Abstract
Background
Symptom severity is the largest factor in determining subjective health in COPD. Symptoms (eg, chronic cough, dyspnea) are associated with decreased health-related quality of life (HRQoL). We evaluated the impact of arformoterol on HRQoL in COPD patients, measured by St George’s Respiratory Questionnaire (SGRQ). Post hoc growth mixture model (GMM) analysis examined symptom response profiles.
Methods
We examined data from a randomized, double-blind, parallel-group, 12-month safety trial of twice-daily nebulized arformoterol 15 µg (n=420) versus placebo (n=421). COPD severity was assessed by Global Initiative for Chronic Obstructive Lung Disease (GOLD) status. GMM analysis identified previously unknown patient subgroups and examined the heterogeneity in response to SGRQ Symptoms scores.
Results
SGRQ Total score improved by 4.24 points with arformoterol and 2.02 points with placebo (P=0.006). Significantly greater improvements occurred for arformoterol versus placebo in SGRQ Symptoms (6.34 vs 4.25, P=0.031) and Impacts (3.91 vs 0.97, P=0.001) scores, but not in Activity score (3.57 vs 1.75, P=0.057). GMM identified responders and nonresponders based on the SGRQ Symptoms score. End-of-study mean difference in SGRQ Symptoms scores between these latent classes was 20.7 points (P<0.001; 95% confidence interval: 17.6–23.9). Compared with nonresponders, responders were more likely current smokers (55.52% vs 44.02%, P=0.0021) and had more severe COPD (forced expiratory volume in 1 second [FEV1]: 1.16 vs 1.23 L, P=0.0419), more exacerbations (0.96 vs 0.69, P=0.0018), and worse mean SGRQ Total (59.81 vs 40.57, P<0.0001), Clinical COPD Questionnaire (3.29 vs 2.05, P<0.0001), and Modified Medical Research Council Dyspnea Scale (3.13 vs 2.75, P<0.0001) scores. Arformoterol-receiving responders exhibited significantly greater improvements in FEV1 (0.09 vs 0.008, P=0.03) and fewer hospitalizations (0.13 vs 0.24, P=0.02) than those receiving placebo.
Conclusion
In this study, arformoterol treatment significantly improved HRQoL reflected by SGRQ. For the analysis performed on these data, arformoterol may be particularly effective in improving lung function and reducing hospitalizations among patients who are unable to quit smoking or present with more severe symptoms.
Introduction
COPD and other chronic lower respiratory diseases are now the third leading cause of death in the US with 14.8 million people diagnosed with COPD and 12 million estimated to be living with COPD who remain undiagnosed.Citation1,Citation2 The economic burden of COPD is significant, with projected direct and indirect costs of up to $49.9 billion annually in the US;Citation2,Citation3 indirect costs account for $20.5 billion of this Total, which include additional burden on patients and employers due to lost productivity and on individuals as a result of absenteeism, Activity limitation, and disability.Citation4,Citation5
Measures of airflow obstruction (eg, forced expiratory volume in 1 second [FEV1]) have traditionally been used to assess the severity of COPD. Assessing lung function remains important in the diagnosis and design of a treatment plan; however, a more thorough evaluation of the disease including other aspects of impairment and patient-related outcomes may be worthwhile for achieving optimal treatment.Citation6 Exacerbations, a worsening of symptoms that often requires hospitalization, are associated with lung function decline and premature mortality.Citation7 Additionally, symptoms such as chronic cough and dyspnea are associated with a decline in health-related quality of life (HRQoL) in patients with COPD, contributing to the overall impairment caused by the disease.Citation8,Citation9 Dyspnea may also lead patients to reduce activities of daily living, which is a stronger predictor of 3-year survival than even FEV1 and contributes to the impact on overall HRQoL.Citation10 Additionally, because of the progressive nature of COPD, the severity of symptoms, which include dyspnea, cough, and sputum production, increases over the course of the disease.Citation11 Severity of symptoms is known to be the largest factor in determining subjective health, outweighing demographics, physiological variables such as lung function, and physical function such as exercise capacity.Citation12 Thus, successful management of COPD-related symptoms is a vital component of treatment goals.Citation13
Inhaled long-acting beta agonists (LABAs) or antimuscarinic agents are commonly prescribed when short-acting agents alone are no longer sufficient to manage the symptoms of COPD.Citation11 Arformoterol tartrate is a nebulized LABA that has been approved for maintenance treatment of COPD and has previously been shown to be both safe and efficacious (15 µg twice daily [bid]) for improvement of lung function in a 12-month, double-blind, randomized, placebo-controlled study.Citation14 Additionally, in a 12-week, randomized, double-blind, placebo- and active-controlled trial, arformoterol was shown to improve health status in patients with COPD as measured by the St George’s Respiratory Questionnaire (SGRQ).Citation15 These findings suggest that use of arformoterol can contribute to the improvement of not only lung function but also clinical symptoms and health status in patients with COPD. Thus, assessing impact of treatment on health status provides useful information regarding disease severity and response to treatment. Further steps remain necessary to better understand the diversity in patient response among those treated for COPD.
