Abstract
Purpose
Current in-hospital management of exacerbations of COPD is suboptimal, and patient outcomes are poor. The primary aim of this study was to evaluate whether implementation of a care pathway (CP) for COPD improves the 6 months readmission rate. Secondary outcomes were the 30 days readmission rate, mortality, length of stay and adherence to guidelines.
Patients and methods
An international cluster randomized controlled trial was performed in Belgium, Italy and Portugal. General hospitals were randomly assigned to an intervention group where a CP was implemented or a control group where usual care was provided. The targeted population included patients with COPD exacerbation.
Results
Twenty-two hospitals were included, whereof 11 hospitals (n=174 patients) were randomized to the intervention group and 11 hospitals (n=168 patients) to the control group. The CP had no impact on the 6 months readmission rate. However, the 30 days readmission rate was significantly lower in the intervention group (9.7%; 15/155) compared to the control group (15.3%; 22/144) (odds ratio =0.427; 95% confidence interval 0.222–0.822; P=0.040). Performance on process indicators was significantly higher in the intervention group for 2 of 24 main indicators (8.3%).
Conclusion
The implementation of this in-hospital CP for COPD exacerbation has no impact on the 6 months readmission rate, but it significantly reduces the 30 days readmission rate.
Introduction
Exacerbations of COPD are a leading cause of hospital admissions worldwide. Thirty-five percent of COPD patients have at least 1 admission a year, with up to 30% readmitted within the 6 months after discharge.Citation1–Citation3 Adequate in-hospital management is expected to reduce readmission rates.Citation4 Although several worldwide established guidelines are available for the management of COPD,Citation2,Citation5 the current in-hospital management of COPD exacerbations is suboptimal, and outcomes with regard to readmission and mortality are poor.Citation6,Citation7
Care pathways (CPs) are widely used for optimizing adherence to guidelines and improving outcomes.Citation8–Citation10 They are defined as “a complex intervention for the mutual decision making and organization of predictable care for a well-defined group of patients during a well-defined period”.Citation11 Although COPD exacerbations are well suited to be treated in CPs, existing research on effectiveness is limited. Moreover, not one randomized study on COPD CPs has been reported up to date.Citation12–Citation15 As CPs are complex interventions that induce change at different levels of the organization, cluster randomized controlled trials (CRCTs) should be used to study their impact.Citation16
This trial is a CRCT on CP effectiveness launched by the European Pathway Association (E-P-A) in 2009.Citation11 The primary aim of this study was to evaluate whether implementation of a CP improves the 6 months readmission rate for patients with a COPD exacerbation. Secondary outcomes were the 30 days readmission rate, mortality, length of stay and adherence to guidelines.
Patients and methods
Study design and participants
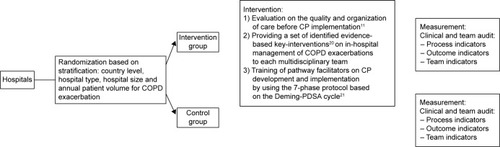
A pragmatic CRCTCitation17 was conducted, and the clusters included general hospitals out of Belgium, Ireland, Italy and Portugal, where patients hospitalized for a COPD exacerbation were cared for by a multidisciplinary team. Hospitals were randomized to either an intervention group, where a CP was developed and implemented, or a control group, where usual care was provided (). Usual care means that team members provide the same care during the study period as they were doing before implementation in the study. The study was registered as a CRCT at ClinicalTrials.gov (identifier: NCT0962468). Ethical approval was obtained on 3 levels. First, ethical approval was sought by the ethical committee of the research center at the country level. This included approval by the ethical committee of the coordinating center at Leuven University (identifier: ML5617), the National Committee of Data Protection for Portugal (6497/2011) and the ethical committee of the AOU Maggiore della Carità di Novara for Italy (625, 21/07/2011). Second, ethical approval was sought with regard to participation in the trial at the cluster level by the ethical committee of each of the participating hospitals (Table S1). Finally, individual written informed consent was obtained from all patients with regard to participation in the study and access to the patient record.
