Abstract
Background
When assessing patients with exacerbation of asthma or COPD, it may be useful to know the drop in forced expiratory volume in 1 second (FEV1) compared with stable state, in particular when considering treatment with oral corticosteroids. The objective of the study was to identify indicators of drop in FEV1 during exacerbations.
Methods
In this prospective multicenter study from primary care, patients diagnosed with asthma or COPD were examined at stable state and during exacerbations the following year. Symptoms, chest findings, and pulse oximetry were recorded, and spirometry was performed. A fixed drop in FEV1 (10% and ≥200 mL) and percentage change in FEV1 were outcomes when possible indicators were evaluated.
Results
Three hundred and eighty patients attended baseline examination, and 88 with a subsequent exacerbation were included in the analysis. Thirty (34%) had a significant drop in FEV1 (10% and 200 mL). Increased wheezing was the only symptom associated with this drop with a likelihood ratio of 6.4 (95% confidence interval, 1.9–21.7). Crackles and any new auscultation finding were also associated with a significant drop in FEV1, as was a ≥2% drop in oxygen saturation (SpO2) to ≤92% in the subgroup diagnosed with COPD. Very bothersome wheezing and severe decrease in SpO2 were also very strong predictors of change in FEV1 in linear regression adjusted for age, gender, and baseline FEV1% predicted.
Conclusion
Increased wheezing, as experienced by the patient, and a decreased SpO2 value strongly indicated a drop in lung function during asthma and COPD exacerbations and should probably be taken into account when treatment with oral corticosteroids is considered.
Background
Patients with COPD or asthma frequently experience exacerbations of their illness. Studies from Norway and the UK have shown that each year ~50% of COPD patients experience an exacerbation that leads to a visit to a general practitioner (GP), in-hospital treatment, or self-administered treatment with antibiotics or systemic corticosteroids.Citation1,Citation2 In a study from the United States, ~25% of asthma patients reported to use a short course with oral corticosteroids during a period of 3 months.Citation3 Treatment with systemic corticosteroids is recommended in both severe COPD exacerbationsCitation4,Citation5 and asthma attacks.Citation6,Citation7 Such medication counteracts the inflammatory response in the bronchial tree, and by doing so, alleviates the accompanying bronchial obstruction. In asthma attacks, there is firm evidence for a favorable effect of systemic corticosteroids,Citation6 for COPD exacerbations the evidence refers mainly to clinical trials in hospitalized patients.Citation4 In COPD guidelines, the criteria for starting such treatment are not clear cut, but it is often recommended when symptoms are not relieved after stepping up treatment with inhaled bronchodilators.Citation8 Benefit from systemic corticosteroid treatment has to be weighed against adverse effects, such as increased fragility of the bones and increased blood levels of glucose.Citation4
Improvement in lung function has been a main outcome measure in studies on the effects of systemic corticosteroids.Citation9,Citation10 This implies that the acute deterioration of lung function associated with the exacerbation is a main target for the systemic corticosteroids. It would, therefore, probably be useful, when deciding on prescribing systemic corticosteroids, to know whether the patient actually suffers from increased bronchial obstruction and if so, to which degree? However, doing spirometry in the acute phase of a COPD exacerbation is not recommended in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines,Citation5 and it might not be convenient in all cases with asthma attacks. Other sources of information that could reflect increased bronchial obstruction could be the symptoms of the patient, as well as chest findings and assessment of oxygen saturation (SpO2) by pulse oximetry. The aim of this study was to describe how these clinical findings are associated with a decrease in lung function during exacerbations. We also wanted to find out whether the strength of these associations would be affected when they could be described in terms of change from stable state.
