Abstract
Chronic obstructive pulmonary disease (COPD) is characterized by an abnormal inflammatory response in the lungs caused by the inhalation of noxious particles and gases. The airway epithelium has a protective function against these harmful agents by maintaining a physical barrier and by secreting defensive proteins, such as bactericidal/permeability-increasing fold-containing (BPIF) proteins, BPIFA1 and BPIFB1. However, inconsistent data regarding BPIFA1 expression in smokers and COPD patients have been reported to date. Therefore, we investigated the expression of BPIFA1 and BPIFB1 in a large cohort of never-smokers and smokers with and without COPD, both on the messenger RNA (mRNA) level in lung tissue and on the protein level in airway epithelium. Furthermore, we examined the correlation between BPIFA1 and BPIFB1 levels, goblet cell hyperplasia, and lung function measurements. BPIFA1 and BPIFB1 mRNA expressions were significantly increased in stage III–IV COPD patients compared with stage II COPD patients and subjects without COPD. In addition, protein levels in COPD patients were significantly increased in comparison with subjects without COPD. BPIFA1 and BPIFB1 levels were inversely correlated with measurements of airflow limitation and positively correlated with goblet cell hyperplasia. In addition, by the use of immunofluorescence double staining, we demonstrated the expression of BPIFB1 in goblet cells. In conclusion, we show that BPIFA1 and BPIFB1 levels are elevated in COPD patients and correlate with disease severity.
Introduction
Chronic obstructive pulmonary disease (COPD) is a highly prevalent lung disease, characterized by gradually progressing and persistent airflow limitation. In subjects prone to develop COPD, the inhalation of noxious particles and gases causes lung inflammation, resulting in small airway obstruction and emphysema.Citation1 The major risk factor for COPD is cigarette smoking,Citation2 and multiple interrelated mechanistic pathways have been identified, varying from exaggerated chronic inflammation to an imbalanced oxidative stress response, trying to explain the damaging nature of cigarette smoke and other detrimental air pollutants.Citation3,Citation4 Unfortunately, currently, no disease-modifying therapy exists for COPD; hence, treatment is mainly focused on relieving the symptoms.
Airway epithelial cells form a first line of defense against harmful pathogens and irritants that enter the respiratory tract. Aside from maintaining a physical barrier, they create a chemical barrier by secreting protective proteins, such as bactericidal/permeability-increasing fold-containing (BPIF) family members BPIFA1 and BPIFB1, formerly known as SPLUNC1 and LPLUNC1, respectively. All the members of the BPIF superfamily share the three-dimensional structure of the bactericidal/permeability-increasing protein (BPI). BPIFA proteins are classified as containing a single BPIF domain, whereas BPIFB proteins contain two of these domains.Citation5,Citation6
BPIFA1 is localized to the respiratory epithelium and the submucosal glands of the upper airways.Citation7,Citation8 BPIFB1 is detected in the trachea and in epithelium and submucosal glands of larger and some smaller airways. BPIFA1 and BPIFB1 are absent from the alveolar regions of healthy lung, and both proteins exhibit limited expression in healthy airways.Citation9,Citation10 Interestingly, the expression and secretion of both proteins are dependent upon the differentiation status of the epithelial cell population, as demonstrated by the study of primary human lung cell cultures grown at air–liquid interface.Citation8,Citation9,Citation11,Citation12 BPIFB1 is localized to goblet cells, whereas BPIFA1 originates from nonciliated nongoblet epithelial cells.Citation7,Citation9,Citation13 This mutually exclusive localization appears to be retained in cystic fibrosis (CF) lung disease.Citation10
Conflicting data regarding BPIFA1 levels in relation to cigarette smoke exposure and COPD status have been reported. For instance, measurements on a limited number of subjects indicated increased levels of BPIFA1 in the airway epithelium and sputum of COPD patients compared with non-COPD patients.Citation14,Citation15 However, lower BPIFA1 levels in airway epithelial cells and nasal lavage fluid of current smokers compared with nonsmokers have also been reported.Citation16–Citation18 These discrepancies may have arisen due to the limited number of patient samples used in the studies and the semi-quantitative nature of the analysis, with most of the data coming from Western blotting. BPIFB1, on the other hand, seems to be consistently upregulated in several respiratory diseases. BPIFB1 levels were higher in the lungs of CF patients, in a mouse model of CFCitation10 and in bronchoalveolar lavage (BAL) fluid of asthma patients after segmental allergen challenge.Citation19 In addition, recent studies showed an elevation of secreted BPIFB1 in the sputum of COPD patients compared with smokers and nonsmokers.Citation13,Citation20 As BPIFB1 is predominantly localized to a population of goblet cells,Citation9 elevations in levels in COPD may be a reflection of the increased number of these cells in the airways.