General hospitals could develop a CP when they provided written agreement to participate in the study. When hospitals were randomized into the control group, they agreed to not develop and implement a COPD CP within the time frame of the study. Eligibility criteria for patients were 1) hospital admission with COPD exacerbation as the primary diagnosis, 2) hospitalized for at least 48 hours, 3) admitted in a ward where COPD exacerbations were usually treated, 4) able to understand and read the native language and 5) provision of written informed consent. Patients could be included in the study only once, specifically at their first admission during the study period. Patients were excluded 1) if already included in another study of which the measurements could influence the measurements or outcomes of this study or 2) if they needed invasive positive pressure ventilation at admission to the hospital.Citation11
Enrollment of hospitals was done by the E-P-A, in close collaboration with the national E-P-A coordinator of each country. After consent, a study coordinator was appointed in each participating hospital.Citation11
Randomization and masking
Allocation concealment at team level was not possible and therefore general hospitals were stratified by country level, hospital type (teaching versus non-teaching), hospital size (<600 and ≥600 beds) and annual patient volume for COPD exacerbation (<300 patients and ≥300 patients)Citation18,Citation19 and then randomly assigned to the intervention group or to the control group. The allocation sequence was computer generated by a principal investigator at the coordination center at Leuven University, using a random number list and statistical software (http://www.randomizer.org). Study coordinators and teams were informed on their allocation after randomization. To minimize the risk for testing bias, the detailed data collection protocol was sent to the study coordinator of each hospital just before the start of the data collection. Furthermore, a logbook was kept for included patients in order to be able to connect the patient identity to the study number. In addition, to check for selection bias, for each patient admitted with COPD exacerbation during the recruitment period but excluded, baseline characteristics and the reason for exclusion were reported in a logbook for excluded patients.
Finally, in order to prevent assessment bias, all data, except for the measurements at discharge, were collected by an external researcher outside the clinical team. At discharge, a structured interview of the patient by a team member was performed to collect information on previous home situation and therapy before admission, which did not imply any risk for bias of the measurements.
Intervention
In the intervention group, a CP was implemented at hospital-level CP, and the intervention was composed of 3 active components ().Citation11 1) An evaluation on the quality and organization of care before CP implementation. In every hospital, a clinical audit was performed during a 2- to 3-month period, 6 months before developing the CP. Hospitals received a feedback report describing their performance compared to evidence-based guidelines and the performance of all other participating hospitals. The purpose of this feedback report was to help the hospitals in understanding the deficiencies in their actual organization of the care. This feedback report was discussed during a workshop, held before the start of the CP development. This workshop, as part of the intervention, was held before the start of the CP development and was attended by pneumologist, (head) nurse, physiotherapists and/or pathway facilitator of each participating hospital. During this workshop, the purpose of the EQCP study was presented, and the feedback report and the key interventions were discussed. This workshop closed with the next steps of the study, and time plans were presented. 2) Providing a set of identified evidence-based key interventionsCitation20 on in-hospital management of COPD exacerbations to each multidisciplinary team. The set of key interventions were based on literature, an international Delphi study and consensus meeting with multidisciplinary expert panel.Citation20 During the workshop, described earlier, these key interventions were presented and discussed. During the implementation phase, teaching sessions were organized by a pneumologist and a respiratory clinical nurse specialist concerning those key interventions for which teams experienced implementation difficulties, ie, administration of corticoid therapy, education on self-management strategies and inhalation therapy. Finally, slide kits were provided to the pathway facilitator of each hospital, ie, regarding administration of oxygen therapy, in order to support the facilitators in the education of their team members regarding the different key interventions. 3) Training of pathway facilitators on CP development and implementation by using the 7-phase protocol based on the Deming-Plan Do Study Act cycle.Citation21 For the development and implementation of the CP, the findings of the evaluation of the care process and the set of evidence-based key interventions were used. Meetings with the pathway facilitators were organized to further discuss the feedback reports and to address problems in implementation. Furthermore, a change expert supported change and exchange of knowledge and best practices.Citation11,Citation22 The teams developed a CP over a 6- to 8-month period.Citation23 In the control group, patients received the usual care, and no intervention was developed or implemented before.