Methods
Study sample
This observational prospective study was carried out in seven general practice offices in the north and south of Norway. Patients aged 40 years or older registered in the electronic medical record with a diagnosis of asthma or COPD (or both) in the previous 5 years were identified, and a random sample of 1,111 patients was invited to participate. Of the 380 patients who met for baseline registration between April 2009 and March 2010, 376 were considered to be in a stable phase of their disease and were included in the study. Out of those, 210 were diagnosed with asthma by their GPs, 74 with COPD, and 92 with both asthma and COPD.Citation11 They were all asked to visit their GP within 2–3 days if they experienced exacerbations of their lung disease the following 12 months.
All participants gave written consent, and the study was approved by the Regional Committee for Medical and Health Research Ethics in North Norway.
Examinations at baseline
Respiratory symptoms the previous week and relevant quality of life items were examined using the Norwegian translation of the Clinical COPD Questionnaire (CCQ).Citation12 In the CCQ, the symptoms “short of breath at rest”, “short of breath doing physical activities”, coughing, and production of phlegm were classified into scores from 0 (never) to 6 (almost all the time), and a mean score of respiratory symptoms was calculated. The CCQ also contains questions on depression and concern about the disease getting worse with the same score system and four questions on activity limitation due to the illness, also with a score system from 0 (not limited at all) to 6 (totally limited). A total CCQ score as the mean of all 10 items was also calculated. Smoking habit and hospitalizations due to the lung disease the previous year were reported on a separate questionnaire. On a pop-up questionnaire in the electronic medical record the examining GP recorded comorbidities and treatment given, including antibiotics and systemic corticosteroids for exacerbations the previous year. Exacerbation of asthma or COPD the year before baseline was defined as exacerbations that were treated with antibiotics and/or systemic corticosteroids or ended with hospitalization, a definition previously used for COPD exacerbations.Citation1 The GPs also reported the presence of wheezes, crackles, diminished breath sounds, and prolonged expiration after auscultation of the lungs. The examinations at baseline were shared between 20 GPs.
C-reactive protein (CRP) was analyzed using near patient tests: Afinion AS100 Analyzer, Orion Quickread CRP, or ABX Micros CRP, which all could display values down to 8 mg/L. SpO2 was measured by a digital handheld pulse oximeter, Onyx II model 0550 (Nonin Medical, Inc., Plymouth, MN, USA). The highest value of the three measurements was recorded.
Spirometry was carried out after the pulse oximetry, following the European Respiratory Society/American Thoracic Society guidelines.Citation13 Spirare SPS310 spirometers (Diagnostica AS, Oslo, Norway) were used. The patients were seated, and nose clip was not used. Post-bronchodilator spirometry was carried out 20 minutes after inhalation of 0.4 mg salbutamol. The post-bronchodilator forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) were used in the analyses. Norwegian reference values for spirometry were applied.Citation14
Examinations at exacerbations
The participants filled in the CCQ, this time concerning the previous 24 hours. Respiratory symptoms the previous 24 hours were also reported by the GPs based on history taking on a pop-up questionnaire and shortness of breath, coughing, phlegm production, and wheezing were classified into three grades: 1) not present or as normal, 2) more bothersome than normal, and 3) very bothersome. The GPs also recorded whether the sputum was yellowish or green. Chest findings, the CRP value, and pulse oximetry were recorded as done at baseline. Lung function testing was also carried out with the same procedure, but post-bronchodilator spirometry was not carried out.
Statistical analysis
A significant drop in FEV1was defined as a drop of ≥10% from baselineCitation15 and of at least 200 mL. How such a drop in FEV1 was associated with baseline characteristics and symptoms, signs and test results at exacerbation was analyzed by chi-square test, Fischer’s exact test (dichotomous variables), and Mann–Whitney test (the CCQ variables). The threshold used for raised CRP value was ≥20 mg/L. Two thresholds were used for SpO2, ≤92% and ≤95%. Changes from baseline were found by subtracting baseline values from the values at exacerbation. Significant increase in CRP was defined as an increase of at least 10 mg/L to a value of ≥20 mg/L. Significant decreases in SpO2 were defined as a reduction of at least 2% to ≤95% and ≤92%, respectively. Likelihood ratios were calculated for findings significantly associated with the fixed drop in FEV1 using online software (M.K. Anders Consulting). Associations with percentage change in FEV1 from baseline were analyzed by linear regression making it possible to have the degree of change as outcome. IBM SPSS Statistics software version 22 was used.
