Abstract
Current guidelines recommend inhaled pharmacologic therapy as the preferred route of administration for treating COPD. Bronchodilators (β2-agonists and antimuscarinics) are the mainstay of pharmacologic therapy in patients with COPD, with long-acting agents recommended for patients with moderate to severe symptoms or those who are at a higher risk for COPD exacerbations. Dry powder inhalers and pressurized metered dose inhalers are the most commonly used drug delivery devices, but they may be inadequate in various clinical scenarios (eg, the elderly, the cognitively impaired, and hospitalized patients). As more drugs become available in solution formulations, patients with COPD and their caregivers are becoming increasingly satisfied with nebulized drug delivery, which provides benefits similar to drugs delivered by handheld inhalers in both symptom relief and improved quality of life. This article reviews recent innovations in nebulized drug delivery and the important role of nebulized therapy in the treatment of COPD.
Introduction
Inhaled pharmacologic therapy is a cornerstone of treatment for patients with COPD.Citation1,Citation2 Four commonly prescribed inhalation devices, pressurized metered dose inhalers (pMDIs), dry powder inhalers (DPIs), slow mist inhalers (SMIs), and nebulizers, have similar efficacies in patients with COPD,Citation3–Citation6 provided they are used appropriately. Although DPIs and pMDIs are the most commonly used devicesCitation7,Citation8 and are recommended for long-term treatment in the vast majority of patients,Citation2 the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy document recommends nebulizers for specific patient populations (eg, patients with very low inspiratory flow rates) in whom nebulizer treatment may provide more benefits than DPIs or MDIs.Citation2 Further, GOLD recommends evaluating the benefits of nebulizer treatment symptomatically and continuing treatment as long as similar benefits are not achievable by simpler, cheaper, and more portable alternatives. In addition, both patients and their caregivers are becoming increasingly satisfied with nebulized drug delivery and have reported benefits in symptom relief, ease of use, and improved quality of life when using this system.Citation9,Citation10 Moreover, several of the emerging medications for COPD (both marketed and under development) utilize nebulizer technology. This article reviews recent innovations in nebulized drug delivery and the important role of nebulized therapy in the treatment of COPD.
Selection of articles for review
After dividing the review topic into specific subsections, articles were selected for inclusion based on comprehensive reviews of the literature according to each subsection. A PubMed search (January 1, 1996 to March 15, 2016) was conducted using multiple primary topic headers combined with appropriate terms for each section of the article (eg, COPD + nebulizers or COPD + nebulizer therapy). The results of the PubMed search were supplemented by relevant papers identified from reference lists of published articles and the author’s knowledge of the literature. Selection of articles for discussion focused on information published within the past 5 years.
COPD
COPD, a common preventable and treatable disease, is characterized by progressive persistent airflow obstruction that is associated with an enhanced inflammatory response to noxious particles or gases in the lung and airways.Citation2,Citation11,Citation12 COPD represents a global health problem, is ranked as the fourth leading cause of death in the world, and significantly affects patient quality of life.Citation2,Citation13,Citation14 The global social and economic burden of COPD is projected to increase, due to aging populations and the continued use of tobacco and exposure to biomass fuels,Citation15,Citation16 underscoring the need for more effective management of this disease. While >12 million people in the US are known to have COPD, it is estimated that up to 24 million may have impaired lung function and undiagnosed disease.Citation17 In 2010, the cost of COPD in the US was projected to be ~$49.9 billion, which included ~$20 billion in indirect costs (eg, loss of work productivity and earnings) and $30 billion in direct health care expenditures (eg, prescription medicines and emergency department visits).Citation17,Citation18
To reduce symptoms, frequency, and severity of COPD exacerbations and improve health status and exercise tolerance, the GOLD strategy documentCitation2 recommends that bronchodilators are the cornerstone of pharmacotherapy for COPD in the majority of patients.Citation1,Citation2 However, physical and/or cognitive symptoms that are common in some COPD patients (eg, the elderlyCitation19,Citation20) could interfere with the proper administration of inhaled therapies via handheld inhalers,Citation21 resulting in insufficient dosing and jeopardizing health outcomes, reducing quality of life, and further adding to the economic burden of COPD.Citation2,Citation22 Further, during exacerbations and in recovery, many COPD patients have decreased peak inspiratory flow rate (PIFR) and are unable to use handheld inhalers effectively. In these populations, inhaled therapies administered via nebulizers may offer improved symptom controlCitation21,Citation23 and quality of lifeCitation9 over non-nebulized bronchodilator therapy.
Pharmacologic therapy
In general, pharmacologic therapy is part of an integrated treatment approach in patients with COPD that begins with smoking cessation and vaccines (influenza and pneumococcal) for all current smokers and progresses to treatment with inhaled therapy.Citation24 Inhaled treatment is tailored to the patient and should be guided by the severity of COPD symptoms, risk of COPD exacerbations, drug availability, and patient response ().Citation2 For patients at low risk of COPD exacerbations with relatively few symptoms (eg, those in GOLD patient category A),Citation2 short-acting bronchodilators are available for acute relief of symptoms or for use before physical activitiesCitation25 to prevent the onset of symptoms (a long-acting inhaled bronchodilator, as well as theophylline, is recommended as an alternative choice).Citation2 For patients with more severe symptoms or who are at a higher risk of COPD exacerbations (eg, those in GOLD patient categories B, C, or D), long-acting bronchodilators are recommended over short-acting bronchodilators for maintenance therapy to improve symptoms, exercise tolerance, and health-related quality of life and reduce the risk of exacerbations.Citation2 As a result, long-acting bronchodilators with or without inhaled corticosteroids (ICS) are the first- or second-choice drugs for the majority of patients with COPD.Citation1
Table 1 Initial pharmacologic management of COPDTable Footnote*
Although handheld pMDIs or DPIs are effective in most patients with COPD, cognitively impaired and elderly patients may benefit more from the use of a nebulizer, since these patient populations may have difficulty synchronizing inhalation with inhaler actuation or may be unable to generate a sufficient inspiratory flow rate against the resistance of a breath-activated DPI to generate an effective aerosol.Citation6,Citation22,Citation26–Citation28 SMIs are compact portable multidose inhalers that use liquid formulations similar to those in nebulizers but, like MDIs and DPIs, require manual manipulation to generate the aerosol and special breathing techniques for effective delivery of the aerosolized medication to the lungs.Citation29 The choice of therapy, however, ultimately depends on a wide range of factors, including the prescribing physician, the availability of specific drug/device pairings, drug cost, and patient preferences and satisfaction.Citation3,Citation22,Citation26,Citation28,Citation30,Citation31 Each of the delivery devices that are available for administering drugs to patients with COPD (eg, pMDIs, DPIs, SMIs, and nebulizers) has advantages and disadvantages ().Citation3,Citation6,Citation29,Citation31,Citation32
Table 2 Advantages and disadvantages of aerosolized formulations
Nebulized drug delivery
In patients with COPD, nebulizers are an alternative to pMDIs and DPIs for providing inhaled therapy, provided the drug is available and chemically stable in liquid form ().Citation6,Citation31 Despite some drawbacks associated with nebulizers (eg, variably long treatment times and daily cleaning), current evidence suggests that the efficacy of treatments administered to patients with moderate to severe COPD via nebulizers is similar to that observed with pMDIs and DPIs.Citation3–Citation6 Further, market analysis indicates that, in the US, ∼45% of patients with COPD have a nebulizer, 69% of whom use it on a regular basis.Citation6
Figure 1 Examples of commercially available nebulizers that incorporate newer aerosol generating technologies.