Measurement
The primary outcome was the 6 months readmission rate. Secondary outcomes included the 30 days readmission rate, the 30 days and the 6 months mortality rate, length of stay and results on 24 main process indicators, categorized in diagnostic, pharmacological and non-pharmacological management, respectively. Twelve of 24 main process indicators were built of 2 or more subcomponents. For the 30 days and the 6 months readmission rate, which refers to COPD-specific readmission, only patients alive at, respectively, the 30 days and the 6 months were included in the analyses.Citation20 Additionally, demographic and COPD-specific data were collected.
The measurement period started 2–3 months after the end of the implementation period.Citation21 Data were collected by structured interviews performed by a team member at discharge, patient questionnaires completed at discharge and 30 days after discharge, structured telephone interviews at 30 days and 6 months after discharge performed by the study coordinator and a patient record analysis after discharge of the patient, performed by the study coordinator, together with a clinician outside the care team. In each hospital, all data were collected centrally and subsequently provided to the national coordinators of all participating countries. Data input was performed in a central database at Leuven University and guided by using a rigorous data input protocol.
Statistical analysis
Sample size calculation was performed according to standard criteria for CRCTs.Citation11,Citation24 Based on a number of 20 consecutive admitted patients in each unit, 20 hospitals needed to be included in both the intervention and the control groups. Briefly, the intracluster correlation coefficient (ICC) for the 6 months readmission rate was estimated to be equal to 0.018, leading to a design effect of 1.342. Using standard 0.05 α error and assuming a reduction of 11% in the 6 months readmission rate (from 41% to 30%),Citation25,Citation26 a sample size of 398 patients per arm was required to obtain a statistical power of 0.80.Citation11
A univariate analysis was carried out testing the baseline characteristics between intervention and control groups by using the chi-square test, Mann–Whitney U-test and independent sample t-test for categorical, ordinal and continuous variables, respectively. The process indicators were analyzed by 2-level mixed-effects logistic regression model, accounting for the clustering effect. The outcome indicators are analyzed by using a 2-level mixed-effects logistic and linear regression model for categorical and continuous variables, respectively, accounting for the clustering effect. For the adjusted outcomes, the significant variables (P<0.1), as determined by the univariate analysis and the intervention, were included in the final model. Multicollineairity was assessed.
Statistical significance was defined as a 2-sided P-value of 0.05. All analyses were intention to treat, performed by using R package lme4 (version 3.1.0) and MPlus 7.3 for ICC calculations.
Results
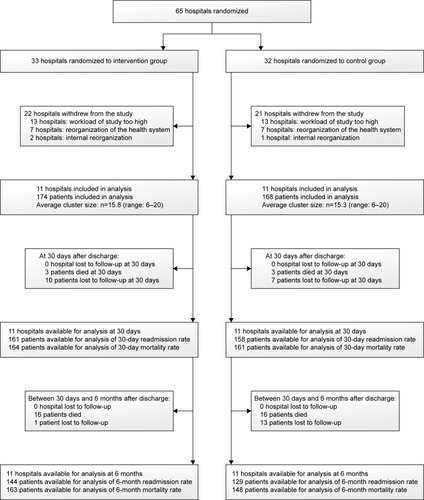
Initially, 65 hospitals were eligible for inclusion. After receiving the detailed study protocol, 22 hospitals decided to participate in the study. Of the 43 hospitals who did not take part in the study, 26 hospitals decided to not participate because they found the workload associated with the study too high. Furthermore, all 14 Irish hospitals dropped out because of reorganization of the Irish health care system and 3 hospitals dropped out because of internal reorganization.
