Abstract
Introduction
The interval from the peak to the end of the electrocardiographic (ECG) T wave (Tp–Te) can estimate cardiovascular mortality and ventricular tachyarrhythmias.
Objectives
In this study, we aimed to define a new ECG parameter in patients with COPD.
Methods
This was a cross-sectional observational study that included COPD patients who were diagnosed previously and followed up in the outpatient clinic. All data of the patients’ demographic features, history, spirometry, and electrocardiographs were analyzed.
Results
We enrolled 134 patients with COPD and 40 healthy volunteers as controls in our study. Patients already known to be having COPD who were under follow-up for their COPD and diagnosed as having COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria were included. Men comprised 82.8% of the COPD group and 73.2% of controls. The mean age in the COPD and control group was 60.2±9.4 and 58.2±6.7 years, respectively. There was no significant difference between the groups for age or sex (p=0.207, p=0.267, respectively). There were 46 (34.3%) patients in group A, 23 (17.2%) patients in group B, 26 (19.4%) patients in group C, and 46 (29.1%) patients in group D as COPD group. There was a significant increase in Tp–Te results in all precordial leads in the COPD group compared with the control group (p<0.05). Precordial V4 lead has the most extensive area under the curve (0.831; sensitivity 76.5%, specificity 89.6%).
Conclusion
We present strong evidence that Tp–Te intervals were increased in patients with COPD, which suggests that there may be an association between COPD and ventricular arrhythmias and cardiac morbidity.
Introduction
COPD and cardiovascular disease are distinctly related. Electrocardiographic (ECG) abnormalities such as bundle branch blocks, axis deviations, and arrhythmias are common in patients with COPD.Citation1 Such patients also have a higher cardiovascular morbidity and mortality rate than the general population.Citation2 In addition, half of the deaths of patients with COPD are attributable to cardiovascular disease.Citation2 Previous studies suggested that patients with COPD have a 2- to 3-fold increased risk of sudden cardiac death (SCD).Citation3 However, underlying mechanisms of the association between COPD and SCD are currently unclear and predictors of malignant cardiac arrhythmias that lead to SCD in COPD have not yet been defined.Citation4 Lahousse et alCitation5 presented the first data showing COPD as an independent risk factor for SCD.
According to recent studies, the interval between the peak and end of the T wave (Tp–Te) on ECG may stand for the index of total (transmural, apicobasal, and global) dispersion of repolarization.Citation6,Citation7 Furthermore, an increased Tp–Te interval could be a practical index to estimate cardiovascular mortality and ventricular tachyarrhythmias.Citation8,Citation9
COPD is associated with some ECG changes including prolonged QT interval, as described previously.Citation10,Citation11 The Tp–Te interval is a new marker for ventricular arrhythmias and repolarization heterogeneity.Citation12–Citation15
To date, no algorithm has been defined for the arrhythmic risk stratification of patients with COPD. Therefore, our aim was to define a new ECG parameter for ventricular arrhythmias and SCD in patients with COPD.
Methods
Study population
We enrolled 134 patients who were admitted to our outpatient clinic. Patients already known as having COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria who were under follow-up for their disease were included.Citation16 Forty healthy age- and sex-matched subjects were enrolled as a control group. Patients were classified using COPD assessment test scores, breathlessness level using the modified Medical Research Council dyspnea scale, and exacerbations history as recommended by GOLD.Citation16
Inclusion criteria included the following: patients were stable, diagnosed previously, and under follow-up for COPD.
The exclusion criteria of the present study were as follows: pregnancy; sepsis; neoplasms; current hemodialysis; acute coronary syndromes; severe electrolyte imbalances; acute cerebrovascular disease; aortic dissections; decompensated heart failure; surgery within the past 30 days; prior pulmonary embolism or deep venous thrombosis; acute or chronic infectious diseases; acute or chronic inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus, or vasculitis; and patients with prior myocardial infarction or heart failure with low ejection fraction (<50%).
The demographic, clinical, and laboratory characteristics of the study groups were taken from the patients’ histories and results of physical examinations, which were collected by chest disease physicians at admission.