Abbreviation: AAD, adaptive aerosol delivery.
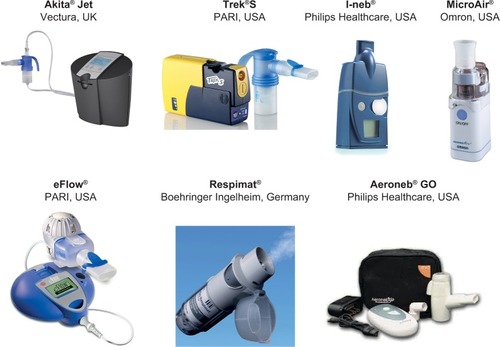
Several options exist for the type of nebulizer (eg, jet, ultrasonic, and vibrating mesh), with many models commercially available (). The Akita® (Vectura, Chippenham, UK) jet nebulizer individualizes aerosol delivery using the adaptive aerosol delivery (AAD) control system (),Citation33 which results in high efficiency and low variability in aerosol drug delivery to patients. Despite these benefits, however, the Akita, in common with older jet nebulizers, is a large, poorly portable nebulizer that has a longer (10 minutes) treatment time than the newer vibrating mesh nebulizers.Citation34 The Trek® S (PARI, Midlothian, VA, USA) portable jet nebulizer is a convenient alternative to larger, more powerful tabletop compressors. In a comparative study of four portable nebulizer systems, the Trek S delivered 33% more respirable dose than the next best system, Mini Elite™ (Philips Healthcare, Andover, MA, USA).Citation35
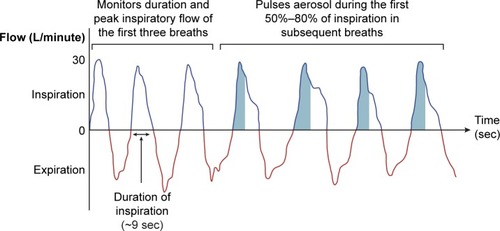
Figure 2 AAD technology used in the Akita® and I-neb® nebulizers.
Abbreviation: AAD, adaptive aerosol delivery.
The Aeroneb® Go (Philips Healthcare) is a portable, compact, handheld ultrasonic nebulizer that is easily assembled, silent, and has short (5 minutes) treatment duration.Citation3,Citation6,Citation34 The eFlow® (PARI) is a battery-operated, compact, portable vibrating mesh nebulizer that has been shown to improve patient compliance due to its comparatively short (5 minutes) treatment time.Citation36 MicroAir® NE-U22 (Omron, Chicago, IL, USA) is a mesh nebulizer that provides efficient aerosol drug delivery with a predominantly fine particle fraction.Citation3,Citation6,Citation34 Like the Aeroneb Go and eFlow devices, the MicroAir is expensive and can be difficult to maintain, as it requires disassembly and cleaning after each use to prevent clogging of the mesh apertures.Citation34
The I-neb® (Philips Healthcare) AAD nebulizer is a small, lightweight, battery-powered, silent smart nebulizer that combines mesh and AAD technologies to deliver a precise, reproducible dose.Citation6,Citation34 With AAD technology, automated timing of aerosol delivery (based on the patient’s breathing pattern) improves the precision and reproducibility of dosingCitation34 () and, compared with previous nebulizers without AAD, significantly improves dyspnea and fatigue in patients with COPD.Citation37 A disadvantage of the newer mesh nebulizers is that little information is available concerning the ideal dose of the bronchodilator solution to add to the nebulizer. Consequently, the potential for overdosing exists if the same dose of the bronchodilator that is conventionally used with jet nebulizers is added to these newer nebulizers. To address this concern, device manufacturers are developing a new generation of closed-system mesh nebulizers that will accept only the ampule containing the specific drug approved for use with a specific device based on the demonstration of safety and efficacy.Citation31
The Respimat® (Boehringer Ingelheim, Ingelheim, Germany) is an SMI that delivers a slow-moving mist, allowing the inhalation of medication independent of inspiratory effortCitation38 (ie, via the release of stored energy from a tensed spring when the tension is released by pushing a button). Although not strictly classified as a nebulizer, the Respimat device, a compact handheld aerosol delivery device similar in size to MDIs and DPIs, is included here because it shares several performance characteristics with the nebulizers discussed earlier, such as liquid formulation, propellant-free function, use of mechanical energy for actuation, generation of an aerosol with a predominantly fine particle fraction (micronebulized), and lack of dependence on high inspiratory flow rates.Citation38 However, in contrast to more conventional nebulizers for which only tidal breathing is required, use of the Respimat requires a special breathing technique (full expiration followed by full inhalation and breath holding).Citation38 Some coordination between inhalation and actuation is also necessary, although the timing for such is more forgiving with the Respimat than for MDI devices, since the aerosol delivered from the Respimat lasts 1.5 seconds (as opposed to a fraction of a second from an MDI).