Regardless of the drop-out, by chance 11 hospitals were allocated to the intervention group and 11 hospitals to the control group. In total, 342 patients, 174 in the intervention group and 168 in the control group, were recruited. The Belgian hospitals included patients between October 2010 and November 2011, while the Italian and Portuguese hospitals included patients between January 2013 and April 2014. In the intervention group, respectively, 10 patients and 1 patient were lost to follow-up at 30 days and 6 months after discharge, because of not reachable by the study coordinator. In the control group, respectively, 7 and 13 patients were lost to follow-up at 30 days and 6 months after discharge for the same reason ().
With regard to patient characteristics, the groups were highly comparable except for COPD severity at admission (higher in control group, P=0.018), diabetes (higher in the control group, P=0.007) and low body mass index (more present in the intervention group, P=0.003). Also, although not significant, cardiac failure and hospitalization in the year before index admission were higher in the control group. At cluster level, both groups were comparable for type, size and annual volume of patients admitted with COPD exacerbation ().
Table 1 Baseline characteristics of patients and hospitals
The 6 months readmission rate was lower in the intervention group (27.3%) compared to the control group (33.0%), though this result was not found statistically significant (adjusted odds ratio [OR] =0.642, 95% CI 0.347–1.188, P=0.158). Readmission rate at 30 days was statistically significantly lower (9.7%) in the intervention group compared to the control group (15.3%) (adjusted OR =0.427, 95% CI 0.222–0.822) ( and ). No significant differences were found for the 30 days and the 6 months mortality rate and length of stay.
Table 2 Results on outcome indicators
Table 3 Two-level mixed-effects regression model
Results on the individual process indicators are presented in . Performance on the individual process indicators was significantly higher for only 2 of 24 main indicators (8.3%) and for 9 of 41 subcomponents (22.0%). The largest differences were determined for the main indicators regarding non-pharmacological management (range of improvements: 0.7–45.9 percentage points). The mean adherence to the total of 24 measured process indicators was 59.4% (range: 18.8%–94.7%) in the intervention group and 49.4% (range: 11.8%–88.2%) in the control group (P=0.071). No patient received all the care they should receive.
Table 4 Results on process indicators: main level and subcomponent levelTable Footnotea
Discussion
The implementation of this CP has no significant effect on the 6 months readmission rate. However, the 30 days readmission rate was significantly lower in the intervention group (9.7%) compared to the control group (15.3%). Performance on process indicators was significantly higher in the intervention group for 2 of 24 main indicators (8.3%) and for 9 of 41 subcomponents (22.0%).
Before the launch of this study, only 5 national CRCTs on CPs had been conducted.Citation23,Citation27–Citation30 This first international trial provides new knowledge on the design of a multicountry CRCT. In comparison to former pathway studies, 1) the impact of the CP on the care itself was comprehensively investigated, while earlier studies focused primarily on outcomes,Citation12–Citation15 2) training of teams was an active component of the intervention and 3) a clinical audit, as part of the intervention, allowed each hospital to focus on those key interventions that showed most room for improvement.Citation21
A weakness of the study is that 43 hospitals withdrew after randomization, which resulted in a smaller sample size than initially targeted. It is reasonable to assume that the lower power of the study may have led to a failure in detecting statistically significant differences. The experience in this study is that a multicountry CRCT poses a major challenge due to standardization of the intervention in order to deliver the “same” intervention at the different sites, logistic, economic and cultural issues. These conditions should be considered carefully before starting an international CRCT. The study was underpowered, but it was decided not to increase the number of patients within the clusters because increasing the sample size per cluster does not increase power.Citation31
Research on COPD CPs is very limited. Previous studies described positive effects on blood sampling, daily weight measurement, arterial blood gas measurement, referral to rehabilitation, feelings of anxiety, readmission and in-hospital mortality. Due to limited statistical analysis and weak study design, the internal validity of results is limited.Citation12–Citation15 Our study confirmed that the implementation of a CP significantly reduces the 30 days readmission rate.Citation32,Citation33