Results
During the 1-year follow-up period, 109 patients visited their GP due to one or more exacerbations. For patients who visited the GP with several exacerbations, the first exacerbation was, as a rule, included in the analysis. However, 21 patients were excluded due to incomplete data from the exacerbations, in most cases because spirometry was not done. Seventeen GPs took part in the examinations of the 88 included patients at baseline, whereas 34 GPs shared the examinations during the exacerbations, and 41 patients (47%) were examined by the same GP both times. The mean age of the patients at baseline was 63 years, and 66% were female. A COPD diagnosis (or both asthma and COPD) had been given to 48 (55%) patients at baseline, and patients with a COPD diagnosis had significantly more frequent history of exacerbation the previous year. They were also different from the patients in the asthma group in other ways ().
Table 1 Baseline characteristics of 88 adult patients with asthma or COPD
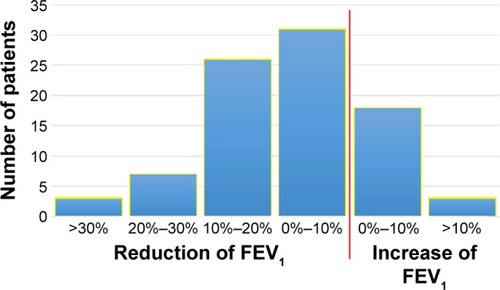
The distribution of FEV1 at baseline and at exacerbation is shown in , with predicted mean values of 76.1% and 70.8%, respectively. Among the asthma patients, the corresponding FEV1% predicted values were 87.5 and 83.0 and among the COPD patients, 61.2 and 54.7. The distribution of change from baseline is shown in . A significant drop in FEV1 at exacerbation (10% and at least 200 mL) was found in 30 patients (34%). The patients with this drop in lung function had more frequent COPD diagnosis at baseline than those without (P=0.04, ). During spirometry at baseline 75 patients (85%) expired for more than 6 seconds, at exacerbation the corresponding number was 67 (76%).
Table 2 Patient characteristics, symptoms, and findings and their association with a significant drop in FEV1 at exacerbation (10% and at least 200 mL from baseline) in 88 patients diagnosed with asthma or COPD
Figure 1 Distribution of FEV1% predicted at baseline and during excerbation in 88 patients diagnosed with asthma or COPD.
Abbreviation: FEV1, forced expiratory volume in 1 second.
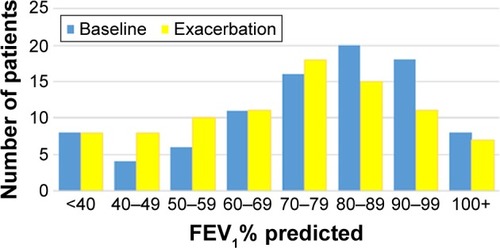
Figure 2 Distribution of change in FEV1% predicted between baseline and exacerbation in 88 patients diagnosed with asthma or COPD.
Increased wheezing was the only symptom associated with a significant drop in lung function. Ten of the 13 patients who reported very bothersome wheezing had a significant drop in FEV1 (P=0.001, ). In the asthma group, only two patients had this symptom, but a significant drop in FEV1 was found in both.