To address some of these inconsistencies, in this study, we quantified the expression of BPIFA1 and BPIFB1 in a large cohort of never-smokers, smokers without airway limitation, and smokers with COPD. We measured the messenger RNA (mRNA) levels of BPIFA1 and BPIFB1 by quantitative real-time polymerase chain reaction (qRT-PCR) in lung tissue, and we analyzed the protein expression levels in airway epithelium using immunohistochemistry (in 92 subjects and 94 subjects, respectively). In addition, we quantified goblet cells in the airway epithelium and correlated their numbers with the levels of BPIFA1 and BPIFB1.
Methods
Human study populations
Lung resection specimens were obtained from patients undergoing surgery for solitary pulmonary tumors (Ghent University Hospital, Ghent, Belgium) or from explant lungs of end-stage COPD patients undergoing lung transplantation (University Hospital Gasthuisberg, Leuven, Belgium). For qRT-PCR and immunohistochemical analysis of paraffin-embedded sections, 92 and 94 subjects were included, respectively ( and ); 68 patients are included in both the qRT-PCR and immunohistochemical analysis. Preoperative spirometry measurements, diffusion capacity tests, and questionnaires were used to categorize patients as never-smokers with normal lung function, smokers without airflow limitation, or patients with COPD. COPD severity was defined according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification.Citation21 Lung tissue of patients diagnosed with solitary pulmonary tumor was obtained at maximum distance from the pulmonary lesions and without signs of retro-obstructive pneumonia or tumor invasion. None of the patients operated for malignancy were treated with neoadjuvant chemotherapy. Written informed consent was obtained from all subjects. This study was approved by the medical ethical committees of the Ghent University Hospital (2011/14) and the University Hospital Gasthuisberg Leuven (S51577).
Table 1 Characteristics of study population (qRT-PCR study)
Table 2 Characteristics of study population (immunohistochemical study)
qRT-PCR
Lung tissue blocks were submersed in RNA later (Qiagen, Hilden, Germany) and stored at −80°C. RNA extraction was performed by using the miRNeasy Mini kit (Qiagen). Next, cDNA was prepared by using the iScript Advanced cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Taq-Man® Gene Expression Assays (Applied Biosystems, Forster City, CA, USA) were used to measure the expression of the target genes BPIFA1 and BPIFB1 and the reference genes glyceraldehyde-3-phosphate dehydrogenase, hypoxanthine phosphoribosyltransferase-1, and succinate dehydrogenase complex flavoprotein subunit A. qRT-PCRs were performed in duplicate, and a standard curve was created by serial dilutions of a mixture of all samples. The amplifications were performed by using a LightCycler 96 detection system (Roche, Basel, Switzerland). The amplification conditions consisted of a preincubation step (600 s at 95°C), followed by 50 cycles of a two-step amplification (10 s at 95°C and 15 s at 60°C). Data were analyzed using the standard curve method, and the expressions of BPIFA1 and BPIFB1 were calculated relative to the expression of the three reference genes, using the geNorm applet (http://medgen.ugent.be/~jvdesomp/genorm/).Citation22
Generation of a human-specific BPIFA1 antibody
An affinity-purified, peptide-specific, rabbit polyclonal antibody against human BPIFA1 was generated by Eurogentec (Seraing, Belgium). The peptide sequences used corresponded to amino acids 192–207 (DGLGPLPIQGLLDSL). This peptide has minimal sequence conservation between man and mouse and exhibits no similarity with other members of the BPIF protein family. It also has no significant sequence identity with any other human proteins. Antibodies were validated by Western blotting using apical secretions from air–liquid interface cultures of human bronchial epithelial cells as previously described.Citation10 The BPIFA1 antibody was used to probe two air–liquid interface wash samples (A84 and A42) at a final dilution of 1:200.