Although the 30 days readmission rate was significantly lower in the intervention group, no differences were found for readmission rate at 6 months. The 6 months readmission was chosen as primary outcome, based on previous studies and expert opinion. However, during the study, it became clear that the outcomes at 30 days after discharge are highly associated with the in-hospital treatment, which was the focus of the CP intervention, while results at 6 months are mainly related to the nature of the disease and the quality of the outpatient and primary care. Therefore, for future studies on in-hospital CPs and readmission, it is recommendable to primarily focus on the 30 days readmission.Citation2 Finally, according to our results, ~6 of every 100 COPD patients treated according to the CP avoid a readmission at 30 days (number needed to treat: 17.0). Worldwide, based on the data of the WHO, this would result in a potential reduction of ~4 million readmissions.Citation34
With regard to process indicators, only 2 of these results were statistically significant, and by implementing a CP, the mean adherence to the guidelines was higher in the intervention group compared to the control group, though this difference was not significant. However, even non-significant results on process indicators may provide valid information regarding quality of care. Indeed, existing clinical practice guidelines on management of COPD exacerbation, which are very congruent and continuously updated, recommend unambiguously that the evaluated processes should be performed in every patient who is hospitalized for COPD exacerbation, regardless of patient characteristics or contextual factors. It is important to notice that, despite large improvements on process indicators after CP implementation, a considerable number of processes, especially with regard to non-pharmacological management, remained suboptimal performed. The non-pharmacological management contains some key interventions regarding education concerning smoking cessation, inhaler therapy and home oxygen therapy. Guidelines recommend more education as education is seen as an important part in the treatment of patients with a COPD exacerbation.Citation2 After implementing the CP, performance of education regarding inhaler therapy was significantly improved (9.1% in the control group and 35.3% in the intervention group; ). Although only 35.3% of the patients received education regarding inhaler therapy, while the other process indicators regarding education were even performed lower. So the care for patients with a COPD exacerbation is suboptimal performed, and therefore, continuous quality improvement will be needed in order to further optimize the care process for in-hospital management of COPD exacerbation and to enhance sustainability of the improved results.Citation35,Citation36 In addition, it is recommended that the team reconsiders the content of the pathway every 6 months.Citation21 For instance, at the beginning of 2014, the evidence concerning optimal duration of glucocorticoid therapy for acute exacerbations of COPD was changed.Citation2,Citation37
Follow-up research is needed to understand why and under which circumstances CPs work in terms of which active components in CPs underlie their effect and what is the role of contextual factors and multidisciplinary teamwork. Furthermore, an economic evaluation should be included to evaluate whether CPs also impact efficiency of care. Finally, in the context of the rising prevalence of COPD, CPs should also include transmural management and community-based treatment of COPD.Citation2
Conclusion
The implementation of this in-hospital CP for COPD exacerbation significantly reduced the 30 days readmission rate. This first international cluster randomized trial on CPs shows that the evidence-based key interventions are better performed after implementation of a CP compared to usual care. Additional studies are needed to understand how CPs are working, what their effect is on long term and how they affect the organization of care for different patient groups.
Acknowledgments
Cathy Lodewijckx is the joint first author with Kris Vanhaecht. We thank all professionals in the participating hospital wards who were involved in the implementation of the CP and the data collection. We thank R Veloso Mendes, P Boto, M Panella, F Leigheb, K Vanhaecht, W Sermeus, S Deneckere and C Lodewijckx for the coordination of the study in their country.