Given the heterogeneous nature of COPD,Citation16–Citation18 methodologies that may offer the ability for a more detailed characterization of COPD and subgroups that exhibit different response patterns to treatment and consequently differential improvements in health status would be valuable.Citation18 Growth mixture models (GMMs) have been used to investigate heterogeneity in treatment response across several diseases.Citation19–Citation22 This method analyzes the variability in the response profiles (eg, health status scores) over the treatment period and detects previously unidentified subgroups (or latent classes) of patients. Identifying differential responders could potentially help to better discern effects of treatment.Citation19 GMMs have been previously used in COPD clinical trials to identify subgroups of responders and nonresponders based on SGRQ Symptoms scores.Citation22
The main objective of this analysis was to compare HRQoL using the SGRQ in patients with moderate-to-severe COPD who were treated with arformoterol or placebo. An additional objective was to identify unknown patient subgroups (latent classes) using the GMM methods. The authors hypothesized that patients would show greater improvement in SGRQ scores with arformoterol treatment compared with placebo and that the GMM analysis would reveal responders and nonresponders with distinct characteristics. Data for these analyses were derived from a Phase III study of efficacy and safety in which patients treated with arformoterol demonstrated a 40% lower risk of respiratory death or COPD exacerbation-related hospitalization compared with placebo.Citation14 The results also indicated that airway function and dyspnea were significantly improved in patients treated with arformoterol versus those treated with placebo and that arformoterol was well tolerated.
Methods
Study design
This study consisted of two parts. The first part was an analysis evaluating improvements in HRQoL as measured by change in SGRQ scores from baseline in the aforementioned Phase III trial. The second part was a post hoc analysis using GMM applied to the SGRQ symptom response profiles.
Data for these analyses were derived from a Phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group, 12-month outpatient safety trial (NCT0909779). The study evaluated time to COPD-related respiratory death or hospitalization for COPD exacerbation as the primary end point.Citation14 Briefly, primary inclusion criteria included FEV1 ≤65% predicted volume, FEV1 >0.50 L, FEV1/forced vital capacity ratio ≤70%, age ≥40 years, smoking history $15 pack-years, and baseline breathlessness severity grade ≥2 based on the Modified Medical Research Council (MMRC) Dyspnea Scale score.Citation14 Patients who were receiving maintenance corticosteroid combination therapy at screening were switched to corticosteroid monotherapy at the same dose as the previous combination therapy.Citation14 Patients were randomly assigned to twice-daily nebulized arformoterol 15 µg (n=420) or matched placebo (n=421). Treatment with other COPD medications was permitted, with the exception of other LABAs. Efficacy assessments in this Phase III trial included spirometry measures and measure of HRQoL using the SGRQ and the Clinical COPD Questionnaire (CCQ). Assessments with the SGRQ were made at randomization and months 3, 6, and 12. COPD-related hospitalizations and exacerbations, defined as an increase in symptoms that necessitates any change in baseline medication other than bronchodilators (eg, anti-inflammatory agents, antibiotics, supplemental oxygen therapy) or causes the patient to require additional medical attention were also recorded over the 12 months of the study. For additional details, please refer to the primary publication of the trial.Citation14
Patient-reported health status
The SGRQCitation23,Citation24 is one of the most widely used patient-reported outcome measures of health status in COPD, designed for use in patients with fixed and reversible airway obstruction to measure impact on overall health, daily life, and perceived well-being. The SGRQ is a validated measure that consists of 50 items that combine to produce a Total score and three domain scores: Symptoms (frequency and severity), Activity (activities that cause or are limited by breathlessness), and Impacts (social functioning and psychological disturbances resulting from airway disease). SGRQ scores range from 0 (no impairment) to 100 (maximum impairment), reflecting health status over the previous 4 weeks. A 4-point change in mean SGRQ Total score is considered to be clinically meaningful.