Nebulized pharmacologic therapy
Many of the drugs used for the treatment of COPD were initially approved for use in pMDIs or DPIsCitation39 and are now available in solution form for use with nebulizers (). The long-acting agents are indicated for maintenance treatment of COPD-associated airflow obstruction, while short-acting bronchodilators are indicated for acute relief of bronchospastic symptoms of COPD. Clinical trials generally have demonstrated significant improvement in forced expiratory volume in 1 second (FEV1) over the dosing interval and reduction in rescue medication use with nebulized therapy.
Table 3 Nebulized medications commonly used for patients with COPD
Long-acting β2-agonists (LABAs)
Arformoterol
Nebulized arformoterol tartrate is of potential benefit to patients with hyperinflation and low PIFR.Citation40 Arformoterol is safe in combination therapy with certain handheld inhalers (eg, ICS, long-acting muscarinic antagonists [LAMAs], and short-acting β2-agonists [SABAs]), but it is contraindicated in combination with a handheld inhaled LABA (alone or in combination with an ICS or an LAMA). A 12-month Phase 4 trial found no increased risk of respiratory death or COPD exacerbation-related hospitalizations with nebulized arformoterol treatment.Citation41 Being a single enantiomer of formoterol, arformoterol may have hypothetically more potent bronchodilator properties, microgram per microgram, than racemic formoterol fumarate, but no major clinical differences between the two drugs have been observed in patients with COPD.Citation42 Maintenance therapy with nebulized arformoterol or formoterol demonstrated a 37% reduction and a 42% reduction in rescue albuterol use, respectively.Citation43,Citation44 Partial tolerance to the bronchodilator effect of arformoterol was noted after 6 weeks of therapy, but the reduction in bronchodilator efficacy did not progress beyond 6 weeks and was not considered clinically significant.Citation44 Finally, arformoterol can improve lung function in combination with LAMAs. In patients with COPD who were receiving twice-daily nebulized arformoterol, tiotropium bromide given in combination with arformoterol produced significantly greater bronchodilation than either arformoterol or tiotropium monotherapies (P<0.001).Citation45
Formoterol
Formoterol differentiates from some other β2-agonists by its rapid onset of significant bronchodilation within 5 minutes of administration.Citation46,Citation47 In patients with COPD, nebulized formoterol fumarate significantly increased FEV1 relative to placebo (P<0.001) when administered for 12 weeks and had similar efficacy and safety compared with the original formoterol fumarate dry powder formulation.Citation46 Quality of life at week 12, as measured by the St George’s Respiratory Questionnaire, demonstrated significant and clinically meaningful improvements in total score, symptom, and impact scores for formoterol vs placebo. Patients treated with formoterol reported greater treatment satisfaction and perception of disease control compared with treatment with short-acting bronchodilators delivered 4 times daily.Citation48 Furthermore, similar to arformoterol,Citation45 nebulized formoterol significantly increased bronchodilation in patients receiving the LAMA tiotropium bromide,Citation49 which indicates that formoterol can improve lung function in combination with antimuscarinics. With regard to tachyphylaxis to the bronchodilator effect of formoterol + tiotropium, tachyphylaxis was not observed during 6 weeks of formoterol add-on treatment in patients receiving tiotropium maintenance therapy,Citation50,Citation51 which is consistent with 12-week trials that did not show any tolerance to the effect of formoterol alone in patients with COPD.Citation46
Olodaterol SMI
Olodaterol hydrochloride SMI is a long-term, once-daily maintenance treatment for controlling symptoms in adults with COPD.Citation52,Citation53 In Phase 3 trials, once-daily olodaterol improved lung function (FEV1) compared with placebo over 48 weeks of treatment, with bronchodilation being achieved and maintained within the 24-hour dosage interval, supporting its once-daily administration.Citation52,Citation54 Olodaterol SMI is not indicated to treat either acute deterioration of COPD or asthma.
LAMA
Tiotropium SMI
Tiotropium bromide SMI provides a solution form of tiotropium bromideCitation55 that is efficacious at lower doses compared with the tiotropium bromide HandiHaler® (Boehringer Ingelheim).Citation56 In patients with COPD, tiotropium SMI improved lung function, health-related quality of life, and dyspnea, reduced acute exacerbations of COPD, and was as effective and safe as the tiotropium HandiHalerCitation57,Citation58 Tiotropium is generally well tolerated in patients with COPD, but antimuscarinic side effects (eg, dry mouth) are among the most commonly reported adverse events.Citation59
LABA–LAMA fixed-dose combination
Tiotropium–olodaterol SMI
Tiotropium bromide–olodaterol hydrochloride SMI, a fixed-dose combination daily maintenance treatment for patients with COPD,Citation60,Citation61 has demonstrated superior efficacy compared with the individual LABA and LAMA components alone.Citation62 Two replicate 52-week trials showed significantly greater improvement in FEV1 area under the curve (AUC) from time 0 to 3 hours, trough FEV1, quality of life (as measured by the St George’s Respiratory Questionnaire), and dyspnea with tiotropium–olodaterol SMI compared with the individual components delivered by SMI.Citation62 Moreover, in a placebo-controlled trial, tiotropium–olodaterol SMI has been shown to result in a clinically meaningful improvement in quality of life compared with placebo.Citation63
SABA
Nebulized albuterol sulfate and levalbuterol hydrochloride are short-acting medications commonly used to treat acute episodes of bronchospasm and acute exacerbations in patients with COPD.Citation64,Citation65 Randomized, controlled clinical studies generally have not demonstrated any significant differences between levalbuterol and albuterol in efficacy, occurrence of adverse effects, or hospital admissions.Citation66 Levalbuterol may have advantages over albuterol in patients with COPD admitted to the hospital, including shorter (1 day) length of stay,Citation67 but albuterol was found to be 3-fold less expensive than levalbuterol in a 2009 study.Citation66
Short-acting muscarinic antagonist (SAMA)
Ipratropium
Ipratropium bromide, an SAMA in a nebulized inhalation solution administered either alone or with other bronchodilators (eg, β2-agonists), is indicated as a bronchodilator for the maintenance treatment of bronchospasm associated with COPD, including chronic bronchitis and emphysema, when administered on a regularly scheduled four times daily schedule.Citation68 In 12-week clinical studies in patients with COPD-associated bronchospasm associated with COPD, significant improvements in pulmonary function (FEV1 increases of 15% or more) occurred within 15–30 minutes and persisted for periods of 4–5 hours in the majority of patients.