Supplementary material
Table S1 List of participating hospitals that gave ethical approval for this study
Disclosure
European Pathway Association obtained an unrestricted education grant from Pfizer SA, and this study was partially funded by Clinical Research Fund of University Hospitals Leuven. The funders had no role in the design, data collection, analysis, interpretation of data, writing of the report or decision to submit the report for publication. The autonomy of E-P-A and all involved academic institutions with regard to scientific independence and intellectual property on the methodology was guaranteed. MD has been part of Advisory Board for AstraZeneca, Boehringer-Pfizer, GSK, Nycomed, Novartis, Altana and Dompé. He has performed consulting work for Boehringer-Pfizer, GSK and Novartis. He also received lecture fees from these companies. All of the above amounted to <10,000 euro per annum. He received a research grant of 45,000 euro/year from AstraZeneca and 25,000 euro/year from GSK. KV, WS and MP are board members of European Pathway Association. KV, CL, WS, SD, FL, PB, SK, DS and MP report no other conflicts of interest in this work.
References
- DumanDAksoyEAgcaMCThe utility of inflammatory markers to predict readmissions and mortality in COPD cases with or without eosinophiliaInt J Chron Obstruct Pulmon Dis2015102469247826648709
- Global Initiative for Chronic Obstructive Lung DisesaseGlobal Strategy for Diagnosis, Management, and Prevention of COPD2015 Available from: http://www.goldcopd.it/materiale/2015/GOLD_Report_2015.pdfAccessed February 11, 2015
- IzquierdoJLBarcinaCJimenezJMuñozMLealMStudy of the burden on patients with chronic obstructive pulmonary diseaseInt J Clin Pract2009631879719125996
- WedzichaJASeemungalTACOPD exacerbations: defining their cause and preventionLancet2007370958978679617765528
- Map of Medicine (MOM) [homepage on the Internet]Specialist management of acute exacerbation Available from: http://mapofmedicine.comAccessed November 2014
- LodewijckxCSermeusWVanhaechtKInhospital management of COPD exacerbations: a systematic review of the literature with regard to adherence to international guidelinesJ Eval Clin Pract20091561101111020367712
- RobertsCMLopez-CamposJLPozo-RodriguezFHartlSEuropean COPD Audit TeamEuropean hospital adherence to GOLD recommendations for chronic obstructive pulmonary disease (COPD) exacerbation admissionsThorax2013681169117123729193
- PearsonSDGoulart-FisherDLeeTHCritical pathways as a strategy for improving care: problems and potentialAnn Intern Med19951239419487486490
- RotterTKinsmanLJamesEClinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costsCochrane Database Syst Rev201039913CD006632
- CostantiniMRomoliVLeoSDLiverpool care pathway for patients with cancer in hospital: a cluster randomised trialLancet201438322623724139708
- VanhaechtKSermeusWPeersJThe impact of care pathways for exacerbation of chronic obstructive pulmonary disease: rationale and design of a cluster randomized controlled trialTrials20101111121092098
- LodewijckxCSermeusWPanellaMImpact of care pathways for in-hospital management of COPD exacerbation: a systematic reviewInt J Nurs Stud2011481445145621798538
- BanAIsmailAHarunRAbdul RahmanASulungSSyed MohamedAImpact of clinical pathway on clinical outcomes in the management of COPD exacerbationBMC Pulm Med2012122722726610
- NishimuraKYasuiMNishimuraTOgaTClinical pathway for acute exacerbations of chronic obstructive pulmonary disease: method development and five years of experienceInt J Chron Obstruct Pulmon Dis2011636537221760723
- Abd-ElwaneesAEl-SoussiAOthmanSAliREffect of implementing clinical pathway on the clinical outcomes of patients with acute exacerbations of chronic obstructive pulmonary diseaseInt J Nurs Sci20144110
- CraigPDieppePMacintyreSDeveloping and evaluating complex interventions: the new Medical Research Council guidanceBMJ2008337a165518824488