SGRQ outcomes
SGRQ outcomes were analyzed using mixed models for repeated measures (MMRMs) that included terms for treatment, baseline smoking status, baseline SGRQ score, baseline SGRQ-by-visit interaction, visit, and treatment-by-visit interaction. The mean change at each visit was based on the least-square means (LSMs) from the MMRMs. The difference in clinically significant change between arformoterol and placebo was assessed using logistic regressions that included terms for treatment, baseline smoking status, and baseline SGRQ Total score. At each visit, logistic regressions were performed using the observed cases. The last available SGRQ measure for each individual was used for the end point comparison. To characterize the relationship between changes in SGRQ and FEV1, we examined change from baseline to end point in SGRQ Total score for subgroups defined by percentage change in FEV1. For all analyses, the two-tailed significance level was set to α=0.05.
GMM analysis
A GMM analysis was conducted to examine the heterogeneity in response to SGRQ Symptoms scores across the four assessment points (baseline, 3 months, 6 months, and 12 months) while controlling for key risk factors (age and smoking status) at baseline. SGRQ Symptoms scores were analyzed in the post hoc GMM analysis because symptoms are most proximal to treatment and thus most likely to show a direct relationship with treatment.
Given large variability in SGRQ Symptoms scores, we hypothesized that there may be subgroups with differential response profiles. The GMM analysis was used to determine whether latent classes (previously unknown or unspecified subgroups) exist in the trial data; subgroup membership was not known a priori but was inferred from the differential patterns of change in data. The heterogeneity of the overall data was treated as being made up of several homogeneous subgroups or classes, each of which was meaningfully different from other classes. Therefore, each post hoc-identified subgroup of patients had distinct SGRQ symptom response profiles. In addition, other characteristics of patients belonging to these latent classes were also evaluated. Stull and HoughtonCitation19 had provided a detailed description of the GMM methodology.
In the present analyses, GMMs examined the heterogeneity within each treatment arm, controlling for age and smoking status, to see if subgroups of differential responders (eg, responders and nonresponders) could be identified. The best model fit was determined by testing different models specified with different numbers of latent classes. Model fit was evaluated using visual examination and empirical indices: Bayesian information criterion (BIC), sample size-adjusted BIC, and accuracy of latent class assignment using posterior probabilities (entropy). Smaller values of BIC and sample size-adjusted BIC indicate a better model performance and are preferred when choosing the number of latent classes.Citation25 Although there is no conventional level for the threshold value for entropy, values closer to 1 indicate greater accuracy of latent class assignment. In addition, visual inspection of the latent class trajectories provided insight into the homogeneity of the trajectories within each latent class, as well as the differences between the latent classes. Fit statistics, visual inspection, and descriptive statistics for each latent class were used to determine the number of latent classes and whether the identified classes were clinically meaningful.
Patients were assigned to a single latent class based on the final model’s posterior probabilities.Citation25 Differences between latent class subgroups were explored. Bivariate comparisons between latent classes of responders, using one-way analyses of variance and chi-square tests, compared the following baseline variables: FEV1, number of exacerbations, SGRQ Total score, CCQ score, and MMRC Dyspnea Scale score.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. The following central/local institutional review boards (IRBs) approved the protocol: Copernicus Group IRB, Crescent City IRB, National Jewish Health IRB, Cooper Green Mercy Hospital IRB, Human Subject Research for Baylor College of Medicine and Affiliated Hospitals IRB, Western IRB, and Johns Hopkins IRB. Written informed consent was obtained from all patients.
