J20144351298130524177002
- JonesSEKonSSCanavanJLThe five-repetition sit-to-stand test as a functional outcome measure in COPDThorax201368111015102023783372
- KawagoshiAKiyokawaNSugawaraKQuantitative assessment of walking time and postural change in patients with COPD using a new triaxial accelerometer systemInt J Chron Obstruct Pulmon Dis2013839740424039414
- Tudor-LockeCCraigCLThyfaultJPSpenceJCA step-defined sedentary lifestyle index: <5000 steps/dayAppl Physiol Nutr Metab201338210011423438219
- Donaire-GonzalezDGimeno-SantosEBalcellsEPhysical activity in COPD patients: patterns and boutsEur Respir J201342993100223258786
- AoyagiYShephardRJHabitual physical activity and health in the elderly: the Nakanojo StudyGeriatr Gerontol Int201010Suppl 1S236S24320590838
- PrioliACFreitas JúniorPBBarelaJAPhysical activity and postural control in the elderly: coupling between visual information and body swayGerontology200551314514815832038
- BeauchampMKBrooksDGoldsteinRSDeficits in postural control in individuals with COPD – emerging evidence for an important secondary impairmentMultidiscip Respir Med20105641742122958342
- VermeulenJNeyensJCvan RossumESpreeuwenbergMDde WitteLPPredicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic reviewBMC Geriatr2011113321722355
- DurutürkNTongaEThe association among balance, activity limitation, dyspnea and vulnerability in chronic obstructive pulmonary diseasesEur Respir J201342Suppl 57P1298
- GarrodRBestallJCPaulEAWedzichaJAJonesPWDevelopment and validation of a standardized measure of activity of daily living in patients with severe COPD: the London Chest Activity of Daily Living scale (LCADL)Respir Med200094658959610921765
- FriskBEspehaugBHardieJAPhysical activity and longitudinal change in 6-min walk distance in COPD patientsRespir Med20141081869424075305
- BeauchampMKO’HoskiSGoldsteinRSBrooksDEffect of pulmonary rehabilitation on balance in persons with chronic obstructive pulmonary diseaseArch Phys Med Rehabil2010911460146520801268