- PatsopoulosNAA pragmatic view on pragmatic trialsDialogues Clin Neurosci201113221722421842619
- HoskerHAnsteyKLoweDPearsonMRobertsCMVariability in the organisation and management of hospital care for COPD exacerbations in the UKRespir Med200710175476117045788
- RobertsCMBarnesSLoweDEvidence for a link between mortality in acute COPD and hospital type and resourcesThorax20035894794914586045
- LodewijckxCDecramerMSermeusWPanellaMDeneckereSVanhaechtKEight-step method to build the clinical content of an evidence-based care pathway: the case for COPD exacerbationTrials20121322923190552
- VanhaechtKVan GervenEDeneckereSThe 7-phase method to design, implement and evaluate care pathwaysInt J Per Centered Med20122341351
- DeneckereSEuwemaMLodewijckxCBetter interprofessional teamwork, higher level of organized care, and lower risk of burnout in acute health care teams using care pathways: a cluster randomized controlled trialMed Care20135119910723132203
- PanellaMMarchisioSDemarchiMLManzoliLDi StanislaoFReduced in-hospital mortality for heart failure with clinical pathways: the results of a cluster randomised controlled trialQual Saf Health Care20091836937319812099
- CampbellMKMollisonJGrimshawJMCluster trials in implementation research: estimation of intracluster correlation coefficients and sample sizeStat Med200120339139911180309
- AlmagroPBarreiroBOchoa de EchaguenARisk factors for hospital readmission in patients with chronic obstructive pulmonary diseaseRespiration200673631131716155352
- BratzlerDWOehlertWHMcAdamsLMLeonJJiangHPiattDManagement of acute exacerbations of chronic obstructive pulmonary disease in the elderly: physician practices in the community hospital settingJ Okla State Med Assoc20049722723215346799
- CunninghamSLoganCLockerbieLDunnMJMcMurrayAPrescottRJEffect of an integrated care pathway on acute asthma/wheeze in children attending hospital: cluster randomized trialJ Pediatr2008152331532018280833
- KinsmanLDRotterTWillisJSnowPCBuykxPHumphreysJSDo clinical pathways enhance access to evidence-based acute myocardial infarction treatment in rural emergency departments?Aust J Rural Health2012202596622435765
- PanellaMMarchisioSBarbieriADi StanislaoFA cluster randomized trial to assess the impact of clinical pathways for patients with stroke: rationale and design of the Clinical Pathways for Effective and Appropriate Care Study [NCT0673491]BMC Health Serv Res2008822318980664
- De LucaAToniDLauriaLAn emergency clinical pathway for stroke patients – results of a cluster randomised trial (isrctn41456865)BMC Health Serv Res200991419159477
- Medical Research Council (MRC)Cluster Randomised Trials: Methodological and Ethical Considerations2002 Available from: http://www.cebma.org/wp-content/uploads/Cluster-randomised-trials-Methodological-and-ethical-considerations.pdfAccessed April 2015
- SantamariaNConnersAOsteraasJHamJBoodramBA prospective cohort study of the effectiveness of clinical pathways for the in-patient management of acute exacerbation of chronic obstructive pulmonary disease (COPD)Collegian2004111216
- McManusTEMarleyAKidneyJCThe Mater Hospital multiprofessional care pathway for acute exacerbations of chronic obstructive pulmonary diseaseJ Integr Care Pathways200593236
- WHO [webpage on the Internet]Chronic obstructive pulmonary disease (COPD): facts Available from: http://www.who.int/respiratory/copd/en/Accessed February 2015
- ShortellSMBennettCLByckGRAssessing the impact of continuous quality improvement on clinical practice: what it will take to accelerate progressMilbank Q1998765936249879304
- WiltseySSKimberlyJCookNCallowayACastsroFCharnsMThe sustainability of new programs and innovations: a review of the empirical literature and recommendations for future researchImplement Sci201271722417162
- VogelmeierCFVestboJHurdSSChanges in GOLD: today and tomorrowLancet Respir Med2015342442626065970