Results
As previously reported in the original publication,Citation14 there were no significant differences in baseline characteristics between the patients randomized to placebo or arformoterol.
Briefly, patients were in their early 60s and predominantly White. A large majority reported smoking for over 30 years, and approximately half of the patients reported being current smokers. The mean SGRQ (SD) Symptoms score at baseline was 65.8 (18.2) for the arformoterol group and 67.0 (17.6) for the placebo group. The number of patients who completed the 3-, 6-, and 12-month visits was 335 (79.8%), 304 (72.4%), and 255 (60.7%) and 295 (70.1%), 253 (60.1%), and 211 (50.1%) for the arformoterol and placebo groups, respectively.Citation14
SGRQ outcomes
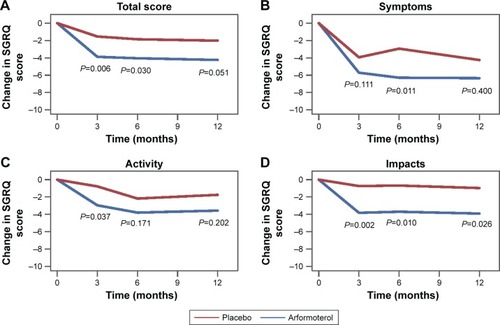
Results of the MMRMs showed that the SGRQ Total score at end point improved by 4.24 points from baseline for the arformoterol-treated patients and 2.02 points for the placebo-treated patients (LSM difference vs placebo: −2.22 [95% confidence interval {CI}: −3.78 to −0.66], P=0.006; ). Similarly, on the SGRQ domains, significantly larger improvements were observed for the arformoterol-versus placebo-treated patients in the Symptoms score (6.34 vs 4.25 points; LSM difference vs placebo: −2.09 [95% CI: −3.99 to −0.19], P=0.031) and Impacts score (3.91 vs 0.97 points; LSM difference vs placebo: −2.94 [95% CI: −4.70 to −1.17], P=0.001), but the Activity score differences did not reach statistical significance (3.57 vs 1.75 points; LSM difference vs placebo: −1.822 [95% CI: −3.70 to −0.06], P=0.057; , respectively). At the end of the study, 42.7% of the arformoterol-treated patients and 37.3% of the placebo-treated patients achieved the criteria for minimal clinically important improvement (P>0.05).
Figure 1 Change in SGRQ scores.
Abbreviations: LS, least squares; SGRQ, St George’s Respiratory Questionnaire.
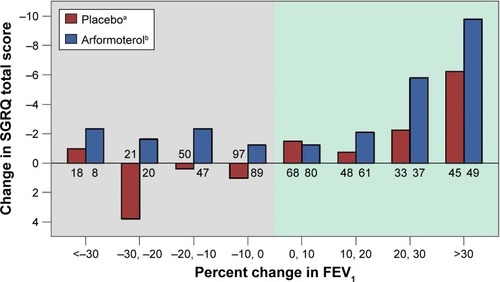
The relationship between SGRQ Total score improvement and percentage change in FEV1 is displayed in . Although SGRQ Total score improved the most for patients with greatest percentage increases in FEV1, the numerically greater improvement in SGRQ for arformoterol-treated patients was observed across nearly all levels of FEV1 change, including patients with worsening FEV1. The SGRQ Total score appears to be capturing an aspect of treatment response that supplements the information obtained by measuring change in FEV1.
Figure 2 SGRQ Total score change by percentage change in FEV1.
Abbreviations: FEV1, forced expiratory volume in 1 second; SGRQ, St George’s Respiratory Questionnaire.
Exploratory GMMs
The post hoc GMM analysis identified two latent classes of differential responders among the arformoterol- and placebo-treated patients based on the SGRQ Symptoms scores. According to the measures of fit (BIC, adjusted BIC, and entropy indices), a two-class solution provided a better fit to the data than a three-class solution (BIC: 20959.8 vs 20972.8, sample size-adjusted BIC: 20880.4 vs 20871.2, entropy: 0.80 vs 0.76). Thus, there were two latent subgroups in each study arm that showed differential SGRQ Symptoms scores at baseline and different trajectories of change. The two latent classes of differential responders identified by GMM will be referred to as responders and nonresponders ().