ECG measurements
A 12-lead ECG was recorded on paper at 25 mm/s and 10 mm/mV gain at rest in the supine position. All ECGs were scanned and analyzed. All QT intervals were measured by 2 different cardiologists. Interobserver correlation for determining Tp–Te interval was assessed using the κ test, and there was a strong correlation between the observers (κ: 0.864, p<0.001). The Tp–Te interval was identified as the interval from the peak of the T wave to the end of the T wave. Tp–Te intervals were taken from precordial leads.Citation8 An average value of 3 readings was analyzed for each lead.
Ethical approval
The Local Ethics Committee of Istanbul University, Turkey, approved the study protocol. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study.
Statistical analysis
Kolmogorov–Smirnov test was used for testing normal distribution. Mann–Whitney U-test and the independent sample t-test were used for quantitative variables. χ2 test was used for the analysis of qualitative data, and Fischer’s test was used when χ2 test conditions were not formed. Statistical analyses were made using SPSS 22.0 (IBM Corporation, Armonk, NY, USA).
Results
The patients’ and control groups’ baseline demographic parameters, COPD stages, and dyspnea scores are shown in and . There was no significant difference between the controls and study group for age or sex (p=0.207, p=0.267, respectively). The cigarette smoking was estimated to be 44.29±2.7 pack-years. All of our patients had persistent airflow limitation, which was shown using spirometry. Patients’ forced expiratory volume in 1 second/forced vital capacity ratio was 52.78%±15.99%. Thirteen (9.7%) of our patients used biomass and coal as their main source of energy for cooking. Forty-five (33.6%) patients were still smoking cigarettes; however, 78 (58.2%) quit smoking. Twenty-one (15.7%) patients had been hospitalized because of COPD exacerbation in the previous year, and 88 (65.7%) visited outpatient clinics. Hypertension was the most frequent comorbidity among the study population.
Table 1 Baseline demographic and respiratory test findings in the control and COPD groups
Table 2 Baseline demographic and biochemical findings in study population
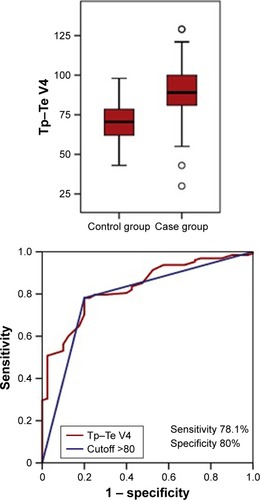
The Tp–Te measured from chest leads V1–6 of COPD and control group are compared in . There were significant differences in all leads (). Receiver operating characteristic curve analyses showed that the most sensitive and specific area under the curve was for lead V4 with a value of 0.83 ().
Table 3 Tp–Te values compared between patients with COPD and controls
Table 4 AUC values of Tp–Te compared between control and COPD groups
For Tp–Te >80, the sensitivity was 78.1% and the specificity was 80%. The receiver operating characteristic curve for Tp–Te V4 is shown in .
Figure 1 ROC curve for Tp–Te V4.
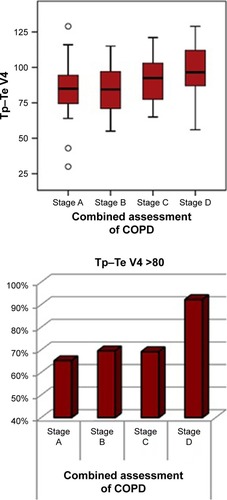
All patients with COPD were divided into 4 groups according to the recommendation by GOLD. When examining the COPD groups according to lead V4, there was only a statistically significant increase in patients who were Stage D (). There were no significant differences between patients who were Stage A, B, or C (; ). Patients were grouped in the light of revised GOLD 2017, and there was no relation between Tp–Te intervals and FEV values.