SABA–SAMA fixed-dose combination
Albuterol–ipratropium
Nebulized albuterol sulfate–ipratropium bromide, a fixed-dose combination product, is indicated for the treatment of bronchospasm associated with COPD in patients requiring more than one bronchodilator.Citation69,Citation70 Research has shown that patients with COPD treated with albuterol–ipratropium have lower hospital expenditures and therapy interruptions than patients taking the individual components as dual single agents (DSAs).Citation71 In a population-based retrospective claims analysis, patients who were taking nebulized albuterol–ipratropium (n=468) had 31% fewer emergency department visits and costs compared with patients taking a DSA (P=0.03 and P<0.001, respectively). In addition, the albuterol–ipratropium cohort was associated with statistically fewer individuals who reported treatment interruptions (10%; P=0.003).
Albuterol–ipratropium SMI
Albuterol sulfate–ipratropium bromide SMI is indicated for patients with COPD on a regular aerosol bronchodilator who continue to have evidence of bronchospasm and who require a second bronchodilator.Citation72 In a controlled clinical study, 652 patients with moderate to severe COPD received either albuterol, ipratropium, or albuterol–ipratropium SMI for 85 days.Citation70 Over the course of the study, the acute pulmonary function response (peak expiratory flow rate) was significantly better with albuterol–ipratropium compared with albuterol or ipratropium alone; quality of life and symptoms, however, were unchanged over the course of the study in all treatment groups. The use of an SAMA either as a single agent (eg, ipratropium) or in combination with a short-acting β-agonist (eg, albuterol) is not recommended in patients receiving concomitant therapy with an LAMA because of concern regarding possible additive anticholinergic side effects and, hypothetically, displacement of the more effective long-acting agent by the short-acting drug from the muscarinic receptor.
Nebulized therapy in development
Despite the benefits of combination therapies, the late-stage development pipeline of nebulized medications for the treatment of COPD currently comprises two LAMA monotherapies, SUN-101 (Sunovion, Marlborough, MA, USA) and TD-4208 (Theravance, South San Francisco, CA, USA), that could provide improvements over existing drugs ().
Table 4 Nebulized therapies in clinical development for the treatment of COPD
SUN-101
SUN-101, a soluble glycopyrrolate bromide formulation in Phase 3 development, is rapidly (within 2 minutes) delivered to the lungs using a novel custom-designed, portable electronic nebulizer device (eFlow). SUN-101 is in Phase 3 development as a twice-daily maintenance treatment of bronchoconstriction in patients with COPD, including those with chronic bronchitis and emphysema. In controlled Phase 2 studies, SUN-101 demonstrated the rapid onset (≤5 minutes) of dose-related bronchodilation following single-dose administration (12.5–400 μg) in patients with moderate to severe COPD.Citation73 SUN-101 produced clinically meaningful improvements in lung function (FEV1) that were maintained over a 24-hour period at all doses >50 μg. Phase 2 clinical studies also demonstrated that SUN-101 has a safety profile similar to tiotropium,Citation74 with no clinically relevant changes in heart rate, systolic and diastolic blood pressure, or in electrocardiographic parameters including QTc interval.Citation73 The SUN-101 Phase 3 program consists of three clinical trials that will enroll ~2,340 adults with moderate to very severe COPD.Citation75–Citation77
Revefenacin (TD-4208)
Revefenacin is a nebulized LAMA with similar potency to tiotropium bromide but with less potential for antimuscarinic side effects (eg, dry mouth).Citation78,Citation79 Revefenacin, administered via the PARI Trek S nebulizer, is in clinical development as a once-daily maintenance treatment for COPD. The results of several Phase 2 studies support the ongoing Phase 3 program. Evaluation of the pharmacokinetics of revefenacin (n=127) demonstrated low plasma concentrations after inhaled administration, consistent with high systemic clearance and a lack of systemic antimuscarinic activity.Citation80 A randomized, crossover, 7-day, multiple-dose study demonstrated that the bronchodilator effect of once-daily revefenacin was sustained for more than 24 hours in patients with COPD.Citation81 In a 28-day dose-ranging Phase 2 study in patients with COPD, revefenacin-treated patients’ use of rescue medication was significantly reduced by more than one puff per day in a dose-dependent manner (P<0.01).Citation82 The Phase 3 program consists of three clinical trials that will enroll >2,000 patients with moderate to very severe COPDCitation83–Citation85 and is designed to support regulatory approval of the drug in the US.
Discussion
With patients becoming increasingly satisfied with nebulized drug delivery,Citation9 improved integration of nebulizers and nebulized therapies into the COPD treatment paradigm should lead to improved clinical and health economic outcomes for patients with COPD. Certain COPD patient populations may especially benefit from the use of nebulizer therapy (eg, patients with low PIFR, the elderly, and those with cognitive or visual impairment or diminished manual dexterity). It is therefore important to select the appropriate device for each patient, particularly in older or more severely impacted patients who may be unable to use handheld devices reliably or those who prefer the feeling of control with a nebulized product.Citation48
Many emerging COPD medications employ portable nebulizers that are typically battery operated, making them less cumbersome to carry. Compared with pMDIs and DPIs, these nebulizers require no hand-breath coordination or extra effort during inhalation.Citation6 Further, the wider availability of high-efficiency nebulizers will ensure accurate delivery of emerging nebulized medications in patients with COPD, which may lead to further reductions in symptoms and exacerbation rates in these patients. However, safety and efficacy studies will be required to define the optimal doses of medications using these high-efficiency nebulizers. Moreover, patient education is crucially important to foster adherence to regular use of nebulized long-acting bronchodilators as part of maintenance therapy, rather than relying on short-acting nebulized agents that should be reserved for rescue treatment of acute symptoms.