An abnormal finding by chest auscultation was found in 68 patients (77%), and a new sign (not recorded at baseline) was found in 48 patients (55%). Any abnormal chest sign, whether new or not, was recorded more frequently in patients with a significant drop in FEV1 () compared with other patients. The sign “crackles” was associated with a drop in FEV1, whereas “wheezes” was not. In 26 patients (30%), one or more of the four chest signs were recorded both at baseline and exacerbation, and with similar frequency whether the same GP performed the auscultation at both occasions, 24% and 34%, respectively (P=0.3). SpO2 values ≤92% were found in 11 patients, and in 10 of these, the saturation had dropped 2% or more between baseline and exacerbation. This drop in SpO2 was associated with a significant drop in FEV1 (P=0.02).
There was a median increase in CCQ score of 1.25 for respiratory symptoms between baseline and exacerbation, but no difference was found between the patients with a significant drop in FEV1 and the other patients ().
Table 3 Median CCQ scores at exacerbation and change in median CCQ score compared with baseline, and significant drop in FEV1 from baseline (10% and ≥200 mL) in 78 patientsTable Footnotea diagnosed with asthma or COPD
The diagnostic value of findings, significantly indicating a drop in FEV1, is shown in . The asthma and COPD subgroups did not differ when it came to likelihood ratios of very bothersome wheezing experienced by the patients and crackles on auscultation. A diagnostic value of SpO2 dropping to below 93% was only found in the COPD group.
Table 4 Likelihood ratio of selected findings for a significant drop in FEV1 at exacerbation (10% and at least 200 mL from baseline) in 88 patients diagnosed with asthma or COPD
When the percentage drop in FEV1 was used as outcome in univariable linear regression, the FEV1/FVC ratio was the strongest predictor among the baseline characteristics (). Treatment with long-acting beta-agonist/inhaled corticosteroids seemed to slightly counteract the drop in FEV1, but this association was not statistically significant. Very bothersome wheezing during exacerbation, as experienced by the patient, was the strongest indicator of change in FEV1 among the symptoms, and prolonged expiration and any abnormal auscultation finding were now stronger indicators than crackles. A decrease in SpO2 of at least 2% to a value of ≤92% was still strongly associated with a drop in FEV1.
Table 5 Associations analyzed by linear regressionTable Footnotea between baseline characteristics and symptoms and findings at exacerbation and the percentage drop in FEV1 from baseline in 88 patients diagnosed with asthma or COPD
Discussion
Main findings
We have attempted to find alternatives to spirometry in assessing drop in lung function during asthma and COPD exacerbations. One strong indicator of a significant drop in lung function was increased wheezing as experienced by the patient. As far as we know, this finding has not been reported previously to be a sign of a drop in lung function. Patient experienced wheezing was a stronger predictor of drop in lung function than were wheezes on auscultation.
Decreased SpO2 measured as SpO2 ≤92% was also strongly associated with a drop in FEV1. Only one COPD patient had SpO2 ≤92% at baseline, telling us that this is a relevant finding for primary care. A drop in FEV1 was most frequently found in the patients with a COPD diagnosis, and the FEV1/FVC ratio at baseline was strongly associated with a change in lung function in the linear regression, indicating that the severity of the COPD should be taken into account.
Strengths and limitations
As part of a prospective study with baseline examinations, we were able to collect post-bronchodilator spirometry at stable state in all patients. The spirometry was well performed, and although fewer patients expired for more than 6 seconds during exacerbation than at baseline, there are reasons to believe that valid FEV1 values were obtained. The cutoff level of 10% for drop in FEV1 may be regarded as arbitrary, and the results are strengthened by using the percentage drop in FEV1 as continuous outcome variable in linear regression.
Findings at exacerbation could also be shown as new findings compared to stable state. However, we did not find any great benefit of taking the baseline findings into account.
One may question whether including both COPD and asthma patients was a good idea. One good reason for doing this is the difficulties in differentiating between asthma and COPD in many patients with obstructive lung disease.Citation11,Citation16 A second justification is the similar challenges in the two diseases when it comes to treating exacerbations with oral corticosteroids.