Figure 3 Growth curves of SGRQ Symptoms scores for the two-class solution.
Abbreviations: GMM, growth mixture model; SGRQ, St George’s Respiratory Questionnaire.
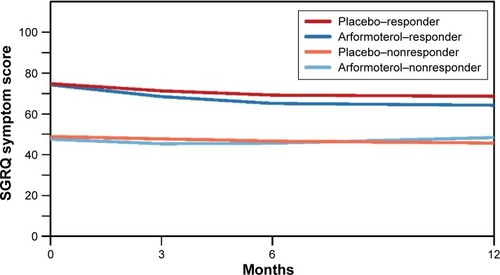
In , the two responder groups (one each for arformoterol and placebo) were significantly different from their nonresponder counterparts at baseline (arformoterol: responder SGRQ Symptoms score =75.3, nonresponder SGRQ Symptoms score =45.9, P<0.001; placebo: responder SGRQ Symptoms score =75.5, nonresponder SGRQ Symptoms score =46.1, P<0.001) and at 12 months (arformoterol: responder SGRQ Symptoms score =65.0, nonresponder SGRQ Symptoms score =48.3, P<0.001; placebo: responder SGRQ Symptoms score =68.3, nonresponder SGRQ Symptoms score =42.3, P<0.001). Moreover, when we compared the two responder classes of arformoterol and placebo with the corresponding nonresponder classes, there was a significant difference in the SGRQ Symptoms score change over 12 months between these two responder classes (responder SGRQ Symptoms score change =−8.8, nonresponder SGRQ Symptoms score change =−1.6, P<0.001).
A review of the baseline characteristics revealed multiple significant differences between the latent classes of responders and nonresponders (). Responders exhibited a greater number of symptoms and greater severity of COPD at baseline as measured by FEV1 (1.16 vs 1.23 L, P=0.0419), number of exacerbations (0.96 vs 0.69, P=0.0018), mean SGRQ Total score (59.81 vs 40.57, P<0.0001), CCQ score (3.29 vs 2.05, P<0.0001), and MMRC Dyspnea Scale score (3.13 vs 2.75, P<0.0001). Responders were also more likely to be current smokers (55.5% vs 44.02%, P=0.0021) and fall under Global Initiative for Chronic Obstructive Lung Disease (GOLD) status D (21.0% vs 13.6%).
Table 1 Descriptive statistics for baseline characteristics by SGRQ symptoms latent class responder status
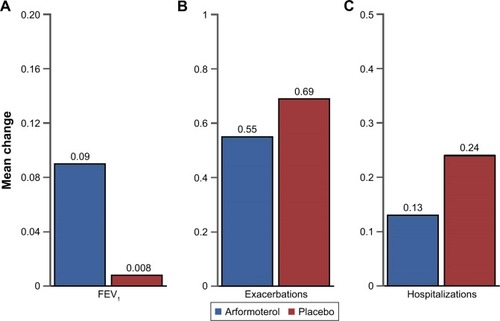
Latent class responders treated with arformoterol were compared with latent class responders treated with placebo on patient outcome measures (). The arformoterol and placebo responders demonstrated comparable improvements in symptoms (−10.3 vs −7.2, P>0.05) and a similar mean number of exacerbations (0.55 vs 0.69, P>0.05; ). However, the arformoterol-treated responders exhibited significantly greater improvements in FEV1 (0.09 vs 0.008 L, P=0.03) and significantly fewer hospitalizations (0.13 vs 0.24, P=0.02; ).
Figure 4 Change in mean FEV1, exacerbations, and hospitalizations for latent class responders treated with arformoterol versus latent class responders treated with placebo.
Abbreviation: FEV1, forced expiratory volume in 1 second.
Discussion
In this analysis of patient-reported outcomes from a large safety study, COPD patients treated with arformoterol reported significant improvement in HRQoL as measured by the SGRQ Total score as well as the Symptoms and Impacts domain scores. In addition, while not significantly different between groups, a higher proportion of patients in the arformoterol group than in the placebo group met the criterion for the minimal clinically important difference of 4% (≥−4 units) on these scores.