When evaluating a nebulized drug delivery option for patients with COPD, important considerations include the availability of specific drug/nebulizer pairings, the need for drug combinations, the ability to use the selected device correctly, drug/nebulizer cost and reimbursement, patient preference and satisfaction, and clinical scenario.Citation3,Citation22,Citation26,Citation28,Citation30 Nebulized drug delivery is generally preferred by patients discharged from hospitals after an inpatient stay, who have demonstrated consistent difficulty using handheld inhalers, and who have impaired manual dexterity, impaired cognition, or chronic muscle weakness.Citation6 In these scenarios, the benefits of nebulization therapy can outweigh potential inconveniences and lead to improved adherence and outcomes in patients with COPD.Citation9 For some patients, use of both a nebulizer (as maintenance therapy) and a handheld inhaler (as rescue medication, particularly when outside the home) may provide the best combination of efficacy and convenience.Citation6,Citation26,Citation86
Long-acting agents formoterol fumarate and arformoterol tartrate, which currently serve as the mainstays of nebulized maintenance therapy for COPD, have demonstrated a significant reduction in FEV1, but there are no head-to-head clinical trials comparing the efficacy and safety of these two nebulized therapies. While tolerance (tachyphylaxis) to the bronchodilator effect of arformoterol has been reported in clinical trials,Citation40,Citation44,Citation87 no other clinical manifestations of tolerance were evident. In contrast, clinical trials with nebulized formoterol failed to show any evidence of tolerance, as indicated by maintained FEV1 AUC and reduced rescue inhaler use with up to 12 weeks of treatment.Citation46
Combination therapy involving two long-acting bronchodilators with differing mechanisms of action is recommended in patients whose COPD is not well controlled with one drug alone.Citation1,Citation88 LABA and LAMA combinations, for example, have shown additive bronchodilator effects at doses used for monotherapy without additional safety concernsCitation89 and may increase patient adherence.Citation90 Approval of the two nebulized LAMA compounds in Phase 3 clinical trials, SUN-101 and TD-4208, will likely increase the use of combination LABA/LAMA nebulized therapy, although a fixed-dose LABA/LAMA combination nebulized product would also be welcome from a patient compliance perspective. Further, the development of a nebulized version of the widely used fixed-dose combination therapy, ICS/LABA, would benefit patients who need or prefer nebulized treatments.
The recent approval of tiotropium–olodaterol SMICitation62 illustrates that demand for combination therapy is driving device innovation. Cosuspension-based pMDIs, for example, are in development,Citation89,Citation91,Citation92 which may facilitate further innovation in fixed-dose combination inhaler products. For example, the US Federal Drug Administration recently approved a novel LAMA/LABA cosuspension-based pMDI (glycopyrrolate and formoterol fumarate) for patients with COPD.Citation93 Triple therapy for COPD (ie, treatments containing LABA, LAMA, and ICS) has also been proposed as a convenient treatment option for COPD.Citation94,Citation95 Indeed, the first triple inhaler containing formoterol, tiotropium, and ciclesonide is already on the market in India.Citation96
Future treatment of patients with COPD will require the continued development of novel nebulizer devices and drugs for patient groups and clinical scenarios where existing pMDI/DPI therapy is inadequate. Health care providers should stay up to date regarding emerging nebulized treatment options that could provide additional clinical benefits for their patients. In daily practice, prescribing the most appropriate nebulized therapy should take into consideration the available drug formulations, combinations, and devices, as well as the patients’ pulmonary function, skills, and preferences. Thus, health care providers and patients together can optimize the benefits of available nebulized treatments for patients with COPD.
Acknowledgments
Mylan Inc. (Canonsburg, PA, USA) funded medical writing support. Roger J Hill, PhD, of Ashfield Healthcare Communications (Haddam, CT, USA) drafted and revised the manuscript based on the input from the author, and Paula Stuckart of Ashfield Healthcare Communications copyedited and styled the manuscript as per journal requirements.
Disclosure
The author reports personal fees from Mylan, grants and personal fees from Sunovion, grants and personal fees from Boehringer-Ingelheim, and personal fees from Theravance during the conduct of this study. Outside the conduct of this study, the author reports grants and personal fees from AstraZeneca and personal fees from Novartis.
References
- BarjaktarevicIZArredondoAFCooperCBPositioning new pharmacotherapies for COPDInt J Chron Obstruct Pulmon Dis2015101427144226244017
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) [web-page on the Internet]Global Strategy for Diagnosis, Management, and Prevention of COPD –2016 Available from: http://goo.gl/ItQL3aAccessed May 4, 2016
- DolovichMBAhrensRCHessDRAmerican College of Chest Physicians; American College of Asthma, Allergy, and ImmunologyDevice selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and ImmunologyChest2005127133537115654001
- RamFSBrocklebankDMMuersMWrightJJonesPWPressurised metered-dose inhalers versus all other hand-held inhalers devices to deliver bronchodilators for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20021CD00217011869627
- TurnerMOPatelAGinsburgSFitzGeraldJMBronchodilator delivery in acute airflow obstruction. A meta-analysisArch Intern Med199715715173617449250235
- DhandRDolovichMChippsBMyersTRRestrepoRFarrarJRThe role of nebulized therapy in the management of COPD: evidence and recommendationsCOPD201291587222292598
- BoniniMUsmaniOSThe importance of inhaler devices in the treatment of COPDCOPD Res Pract20151119
- LavoriniFCorriganCJBarnesPJAerosol Drug Management Improvement TeamRetail sales of inhalation devices in European countries: so much for a global policyRespir Med201110571099110321489771