This is rather a small clinical study, and the results should be interpreted with caution. Not all patients who were assessed during exacerbation were included, due to incomplete data collection in the practices. Whereas the collection of baseline data was well organized, the participating practices were not always as prepared when it came to the exacerbations. This has probably not resulted in any systematic selection bias influencing the association between findings and drop in lung function.
Comparisons with existing literature
We have not found studies evaluating wheezing, as experienced by patients, as a sign of drop in lung function, only as an independent predictor of COPD in general.Citation17 Wheezing is not one of the items in score sheets for health status in COPD patients, such as the CCQCitation12 and the COPD assessment test (CAT)Citation18 and neither in the exacerbations of chronic pulmonary disease tool (EXACT).Citation19
Decreased SpO2 during COPD exacerbations has been shown in previous studies,Citation20,Citation21 but decreased SpO2 has not previously been linked to the magnitude of drop in FEV1. A strong link between reduced FEV1 and reduced pulse oximetry values has been shown in several studies, also among asthma and COPD patients in general practice.Citation22 Introduction of pulse oximeters in primary care was recommended in 2010,Citation23 and our results support this development.
In a previous publication from this project, we showed that decreased SpO2 predicted treatment with oral corticosteroids.Citation24 There was, however, no association between patient experienced wheezing and the prescribing of oral corticosteroids (among the 13 patients with very bothersome wheezing, 6 [46.2%] were treated with oral corticosteroids, compared to 40% in the remaining patients).
One may question why not just spirometry can be used in the assessment of lung function during asthma and COPD exacerbations. It was successfully applied in this study and has also been performed in other studies without reports of adverse experiences.Citation9,Citation10 The recommendation in the GOLD guidelines, not to use spirometry during exacerbations,Citation5 is probably based on experiences from hospital and with severely ill outpatients. In primary care, the majority of COPD patients have a mild or moderate disease, as was also the case in this study. When a spirometry result from stable state is available and performed within the previous year, a new spirometry can usually give a valid measure of drop in lung function. However, coughing may force the patient to terminate the expiratory maneuver too early, and this may explain why the expiration lasted less than 6 seconds more frequently during exacerbation than at baseline in this study. Clinical assessment without spirometry is often needed.
To find wheezes by auscultation of the chest might be more useful in clinical practice than shown in this study. Wheezes were recorded as present or absent, but their intensity in terms of number and duration was not documented. Consequently, when wheezes were recorded as present in both stable state and during exacerbation, we had no information about the change in the intensity. This can also explain why comparison with stable state was of little help. The difficulties in interpreting auscultation findings also relate to crackles and prolonged expiration, which were stronger indicators of a drop in FEV1 than wheezes in this study.
To ask primary care patients with asthma or COPD exacerbations about wheezing seems to be a simple and a rational approach when assessing change in lung function. Measuring SpO2 with pulse oximetry is useful, particularly in COPD patients with severe symptoms. Increased “wheezing” and low pulse oximetry values are probably useful to take into account when treatment with oral corticosteroids is considered. However, we do not know to which degree treatment with oral corticosteroids should be based on a drop in FEV1. More studies are needed to help identify the clinical findings that can be helpful in the difficult decision to prescribe this potent medication.
Author contributions
HM designed the study and conducted the collection of data. All authors contributed toward data analysis, drafting, and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We thank participating patients and doctors at the following GP offices: Nordbyen legesenter, Tromsø, Allmed legesenter, Hammerfest, Alta helsesenter, Skedsmokorset legesenter, Lillestrøm legesenter, Langbølgen legesenter, Oslo, and Gransdalen legesenter, Oslo. The work was funded by the Norwegian Research Council, project 202650.
Disclosure
The authors report no conflicts of interest in this work.