The HRQoL improvements reported here are consistent with a previous study of COPD patients treated with arfor-moterol.Citation15 Although the previous trial was of shorter duration (12 weeks), Baumgartner et al reported mean SGRQ Total score changes from baseline to week 6 of −2.6 and −3.6 for patients treated with arformoterol 15 and 25 µg twice daily, respectively, and −1.2 in the placebo group.Citation15 In both arformoterol groups, the greatest improvements were observed in the Symptoms domain of the SGRQ. The current study found a similar pattern of results. The greatest improvement was in the Symptoms domain, with a mean change from baseline of −6.33 in the arformoterol group and −4.25 in the placebo group at trial end.
At the end of the present study, 42.7% of patients treated with arformoterol achieved at least a 4-point improvement in SGRQ Total score (minimal clinically important difference) versus 37.3% of patients treated with placebo. The improvement in SGRQ Total score at 12 months (−2.2 vs placebo) is consistent with the mean improvement in SGRQ Total score (−2.3 vs placebo) reported in a review of 26 randomized controlled trials that compared LABAs with placebo in the treatment of COPD.Citation26 The trials from this review had a large range for observation periods; reports were as early as 3 months to as long as 3 years. However, the majority of the results described were after 6 months.
Our analysis also showed greater improvements on the Impacts domain of the SGRQ in patients treated with arformoterol compared with patients treated with placebo. While improvements were observed in Activity score in the arformoterol group compared with the placebo group, the difference did not reach statistical significance. This could potentially be explained by the generally older population (≥63 years old) in this study and the long smoking history of the patients (30+ years in 80% of patients), with approximately half of patients reported to be current smokers.
The exploratory GMM analyses conducted as part of this post hoc study efficiently identified differential responders on the SGRQ Symptoms domain within all patient responses over time, revealing two distinct and internally homogeneous cohorts. The groups identified in these GMM analyses were labeled as responders and nonresponders due to their different observed patterns of symptom improvement. GMM analyses do not require establishing an a priori threshold for “responder”, and they eliminate the need to try different cut-points on outcomes variables to identify differential responders, which results in multiplicity and violates assumptions of independence of tests.
The responders and nonresponders differed on multiple important baseline variables. The responder group could be characterized as experiencing greater initial COPD severity that had a greater impact on HRQoL. In addition, the responder group was represented by a significantly higher percentage of current smokers and GOLD status D patients and exhibited significantly higher CCQ and MMRC scores compared with nonresponders.
Among the symptom responders, arformoterol-treated patients had greater improvements in lung function and fewer hospitalizations than placebo-treated patients. Hospitalizations represent a worse clinical outcome for patients and make up the largest portion of the direct treatment costs of COPD. Reducing symptoms and hospital admissions could help improve patients’ outcomes and quality of life while reducing the direct and indirect economic burden of COPD. Treating patients who have a baseline profile similar to the responders with arformoterol could help reduce symptoms and reduce hospitalizations, but more research is needed to replicate and extend these findings.
Stull et alCitation22 conducted a GMM analysis across three studies of indacaterol, one 12-month study and two 6-month studies. The 12-month study resulted in three latent class assignments, while the two 6-month studies resulted in two latent class assignments (nonresponder and responder) similar to the present study. While the findings in the present study are generally consistent with those reported by Stull et al, there were some important differences between the studies with regard to baseline SGRQ Symptoms domain scores. In the report by Stull et al, the responder groups in the two 6-month studies were less symptomatic than the non-responder groups at baseline (SGRQ Symptoms score: 50.5 and 48.3 vs 73.7 and 79.3, respectively, P<0.001 for both). In the present study, the inverse was found. In the present study, the responder group was more symptomatic than the nonresponder group at baseline (75.4 vs 46.0, respectively). One could speculate that the different pattern of GMM results across the current study and the indacaterol study suggests that arformoterol may be more effective among patients with a more severe baseline profile and indacaterol may be more effective among those with less severe symptoms, but head-to-head studies are needed to draw any meaningful conclusions.
Our study adds to the present knowledge of COPD management, highlighting the importance of assessing health status in addition to lung function to obtain a more accurate evaluation of patient outcome and treatment. In addition, identifying and characterizing differential responders may potentially help to better assess treatment effects.