- SharafkhanehAWolfRAGoodnightSHananiaNAMakeBJTashkinDPPerceptions and attitudes toward the use of nebulized therapy for COPD: patient and caregiver perspectivesCOPD201310448249223875742
- BartaSKCrawfordARobertsCMSurvey of patients’ views of domiciliary nebuliser treatment for chronic lung diseaseRespir Med200296637538112117035
- QaseemAWiltTJWeinbergerSEAmerican College of PhysiciansAmerican College of Chest PhysiciansAmerican Thoracic SocietyEuropean Respiratory SocietyDiagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory SocietyAnn Intern Med2011155317919121810710
- AngelisNPorpodisKZarogoulidisPAirway inflammation in chronic obstructive pulmonary diseaseJ Thorac Dis20146suppl 1S167S17224672691
- VosTFlaxmanADNaghaviMYears lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010Lancet201238098592163219623245607
- LozanoRNaghaviMForemanKGlobal and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010Lancet201238098592095212823245604
- LandisSHMuellerovaHManninoDMContinuing to confront COPD international patient survey: methods, COPD prevalence, and disease burden in 2012–2013Int J Chron Obstruct Pulmon Dis2014959761124944511
- ManninoDMBuistASGlobal burden of COPD: risk factors, prevalence, and future trendsLancet2007370958976577317765526
- Lung.org [webpage on the Internet]American Lung Association COPD fact sheet [updated 2011]. Available from: http://www.lung.org/lung-disease/copd/resources/facts-figures/COPD-Fact-Sheet.htmlAccessed March 3, 2016
- Nhlbi.nih.gov [webpage on the Internet]National Heart Lung and Blood Institute. Morbidity and mortality: 2012 chart book on cardiovascular, lung, and blood diseases Available from: http://www.nhlbi.nih.gov/resources/docs/cht-book.htmAccessed March 3, 2016
- AkinbamiLJLiuXChronic obstructive pulmonary disease among adults aged 18 and over in the United States, 1998–2009NCHS Data Brief20116318
- CDC.gov Centers for Disease Control and Prevention. National Center for Health StatisticsData brief 63: chronic obstructive pulmonary disease among adults aged 18 and over in the United States, 1998–2009 Available from: http://www.cdc.gov/nchs/data/databriefs/db63_tables.pdf#2Accessed March 4, 2016
- QuinetPYoungCAHeritierFThe use of dry powder inhaler devices by elderly patients suffering from chronic obstructive pulmonary diseaseAnn Phys Rehabil Med2010532697620018583
- TaffetGEDonohueJFAltmanPRConsiderations for managing chronic obstructive pulmonary disease in the elderlyClin Interv Aging20149233024376347
- MahlerDAWatermanLAWardJGiffordAHComparison of dry powder versus nebulized beta-agonist in patients with COPD who have suboptimal peak inspiratory flow rateJ Aerosol Med Pulm Drug Deliv201427210310923745526
- CelliBRUpdate on the management of COPDChest200813361451146218574288
- Mayoclinic.org [webpage on the Internet]Diseases and conditions: COPD: treatments and drugs Available from: http://www.mayoclinic.org/diseases-conditions/copd/basics/treatment/con-20032017Accessed March 18, 2016
- BarronsRPegramABorriesAInhaler device selection: special considerations in elderly patients with chronic obstructive pulmonary diseaseAm J Health Syst Pharm201168131221123221690428
- DekhuijzenPNBjermerLLavoriniFNinaneVMolimardMHaughneyJGuidance on handheld inhalers in asthma and COPD guidelinesRespir Med2014108569470024636812
- MelaniASBonaviaMCilentiVGruppo Educazionale Associazione Italiana Pneumologi OspedalieriInhaler mishandling remains common in real life and is associated with reduced disease controlRespir Med2011105693093821367593
- NewmanSPInhaler treatment options in COPDEur Respir Rev20051496102108
- DolovichMBDhandRAerosol drug delivery: developments in device design and clinical useLancet201137797701032104521036392
- BerlinskiAAssessing new technologies in aerosol medicine: strengths and limitationsRespir Care2015606833847 discussion 847–84926070578
- RauJLPractical problems with aerosol therapy in COPDRespir Care200651215817216441960
- RubinBKPediatric aerosol therapy: new devices and new drugsRespir Care201156914111421 discussion 1421–142321944688
- AriAJet, ultrasonic, and mesh nebulizers: an evaluation of nebulizers for better clinical outcomesEurasian J Pulmonol20141617
- ZemanKTiffinNEfficiency of portable jet nebulizer systems using budesonideAm J Respir Crit Care Med2010181A1347
- LenneyWEdenboroughFKhoPKovarikJMLung deposition of inhaled tobramycin with eFlow rapid/LC Plus jet nebuliser in healthy and cystic fibrosis subjectsJ Cyst Fibros201110191420884302
- GoodmanNMorganMNikanderKHinchSCoughlinSEvaluation of patient-reported outcomes and quality of life with the I-neb AAD system in patients with chronic obstructive pulmonary diseaseJ Aerosol Med Pulm Drug Deliv201023suppl 1S61S7020373911
- AndersonPUse of Respimat® Soft Mist™ Inhaler in COPD patientsInt J Chron Obstruct Pulmon Dis20061325125918046862
- MeyerKCCOPD 2013: an update on treatment and newly approved medications for pharmacistsJ Am Pharm Assoc (2003)2013536e219e229 quiz e230-124185438
- LohCHDonohueJFOharJAReview of drug safety and efficacy of arformoterol in chronic obstructive pulmonary diseaseExpert Opin Drug Saf201514346347225563342
- DonohueJFHananiaNAMakeBOne-year safety and efficacy study of arformoterol tartrate in patients with moderate to severe COPDChest201414661531154225451347
- KingPRole of arformoterol in the management of COPDInt J Chron Obstruct Pulmon Dis20083338539118990965
- HanrahanJPHananiaNACalhounWJSahnSASciarappaKBaumgartnerRAEffect of nebulized arformoterol on airway function in COPD: results from two randomized trialsCOPD200851253418259972
- BaumgartnerRAHananiaNACalhounWJSahnSASciarappaKHanrahanJPNebulized arformoterol in patients with COPD: a 12-week, multicenter, randomized, double-blind, double-dummy, placebo- and active-controlled trialClin Ther200729226127817472819
- TashkinDPDonohueJFMahlerDAEffects of arformoterol twice daily, tiotropium once daily, and their combination in patients with COPDRespir Med2009103451652419208459
- GrossNJNelsonHSLapidusRJFormoterol Study GroupEfficacy and safety of formoterol fumarate delivered by nebulization to COPD patientsRespir Med2008102218919718363201