Immunohistochemistry
To analyze BPIFA1 and BPIFB1 in lung resection specimens, paraffin-embedded sections were subjected to either BPIFA1 or BPIFB1 staining. For this purpose, 3-µm-thick paraffin-embedded sections were cut on poly-L-lysin-coated slides. The sections were subjected to antigen retrieval using either EDTA buffer (BPIFA1) or citrate buffer (BPIFB1). Next, endogenous peroxidase activity was blocked using 3% hydrogen peroxide in phosphate-buffered saline (PBS). Then, tissue sections were incubated with Boehringer blocking agent (Roche) with 0.3% Triton™ X-100 (Sigma-Aldrich, St Louis, MO, USA), followed by 24-hour incubation with rabbit polyclonal anti-BPIFA1 or rabbit polyclonal anti-BPIFB1Citation9 or isotype rabbit IgG (Abcam, Cambridge, UK). Next, the slides were incubated for 30′ with PowerVision poly-horseradish peroxidase anti-rabbit (Immunologic, Duiven, the Netherlands), followed by staining with 3,3′-diaminobenzidine (Dako, Glostrup, Denmark) for 10 minutes. Afterward, the slides were counterstained with Mayer’s hematoxylin (Sigma-Aldrich). Finally, the sections were dehydrated and mounted with distrene plasticizer xylene (Prosan, Merelbeke, Belgium). Photomicrographs of the airways in the sections were taken, and the amount of BPIFA1 and BPIFB1 in the airway epithelium was quantified, in a blinded manner, using the Axiovision software from Zeiss (Oberkochen, Germany). The amount of BPIFA1 and BPIFB1 was normalized to the length of the basement membrane.
Immunofluorescence staining
Immunofluorescence staining was performed on paraffin-embedded sections of human lung tissue of a COPD GOLD II patient. The sections were subjected to antigen retrieval using EDTA buffer (Dako). After washing steps with PBS, the sections were incubated with Boehringer blocking agent (Roche) with 0.3% Triton X-100 (Sigma-Aldrich). Primary antibodies were added for 24 hours: rabbit anti-BPIFA1, rabbit anti-BPIFB1,Citation9 mouse anti-MUC5AC (Abcam), mouse anti-P63 (Abcam), isotype rabbit IgG (Novus Biologicals, Littleton, CO, USA), isotype mouse IgG2a (BD Biosciences, San Diego, CA, USA), and isotype mouse IgG1 (BD Biosciences). Wash steps were performed, and the sections were incubated with secondary antibodies: donkey anti-rabbit Alexa Fluor 555 (Thermo Fisher Scientific, Waltham, MA, USA), donkey anti-mouse Alexa Fluor 488 (Thermo Fisher Scientific), and DAPI (Thermo Fisher Scientific). Finally, the sections were mounted with fluorescence mounting medium (Dako). Images were recorded with the Axiovision software from Zeiss. BPIFA1 and BPIFB1 were visualized as green, whereas MUC5AC and P63 were visualized as red.
Goblet cell analysis
Tissue sections were stained with Periodic Acid–Schiff (PAS). PAS stains neutral mucins and acid mucins that contain significant amounts of sialic acid. Both major airway mucin proteins (MUC5AC and MUC5B) are stained by PAS. Goblet cells were counted using Axiovision software and were expressed as the number of goblet cells per millimeter basal membrane.
Statistical analysis
Statistical analyses were performed by using Sigma Stat software (SPSS Statistics 23, Chicago, IL, USA) including Kruskal–Wallis, Mann–Whitney U-test, Fisher’s exact test, general linear model, and Spearman correlation analysis. Differences at p-values <0.05 were considered to be significant. Characteristics of the study population ( and ) are expressed as median and interquartile range.