Our study has several limitations, which include the fact that there were a meaningful number of treatment discontinuations during the 12-month study. In addition, concomitant medication treatments were allowed (consistent with usual clinical care), which could cloud the assessment of treatment effect. Study findings may not be generalizable to a broader population, partly due to exclusion of some patients based on background LABA/inhaled corticosteroid therapy. However, the consistency of the findings with other studies of the effect of LABAs on the SGRQ lends strength to the conclusions.
The post hoc GMM analysis has inherent limitations. Because it was an exploratory analysis of a single trial, there was no way to confirm the presence of the same number and characteristics of latent classes in a second study. Thus, we relied on the fit statistics and visual examination of the latent classes. GMMs become a powerful tool when they can be used in more than one study to explore and confirm the results. A second limitation is that the SGRQ Symptoms domain as a stand-alone subscale is not validated. However, this domain assesses patients’ symptom experience and is more likely to demonstrate a direct relationship with treatment.Citation19,Citation22 Although not a limitation of the method, a note of caution is in order: use of this methodology requires a robust understanding of latent growth modeling and structural growth modeling to identify nuances of the model design and interpret outputs for possible issues.Citation19 The findings reported here may add information to those reported by Stull et alCitation22 and serve as a partial foundation from which confirmatory analyses or clinical trials could be used.Citation19,Citation20,Citation22 However, having partially replicated previous findings supports our confidence in these results.
Conclusion
In these analyses of data from a large placebo-controlled clinical trial in patients with COPD, improvements in arformoterol-treated patients were observed for the SGRQ Total score as well as the Symptoms and Impacts domain scores. Based on the GMM results, we identified two underlying subgroups that determined pattern of symptoms change: responders who at baseline were more impaired in FEV1 (mean: 1.16 vs 1.23 L, P=0.042) and demonstrated more limitations based on SGRQ scores (mean: 59.81 vs 40.57, Symptoms score 75.43 vs 45.97, Impacts score 46.13 vs 27.43, and Activity score 74.54 vs 59.56, all P<0.0001) compared with nonresponders. Treatment with arformoterol appeared to decrease hospitalizations and increase FEV1 for patients with more severe symptoms at baseline who were unable to quit smoking. More research is needed to better understand and optimize treatment for patients with COPD.
Data sharing statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
All authors have contributed substantially to the design of the study, acquisition, analysis, and interpretation of data and to the drafting of the manuscript or revising it critically and gave final approval of the version to be submitted.
Acknowledgments
The study was funded by Sunovion Pharmaceuticals Inc. The authors wish to thank Claudette Knight, PharmD, and D. Michele Nikoloff, PhD, of Percolation Communications LLC for providing medical editorial assistance. Development of this manuscript was sponsored by Sunovion Pharmaceuticals Inc.
Disclosure
JFD receives consultancy and advisory fees from Sunovion Pharmaceuticals Inc. MDS is a full-time employee and sole stockholder in Agile Outcomes Research, Inc., a research consulting firm contracted by Sunovion Pharmaceuticals Inc. to assist with this research. DES, LMN, and VSLW are employees of RTI Health Solutions, a research consulting firm contracted by Sunovion Pharmaceuticals Inc. to assist with this research. RTI Health Solutions is a research unit of RTI International, a not-for-profit research institute. At the time of this research, VKB was an employee of Sunovion Pharmaceuticals Inc. NAH has served as a consultant for Sunovion Pharmaceuticals Inc., and his institution has received research grant funding on his behalf. The authors report no other conflicts of interest in this work.