- KottakisJCioppaGDCreemersJFaster onset of bronchodilation with formoterol than with salmeterol in patients with stable, moderate to severe COPD: results of a randomized, double-blind clinical studyCan Respir J20029210711511972164
- SutherlandERBrazinskySFeldmanGMcGintyJTomlinsonLDenis-MizeKNebulized formoterol effect on bronchodilation and satisfaction in COPD patients compared to QID ipratropium/albuterol MDICurr Med Res Opin200925365366119232039
- TashkinDPHananiaNAMcGintyJDenis-MizeKChaudryINebu-lized formoterol provides added benefits to tiotropium treatment in chronic obstructive pulmonary diseaseAdv Ther200926111024103419953349
- HananiaNABootaAKerwinETomlinsonLDenis-MizeKEfficacy and safety of nebulized formoterol as add-on therapy in COPD patients receiving maintenance tiotropium bromide: results from a 6-week, randomized, placebo-controlled, clinical trialDrugs20096991205121619537837
- TashkinDPLittnerMAndrewsCPTomlinsonLRinehartMDenis-MizeKConcomitant treatment with nebulized formoterol and tiotropium in subjects with COPD: a placebo-controlled trialRespir Med2008102447948718258423
- DeeksEDOlodaterol: a review of its use in chronic obstructive pulmonary diseaseDrugs201575666567325773742
- Boehringer IngelheimNewly published head-to-head data show stiolto™ respimat® (tiotropium bromide and olodaterol) improved lung function across range of measures [press release]Boehringer Ingelheim201629 Available from: https://goo.gl/VpstiJAccessed September 28, 2016
- FeldmanGJBernsteinJAHamiltonANivensMCKorduckiLLaForceCThe 24-h FEV1 time profile of olodaterol once daily via Respimat® and formoterol twice daily via Aerolizer® in patients with GOLD 2–4 COPD: results from two 6-week crossover studiesSpringerplus2014341925187881
- KeatingGMTiotropium Respimat® soft mist inhaler: a review of its use in chronic obstructive pulmonary diseaseDrugs201474151801181625300412
- HochrainerDHolzHKreherCScaffidiLSpallekMWachtelHComparison of the aerosol velocity and spray duration of Respimat soft mist inhaler and pressurized metered dose inhalersJ Aerosol Med200518327328216181002
- IaconoPVelicitatPGuemasELeclercVThebaultJJImproved delivery of ipratropium bromide using Respimat (a new soft mist inhaler) compared with a conventional metered dose inhaler: cumulative dose response study in patients with COPDRespir Med200094549049510868713
- DahlRCalverleyPMAnzuetoASafety and efficacy of tiotropium in patients switching from HandiHaler to Respimat in the TIOSPIR trialBMJ Open2015512e009015
- BarrRGBourbeauJCamargoCARamFSInhaled tiotropium for stable chronic obstructive pulmonary diseaseCochrane Database Syst Rev20052CD00287615846642
- ZuWallackRAllenLHernandezGTingNAbrahamsREfficacy and safety of combining olodaterol Respimat® and tiotropium HandiHaler® in patients with COPD: results of two randomized, double-blind, active-controlled studiesInt J Chron Obstruct Pulmon Dis201491133114425342898
- FergusonGTFlezarMKornSEfficacy of tiotropium + olodaterol in patients with chronic obstructive pulmonary disease by initial disease severity and treatment intensity: a post hoc analysisAdv Ther201532652353626112656
- BuhlRMaltaisFAbrahamsRTiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4)Eur Respir J201545496997925573406
- SinghDFergusonGTBolitschekJTiotropium + olodaterol shows clinically meaningful improvements in quality of lifeRespir Med2015109101312131926320402
- DattaDVitaleALahiriBZuWallackRAn evaluation of nebulized levalbuterol in stable COPDChest2003124384484912970007
- NairSThomasEPearsonSBHenryMTA randomized controlled trial to assess the optimal dose and effect of nebulized albuterol in acute exacerbations of COPDChest20051281485416002915
- BorkowskiJCraderMNebulized albuterol versus levalbuterol in pediatric and adult patients: a reviewFormulary200944108118
- TruittTWitkoJHalpernMLevalbuterol compared to racemic albuterol: efficacy and outcomes in patients hospitalized with COPD or asthmaChest2003123112813512527613
- Ipratropium BromideIpratropium bromide solution [prescribing information]FLCobalt Laboratories Available from: https://dailymed.nlm.nih.gov/dailymed/getFile.cfm?setid=lf69f100f-c766-490f-9143-5e8e0c8cb7c9&type=pdf&name=lf69f100f-c766-490f-9143-5e8e0c8cb7c9Accessed March 13, 2016
- DuoNeb® (Ipratropium Bromide 0.5 mg/Albuterol Sulfate 3.0 mg*) inhalation solution [prescribing information]CADey Pharma, L.P Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020950s037lbl.pdfAccessed February 23, 2016
- The COMBIVENT Inhalation Solution Study GroupRoutine nebulized ipratropium and albuterol together are better than either alone in COPD. The COMBIVENT Inhalation Solution Study GroupChest19971126151415219404747
- YorkJMSmeedingJBrookRAHoehlerFKleinGLExploratory economic evaluation of patients with COPD on a combination product versus individual components (ipratropium bromide and albuterol)Adv Ther200724475777117901025
- Combivent® Respimat® (ipratropium bromide and albuterol) inhalation spray [prescribing information]1996 Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Combivent%20Respimat/CMVTRSPT.pdfAccessed May 24, 2016
- LeakerBRBarnesPJJonesCRTutuncuASinghDEfficacy and safety of nebulized glycopyrrolate for administration using a high efficiency nebulizer in patients with chronic obstructive pulmonary diseaseBr J Clin Pharmacol201579349250025243340
- FogartyCDunnKSinghDTutuncuAKerwinECardiovascular safety of nebulized glycopyrrolate (SUN-101) compared with tiotropium, ipratropium and placebo in patients with COPDClin Res2013234
- Sunovion Respiratory Development IncRandomized, double-blind, placebo-controlled, parallel-group, multicenter, efficacy and safety trial of 12 weeks of treatment with nebulized SUN-101 in patients with COPD: Golden-3 (glycopyrrolate for obstructive lung disease via electronic nebulizer) Available from: https://clinicaltrials.gov/ct2/show/NCT2347761?term=NLMidentifier&rank=1. NCT2347761.NLM identifier: NCT2347761Accessed March 13, 2016