Table 5 Tp–Te V4 values compared between in COPD stages
Discussion
Cardiovascular morbidity and mortality in the population of patients with COPD is higher than in the overall population. For this reason, we need to develop new methods to predict patients who are at risk of SCD. The relationship between COPD and malignant ventricular arrhythmias causing SCD is still unknown. Surface ECG is an easy and cost-effective method to predict SCD. A new parameter, the Tp–Te, which shows the risk of malignant ventricular arrhythmias and SCD, has not previously been studied in patients with COPD. Our study is the first to prove a prolonged Tp–Te interval in patients with COPD.
Lahousse et alCitation5 presented the first study showing COPD as an independent risk factor for SCD. They showed a 2-fold increased risk of SCD in patients with COPD. The study demonstrated that patients with COPD died of SCD more frequently at night, logically due to decreased ventilation and increased hypercapnia, which may cause more nocturnal ventricular arrhythmias.Citation5 In addition, Lahousse et alCitation5 found that SCD was higher in patients with frequent exacerbations of COPD than in patients with stable outcomes of COPD. In our study, significantly increased Tp–Te values observed in group D patients for lead V4 supports this situation. Hawkins et alCitation17 proved that COPD was an independent predictor of death in patients with acute myocardial infarction. COPD can increase both cardiovascular and noncardiovascular events such as pulmonary disease, malignancy, and infection.
Several underlying mechanisms cause the increased risk of SCD in patients with COPD. One of these mechanism is β-adrenergic agonists, which are generally used in the treatment of COPD and can decrease the ventricular refractory period and increase the risk of ventricular arrhythmias.Citation18,Citation19
In addition, sympathetic overactivity and increased oxidative stress, which also exist in the COPD population, have been proven to participate in ventricular arrhythmias and SCD. Increased sympathetic activity has been linked with cardiac electrical instability, and therefore the progression to ventricular arrhythmia.
Previous studies have demonstrated the prolongation of the QTc interval in patients with hypoxemic COPD.Citation20 QTc interval >450 ms is an independent risk for ventricular arrhythmias.Citation4
Smoking is also another well-known underlying arrhythmogenic factor in patients with COPD. It is worth noting that nicotine can activate the release of catecholaminergic chemokines, thus increasing blood pressure and heart rate, and may decrease the threshold for ventricular arrhythmias.Citation21 Oxidative stress and carbon monoxide, as other components of cigarette smoke, probably play a vital role in triggering ventricular arrhythmias.Citation21
Previous clinical trials have demonstrated that prophylactic use of implantable cardioverter–defibrillator (ICD) can reduce SCD in patients with poor left ventricular ejection function (≤35%). This survival benefit has been shown in patients with COPD who have low left ventricular ejection fraction.Citation22 Bilchick et alCitation23 showed that COPD was an independent risk factor for mortality (hazard ratio: 1.70; 95% confidence interval: 1.61–1.80) in a study with primary prevention ICD implantation. However, COPD diagnosis alone is not sufficient for ICD implantation. Some additional criteria that can also be used include a cutoff Tp–Te interval, especially in lead V4 or QTc interval, for predicting COPD patients in whom ICD implantation can be performed.
The current study has some limitations. We performed standard surface 12-lead ECG; it is better to use 24-h ECG monitoring to predict Tp–Te interval change in a study group. In addition, the current study had a cross-sectional study design, and it would be better if it were a prospective study design to establish a relationship between prolonged Tp–Te interval in patients with COPD, malignant ventricular arrhythmias, and SCD. Controlled studies involving large patient groups are needed to establish whether a prolonged Tp–Te interval promotes malignant ventricular arrhythmia and SCD in patients with COPD.
Conclusion
COPD has emerged as an important risk factor for ventricular arrhythmias and SCD. To date, no algorithm has been defined for arrhythmic risk stratification of patients with COPD to protect patients from SCD. So, our aim was to define a new ECG parameter which is connected with ventricular arrhythmias and SCD in patients with COPD. Tp–Te interval is a new and useful parameter which can be assessed using surface ECG easily. We demonstrated strong evidence that Tp–Te intervals were increased in patients with COPD, which suggests that there may be an association among COPD, ventricular arrhythmias, and SCD. Nonetheless, further prospective studies should be done.
Disclosure
The authors report no conflicts of interest in this work.