Results
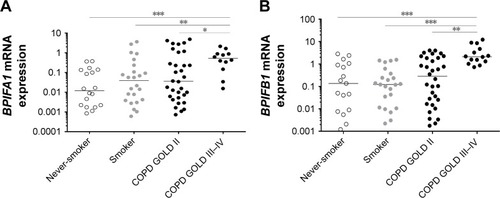
BPIFA1 and BPIFB1 mRNA expressions are increased in lung tissue of patients with severe and very severe COPD
The expressions of BPIFA1 and BPIFB1 in lung tissue were analyzed by qRT-PCR in 92 subjects () and were significantly increased in patients with severe and very severe COPD (GOLD stage III–IV) compared with patients with mild-to-moderate COPD (GOLD stage II), smokers without airway limitation, and never-smokers (). Furthermore, the mRNA expressions of both BPIFA1 and BPIFB1 were significantly and inversely correlated with lung function parameters of airway obstruction (forced expiratory volume in 1 second [FEV1] and FEV1/forced vital capacity [FVC] post-bronchodilator [BD]) and with the diffusing capacity of the lungs (diffusing capacity of the lung for carbon monoxide [DLCO] and the ratio of DLCO to alveolar volume [KCO]; Figure S1). To evaluate the effect of different parameters (age, gender, COPD status, smoking status, and use of inhaled corticosteroids) on the expressions of BPIFA1 and BPIFB1, we performed linear regression analysis. Severe COPD status is the only significant independent determinant for BPIFA1 and BPIFB1 mRNA expressions (Tables S1 and S2).
Figure 1 Pulmonary expression of BPIFA1 and BPIFB1 mRNA levels.
Abbreviations: BPIF protein, bactericidal/permeability-increasing fold-containing protein; COPD, chronic obstructive pulmonary disease; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HPRT-1, hypoxanthine phosphoribosyltransferase-1; qRT-PCR, quantitative real-time polymerase chain reaction; SDHA, succinate dehydrogenase complex flavoprotein subunit A.
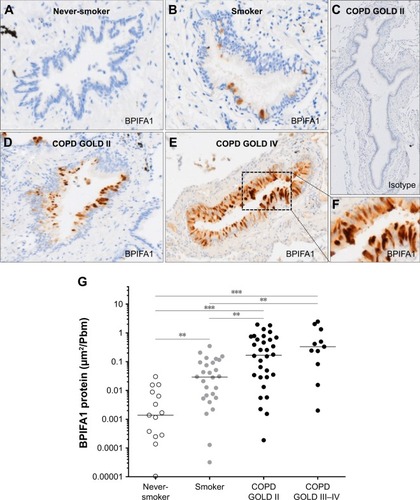
BPIFA1 and BPIFB1 protein levels are increased in airway epithelium of patients with COPD
To study the expression of BPIFA1, we generated an affinity-purified, peptide-specific, rabbit polyclonal antibody against human BPIFA1. To validate the BPIFA1 antibody, it was used to probe two air–liquid interface wash samples (A84 and A42) at a final dilution of 1:200 ().
Figure 2 Generation of a human-specific BPIFA1 antibody.
Abbreviation: BPIF protein, bactericidal/permeability-increasing fold-containing protein.

The protein expressions of BPIFA1 and BPIFB1 in airway epithelium were analyzed by immunohistochemistry in 94 subjects (). Immunohistochemical staining of BPIFA1 is localized to the airway epithelium with no evidence of staining in peripheral lung tissue (). Protein levels of BPIFA1 in airway epithelium of COPD patients (stage II and stage III–IV) were significantly increased in comparison with subjects without COPD. In addition, BPIFA1 protein expression was increased in the airway epithelium of control smokers compared with never-smokers ().
Figure 3 BPIFA1 protein expression in airway epithelium.