- Sunovion Respiratory Development IncA randomized, double-blind, placebo-controlled, parallel-group, multicenter, efficacy and safety trial of 12 weeks of treatment with nebulized SUN-101 in patients with COPD: Golden-4 (glycopyrrolate for obstructive lung disease via electronic nebulizer) Available from: https://clinicaltrials.gov/ct2/show/NCT2347774?term=NCT2347774&rank=1. NLM identifier: NCT2347774Accessed March 13, 2016
- Sunovion Respiratory Development IncA randomized, open-label, active-controlled, parallel-group, multicenter, long-term safety trial of treatment with nebulized SUN-101 in patients with COPD: Golden-5 (glycopyrrolate for obstructive lung disease via electronic nebulizer) Available from: https://clinicaltrials.gov/ct2/show/NCT2276222?term=NCT2276222&rank=1. NLM identifier: NCT2276222Accessed March 13, 2016
- GrossNThe COPD pipeline, XXVIIIJ COPD Found201523259263
- Pulido-RiosMTMcNamaraAObedencioGPIn vivo pharmacological characterization of TD-4208, a novel lung-selective inhaled muscarinic antagonist with sustained bronchoprotective effect in experimental animal modelsJ Pharmacol Exp Ther2013346224125023685545
- LoANichollsAJHaumannBMoranEJBourdetDLA population PK model of once-daily TD-4208, a nebulized long-acting muscarinic antagonist (LAMA) in patients with chronic obstructive pulmonary disease (COPD)Am J Respir Crit Care Med2015191A5764 Abstract
- NichollsAJBarnesCYatesWMoranEJSinghDA randomized, crossover, 7-day study of once-daily TD-4208, a long-acting muscarinic antagonist, in subjects with COPDAm J Respir Crit Care Med2014189A6003 Abstract
- HaumannBKNichollsAJBarnesCBourdetDLMoranEJDose-ranging study of once-daily TD-4208, an inhaled long-acting muscarinic antagonist (LAMA) in patients with chronic obstructive pulmonary disease (COPD)Am J Respir Crit Care Med2015191A5750 Abstract
- Theravance Biopharma R & D, IncA phase 3, 12-week, randomized, double-blind placebo-controlled parallel group study of nebulized TD-4208 in subjects with chronic obstructive pulmonary disease Available from: https://goo.gl/ab2VxL?term=NLMidentifier&rank=NCT2459080. NLM identifier: NCT2459080Accessed March 13, 2016
- Theravance Biopharma R & D, IncA phase 3, 12-week, randomized, double-blind placebo-controlled parallel group study of nebulized TD-4208 in subjects with chronic obstructive pulmonary disease Available from: https://goo.gl/fPyjfM?term=NLMidentifier&rank=NCT2512510. NLM identifier: NCT2512510Accessed March 13, 2016
- Theravance Biopharma R & D, IncA phase 3, 52-week, random-ized, active-controlled parallel group study to evaluate the safety and tolerability of nebulized TD-4208 in subjects with chronic obstructive pulmonary disease Available from: https://goo.gl/83EjwX?term=NLMidentifier&rank=NCT2518139. NLM identifier: NCT2518139Accessed March 13, 2016
- TashkinDPKleinGLColmanSSZayedHSchonfeldWHComparing COPD treatment: Nebulizer, metered dose inhaler, and concomitant therapyAm J Med2007120543544117466655
- BrovanaBrovana medical guide and prescribing information2006 Available from: http://www.brovana.com/brovana-approved-labeling-text.pdfAccessed May 24, 2016
- The Global Initiative for Chronic Obstructive Lung DiseaseThe 2016 Global Strategy for Diagnosis, Management, and Prevention of COPD Available from: http://goo.gl/ItQL3aAccessed March 3, 2016
- TashkinDPFergusonGTCombination bronchodilator therapy in the management of chronic obstructive pulmonary diseaseRespir Res2013144923651244
- CazzolaMSegretiAMateraMGNew developments in the combination treatment of COPD: focus on umeclidinium/vilanterolDrug Des Devel Ther2013712011208
- AdiHYoungPMTrainiDCo-deposition of a triple therapy drug formulation for the treatment of chronic obstructive pulmonary disease using solution-based pressurised metered dose inhalersJ Pharm Pharmacol20126491245125322881437
- Lechuga-BallesterosDNogaBVehringRCummingsRHDwivediSKNovel cosuspension metered-dose inhalers for the combination therapy of chronic obstructive pulmonary disease and asthmaFuture Med Chem20113131703171821942257
- Bevespi Aerosphere™ (glycopyrrolate and formoterol fumarate) inhalation aerosol, for oral inhalation use [prescribing information]2016Holmes ChapelAventis Pharma LTD Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208294s000lbl.pdfAccessed May 24, 2016
- BarnesPJTriple inhalers for obstructive airways disease: will they be useful?Expert Rev Respir Med20115329730021702649
- LipworthBTriple inhaler therapy for COPDThorax2015701099126092923
- TrivediRKChendakeDSPatelMCA rapid, stability-indicating RP-HPLC method for the simultaneous determination of formoterol fumarate, tiotropium bromide, and ciclesonide in a pulmonary drug productSci Pharm201280359160323008808
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- LaubeBLJanssensHMJonghFHDevadasonSGDhandRDiotPWhat the pulmonary specialist should know about the new inhalation therapiesEur Respir J20113761308133121310878
- GrossNJDonohueJFNebulized formoterol: a review of clinical efficacy and safety in COPDInt J Chron Obstruct Pulmon Dis2010522323220714376
- FergusonGTFeldmanGJHofbauerPEfficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2–4 COPD: results from two replicate 48-week studiesInt J Chron Obstruct Pulmon Dis2014962964524966672
- van NoordJACornelissenPJAumannJLPlatzJMuellerAFogartyCThe efficacy of tiotropium administered via Respimat soft mist inhaler or HandiHaler in COPD patientsRespir Med20091031222919022642
- Accuneb® (albuterol sulfate) inhalation solution [prescribing information] Available from: http://dailymed.nlm.nih.gov/dailymed/getFile.cfm?setid=fa5ebedb-069c-4a1e-9a25-b6fa957e6c2b&type=pdf&name=fa5ebedb-069c-4a1e-9a25-b6fa957e6c2bAccessed March 13, 2016
- Xopenex® (levalbuterol HCl) inhalation solution [prescribing information] Available from: https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=20083Accessed March 13, 2016
- Theravance BiopharmaTheravance Biopharma announces positive top-line results from phase 2b dose-ranging study of its investigational LAMA, TD-4208, for the treatment of COPD [press release]. Thera-vance Biopharma; 2014 [September 8] Available from: https://goo.gl/fhudYXAccessed September 28, 2016
- KesserKCGellerDENew aerosol delivery devices for cystic fibrosisRespir Care200954675476719467162