References
- HurstJRVestboJAnzuetoAEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) InvestigatorsSusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- Al-aniSSpigtMHofsetPMelbyeHPredictors of exacerbations of asthma and COPD during one year in primary careFam Pract201330662162824115012
- MillerMKLeeJHMillerDPWenzelSERecent asthma exacerbations: a key predictor of future exacerbationsRespir Med2007101348148916914299
- WaltersJATanDJWhiteCJGibsonPGWood-BakerRWaltersEHSystemic corticosteroids for acute exacerbations of chronic obstructive pulmonary diseaseCochrane Database Syst Rev20149CD001288
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- RoweBHEdmondsMLSpoonerCHDinerBCamargoCAJrCorticosteroid therapy for acute asthmaRespir Med200498427528415072167
- BatemanEDHurdSSBarnesPJGlobal strategy for asthma management and prevention: GINA executive summaryEur Respir J200831114317818166595
- LaueJReierthEMelbyeHWhen should acute exacerbations of COPD be treated with systemic corticosteroids and antibiotics in primary care: a systematic review of current COPD guidelinesNPJ Prim Care Respir Med2015251500225695630
- WedzichaJAOral corticosteroids for exacerbations of chronic obstruc-tive pulmonary diseaseThorax200055Suppl 1S23S2710943634
- WhiteAJO’BrienCHillSLStockleyRAExacerbations of COPD diagnosed in primary care: changes in spirometry and relationship to symptomsCOPD20052441942517147007
- MelbyeHDrivenesEDalbakLGLeinanTHøegh-HenrichsenSOstremAAsthma, chronic obstructive pulmonary disease, or both? Diagnostic labeling and spirometry in primary care patients aged 40 years or moreInt J Chron Obstruct Pulmon Dis2011659760322135492
- van der MolenTWillemseBWSchokkerSten HackenNHPostmaDSJuniperEFDevelopment, validity and responsiveness of the Clinical COPD QuestionnaireHealth Qual Life Outcomes200311312773199
- MillerMRHankinsonJBrusascoVATS/ERS Task ForceStandardisation of spirometryEur Respir J200526231933816055882
- LanghammerAJohnsenRGulsvikAHolmenTLBjermerLForced spirometry reference values for Norwegian adults: the Bronchial Obstruction in Nord-Trondelag StudyEur Respir J200118577077911757626
- AronssonDTufvessonEBjermerLComparison of central and peripheral airway involvement before and during methacholine, mannitol and eucapnic hyperventilation challenges in mild asthmaticsClin Respir J201151101821159136
- BarnesPJAsthma-COPD overlapChest201614917826757281
- BroekhuizenBDSachsAPVerheijTJAccuracy of symptoms, signs, and C-reactive protein for early chronic obstructive pulmonary diseaseBr J Gen Pract201262602e632e63822947584
- JonesPWHardingGBerryPWiklundIChenWHKline LeidyNDevelopment and first validation of the COPD assessment testEur Respir J200934364865419720809
- LeidyNKMurrayLTJonesPSethiSPerformance of the EXAc-erbations of chronic pulmonary disease tool patient-reported outcome measure in three clinical trials of chronic obstructive pulmonary diseaseAnn Am Thorac Soc201411331632524432712
- SchermerTLeendersJin ’t VeenHPulse oximetry in family practice: indications and clinical observations in patients with COPDFam Pract200926652453119815674
- HurstJRDonaldsonGCQuintJKGoldringJJPatelARWedzichaJADomiciliary pulse-oximetry at exacerbation of chronic obstructive pulmonary disease: prospective pilot studyBMC Pulm Med2010105220961450
- DalbakLGStraandJMelbyeHShould pulse oximetry be included in GPs’ assessment of patients with obstructive lung disease?Scand J Prim Health Care201533430531026654760
- PluddemannAThompsonMHeneghanCPriceCPulse oximetry in primary care: primary care diagnostic technology updateBr J Gen Pract20116158635835921619771
- SalwanAASpigtMLaueJMelbyeHPredictors of treatment with antibiotics and systemic corticosteroids for acute exacerbations of asthma and chronic obstructive pulmonary disease in primary careBMC Fam Pract2015164025887285