Abbreviations: BPIF protein, bactericidal/permeability-increasing fold-containing protein; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
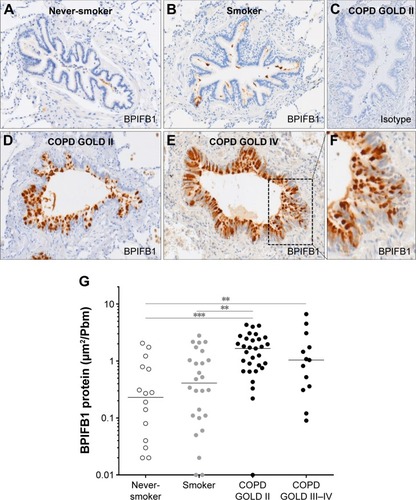
Immunohistochemical staining of BPIFB1 is detected in the airway epithelium (). As with BPIFA1, there was no staining in the alveolar tissues. BPIFB1 protein staining in the airway epithelium was significantly increased in patients with COPD compared with subjects without COPD ().
Figure 4 BPIFB1 protein expression in airway epithelium.
Abbreviations: BPIF protein, bactericidal/permeability-increasing fold-containing protein; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
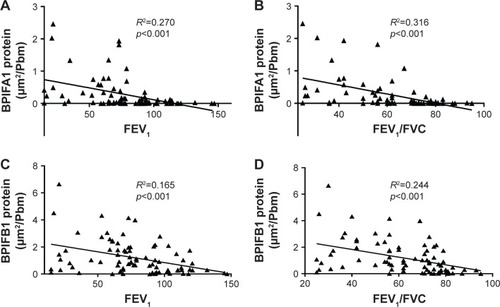
Importantly, both BPIFA1 and BPIFB1 protein staining was significantly inversely correlated with FEV1 and FEV1/FVC post-BD (). In addition, BPIFA1 protein was significantly negatively correlated with DLCO and KCO, while BPIFB1 protein was not (Figure S2). The influence of age, gender, COPD status, smoking status, and use of inhaled corticosteroids on protein expressions of both BPIFA1 and BPIFB1 was determined by linear regression analysis. COPD status was the main determinant for BPIFA1 and BPIFB1 protein staining. BPIFA1 protein was additionally influenced by the smoking status (Tables S3 and S4).
Figure 5 Correlation of BPIFA1 and BPIFB1 protein levels in airway epithelium and lung function parameters of airflow limitation.
Abbreviations: BPIF protein, bactericidal/permeability-increasing fold-containing protein; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
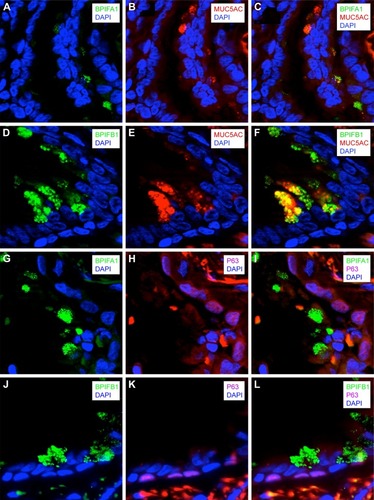
Localization of BPIFA1 and BPIFB1 in the airway epithelium by double immunofluorescence staining
To know which cell types are responsible for the production of BPIFA1 and BPIFB1, we performed double staining of these proteins with two different epithelial cell markers in a COPD GOLD II patient. We performed double staining for P63 (marker for basal cells) and BPIFA1 or BPIFB1. We did not see any BPIFA1 or BPIFB1 staining in P63+ basal cells (). In addition, we also performed double staining for MUC5AC and BPIFA1 or BPIFB1. MUC5AC is an abundantly expressed mucin localized to goblet cells. We observed strongly positive double staining for MUC5AC and BPIFB1 (), implicating the production of BPIFB1 by goblet cells and no or only very faint double staining for MUC5AC and BPIFA1 ().
Figure 6 Localization of BPIFA1 and BPIFB1 expression in lung tissue by double immunofluorescence staining.