References
- KochanekKDMurphySLJiaquanXAriasEMortality in the United States, 2013. National Center for Health Statistics (NCHS) Data Brief. No 1782014 Available from: http://www.cdc.gov/nchs/data/databriefs/db178.pdfAccessed May 9, 2017
- National Heart, Lung and Blood InstituteMorbidity and Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood DiseasesBethesda, MDNational Institutes of Health2012 Available from: https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdfAccessed May 9, 2017
- FosterTSMillerJDMartonJPCaloyerasJPRussellMWMenzinJAssessment of the economic burden of COPD in the U.S.: a review and synthesis of the literatureCOPD20063421121817361502
- American Lung AssociationTrends in COPD: Chronic Bronchitis and Emphysema: Morbidity and Mortality2013 Available at http://www.lung.org/finding-cures/our-research/trend-reports/copd-trend-report.pdfAccessed May 9, 2017
- PatelJGNagarSPDalalAAIndirect costs in chronic obstructive pulmonary disease: a review of the economic burden on employers and individuals in the United StatesInt J Chron Obstruct Pulmon Dis2014928930024672234
- JonesPMiravitllesMvan der MolenTKulichKBeyond FEV(1) in COPD: a review of patient-reported outcomes and their measurementInt J Chron Obstruct Pulmon Dis2012769770923093901
- RennardSIFarmerSGExacerbations and progression of disease in asthma and chronic obstructive pulmonary diseaseProc Am Thorac Soc200412889216113418
- MiravitllesMMolinaJNaberanKFactors determining the quality of life of patients with COPD in primary careTher Adv Respir Dis200712859219124350
- ShavroSAEzhilarasuPAugustineJBechtelJJChristopherDJCorrelation of health-related quality of life with other disease severity indices in Indian chronic obstructive pulmonary disease patientsInt J Chron Obstruct Pulmon Dis2012729129622615528
- ReardonJZLareauSCZuWallackRFunctional status and quality of life in chronic obstructive pulmonary diseaseAm J Med200611910 suppl 1323716996897
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for the Diagnosis, Management and Prevention of COPD2015 Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/Accessed May 9, 2017
- BentsenSBHenriksenAHWentzel-LarsenTHanestadBRWahlAKWhat determines subjective health status in patients with chronic obstructive pulmonary disease: importance of symptoms in subjective health status of COPD patientsHealth Qual Life Outcomes2008611519094216
- van der MolenTMiravitllesMKocksJWCOPD management: role of symptom assessment in routine clinical practiceInt J Chron Obstruct Pulmon Dis2013846147124143085
- DonohueJFHananiaNAMakeBOne-year safety and efficacy study of arformoterol tartrate in patients with moderate to severe COPDChest201414661531154225451347
- BaumgartnerRAHananiaNACalhounWJSahnSASciarappaKHanrahanJPNebulized arformoterol in patients with COPD: a 12-week, multicenter, randomized, double-blind, double-dummy, placebo- and active-controlled trialClin Ther200729226127817472819
- AgustiACalverleyPMCelliBCharacterisation of COPD heterogeneity in the ECLIPSE cohortRespir Res20101112220831787
- AgustiAGCOPD, a multicomponent disease: implications for managementRespir Med200599667068215878483
- WedzichaJAThe heterogeneity of chronic obstructive pulmonary diseaseThorax200055863163210899236
- StullDEHoughtonKIdentifying differential responders and their characteristics in clinical trials: innovative methods for analyzing longitudinal dataValue Health201316116417623337228
- StullDEHoughtonKPetrilloJInnovative data analysis for demonstrating product value: analysis of heterogeneity in treatment response in clinical trialsISPQR Connect20131958
- WillkeRJZhengZSubediPAlthinRMullinsCDFrom concepts, theory, and evidence of heterogeneity of treatment effects to methodological approaches: a primerBMC Med Res Methodol20121218523234603
- StullDEWiklundIGaleRCapkun-NiggliGHoughtonKJonesPApplication of latent growth and growth mixture modeling to identify and characterize differential responders to treatment for COPDContemp Clin Trials20113281882821762787
- JonesPWQuirkFHBaveystockCMThe St George’s Respiratory QuestionnaireRespir Med199185suppl B2531 discussion 33–371759018
- JonesPWQuirkFHBaveystockCMLittlejohnsPA self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory QuestionnaireAm Rev Respir Dis1992145132113271595997
- TofighiDEndersCKIdentifying the correct number of classes in growth mixture modelsHancockGSamuelsonKAdvances in Latent Variable Mixture ModelsCharlotte, NCInformation Age Publishing2008317341
- KewKMMavergamesCWaltersJALong-acting beta2-agonists for chronic obstructive pulmonary diseaseCochrane Database Syst Rev201310CD010177