References
- RodmanDMLowensteinSRRodmanTThe electrocardiogram in chronic obstructive pulmonary diseaseJ Emerg Med1990856076152254610
- HuiartLErnstPSuissaSCardiovascular morbidity and mortality in COPDChest200512842640264616236937
- SinDDManSFChronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortalityProc Am Thorac Soc20052181116113462
- SieviNAClarenbachCFCamenGRossiVAvan GestelAJKohlerMHigh prevalence of altered cardiac repolarization in patients with COPDBMC Pulm Med2014145524690123
- LahousseLNiemeijerMNvan den BergMEChronic obstructive pulmonary disease and sudden cardiac death: the Rotterdam studyEur Heart J201536271754176125920404
- KorsJARitsema van EckHJvan HerpenGThe meaning of the Tp-Te interval and its diagnostic valueJ Electrocardiol200841657558018954608
- AntzelevitchCSicouriSDi DiegoJMDoes Tpeak–Tend provide an index of transmural dispersion of repolarization?Heart Rhythm2007481114111617675094
- Castro HeviaJAntzelevitchCTornés BárzagaFTpeak-Tend and Tpeak-Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndromeJ Am Coll Cardiol20064791828183416682308
- SmetanaPSchmidtAZabelMAssessment of repolarization heterogeneity for prediction of mortality in cardiovascular disease: peak to the end of the T wave interval and nondipolar repolarization componentsJ Electrocardiol201144330130821511064
- LuiCYAcute pulmonary embolism as the cause of global T wave inversion and QT prolongation: a case reportJ Electrocardiol199326191958433060
- PunukolluGGowdaRMKhanIAWilburSLVasavadaBCSacchiTJQT interval prolongation with global T-wave inversion: a novel ECG finding in acute pulmonary embolismAnn Noninvasive Electrocardiol200491949814731221
- TaggartPSuttonPMOpthofTTransmural repolarization in the left ventricle in humans during normoxia and ischaemiaCardiovasc Res200150345446211376621
- OpthofTCoronelRJanseMJIs there a significant transmural gradient in repolarization time in the intact heart? Repolarization gradients in the intact heartCirc Arrhythm Electrophysiol200921899619808447
- AntzelevitchCSicouriSLitovskySHHeterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cellsCirc Res1991696142714491659499
- TopilskiIRogowskiORossoRThe morphology of the QT interval predicts torsade de pointes during acquired bradyarrhythmiasJ Am Coll Cardiol200749332032817239713
- VogelmeierCFCrinerGJMartinezFJGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2017 report. GOLD Executive SummaryAm J Respir Crit Care Med2017195555758228128970
- HawkinsNMHuangZPieperKSValsartan in Acute Myocardial Infarction Trial InvestigatorsChronic obstructive pulmonary disease is an independent predictor of death but not atherosclerotic events in patients with myocardial infarction: analysis of the Valsartan in Acute Myocardial Infarction Trial (VALIANT)Eur J Heart Fail200911329229819176539
- QinMLiuTHuHWangTYuSHuangCEffect of isoprenaline chronic stimulation on APD restitution and ventricular arrhythmogenesisJ Cardiol201361216216823085034
- SalpeterSROrmistonTMSalpeterEECardiovascular effects of β-agonists in patients with asthma and COPD: a meta-analysisChest200412562309232115189956
- StewartAGWaterhouseJCHowardPThe QTc interval, autonomic neuropathy and mortality in hypoxaemic COPDRespir Med199589279847709006
- PrasadDSKabirZDashAKDasBCSmoking and cardiovascular health: a review of the epidemiology, pathogenesis, prevention and control of tobaccoIndian J Med Sci2009631152053320075556
- RazakEKamireddySSabaSImplantable cardioverter-defibrillators confer survival benefit in patients with chronic obstructive pulmonary diseasePacing Clin Electrophysiol20103391125113020487355
- BilchickKCStukenborgGJKamathSChengAPrediction of mortality in clinical practice for medicare patients undergoing defibrillator implantation for primary prevention of sudden cardiac deathJ Am Coll Cardiol201260171647165523021331