Abbreviations: BPIF protein, bactericidal/permeability-increasing fold-containing protein; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
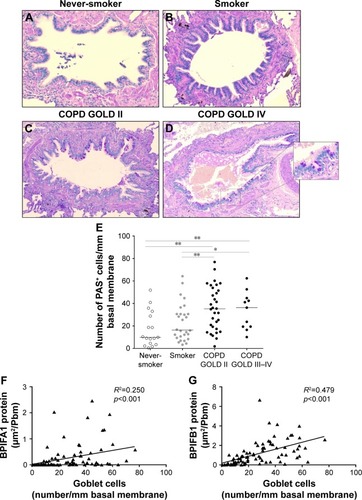
BPIFA1 and BPIFB1 protein levels in airway epithelium correlate significantly with goblet cell hyperplasia
shows representative images of PAS staining of the airway epithelium of a never-smoker, a smoker without COPD, a patient with COPD GOLD stage II, and a patient with COPD GOLD stage IV. The number of PAS-positive cells per millimeter basal membrane is significantly increased in COPD patients compared with subjects without COPD (). Staining of both BPIFA1 and BPIFB1 protein in the airway epithelium was significantly correlated with the number of goblet cells ().
Figure 7 Goblet cell quantification and correlation with BPIFA1 and BPIFB1 protein expression in airway epithelium.
Abbreviations: BPIF protein, bactericidal/permeability-increasing fold-containing protein; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; PAS, Periodic Acid–Schiff.
Discussion
In this study, we used a large cohort to demonstrate that the mRNA expression in lung tissue and protein expression in the airway epithelium of both BPIFA1 and BPIFB1 are increased in patients with COPD compared with those without COPD. Moreover, we show that BPIFA1 and BPIFB1 levels are positively correlated with goblet cell hyperplasia and inversely correlated with lung function parameters of airflow limitation (FEV1 and FEV1/FVC). In addition, BPIFA1 levels are inversely correlated with lung diffusion capacity parameters (DLCO and KCO).
Research into the expression of BPIFA1 in subjects with COPD has previously produced inconsistent results. In accordance with our data, increased BPIFA1 expression in airway epithelium and increased BPIFA1 protein levels in sputum samples were observed in subjects with COPD compared with those without.Citation14,Citation15 In contrast, another study reported reduced levels of BPIFA1 in BAL fluid of smokers with and without COPD compared with controls.Citation18 In addition, Steiling et al demonstrated increased expression of BPIFA1 in airway epithelial cells of never-smokers compared with current smokers.Citation16 Substantial variability in the number of subjects studied and a wide range of techniques used to determine the level of BPIFA1 are probably in part responsible for these discrepancies. In addition, BPIFA1 is degraded by neutrophil elastase,Citation18 and this might influence levels in COPD sputum and BAL fluid. It is important to reiterate that, although both of these proteins are commonly considered to be highly expressed in the airways, in fact neither protein is found at significant levels in the epithelium of the normal lung,Citation10,Citation13 an observation that is confirmed by the systematic quantitative immunohistochemical analysis presented here.
We showed an increase in the expression of BPIFB1 in COPD patients compared with subjects without COPD, at both the mRNA and the protein levels. This is consistent with the reports of significant increases in levels of BPIFB1 detected in sputum and lung tissue of COPD patients as determined by enzyme linked immunosorbent assay, Western blotting, and mass spectrometry.Citation13,Citation20 In addition, increased BPIFB1 expression, especially in the terminal airways within parenchyma tissues, was observed in COPD patients compared with controls.Citation23 Stratification into current smokers and ex-smokers in one study showed that the levels were only significantly higher in COPD patients who continued smoking.Citation13
The levels of BPIFA1 and BPIFB1 are significantly inversely correlated with parameters of airflow limitation, and the levels of BPIFA1 are also significantly inversely correlated with parameters of lung diffusion capacity, which serve as a proxy for COPD disease severity and the amount of emphysema, respectively. Consistent with our data, in the study from Saferali et al,Citation24 which studied a group of 739 patients with smoking-related lung disease (mostly lung cancer and COPD patients), elevated BPIFA1 levels were significantly associated with decreased lung function. In the study by Gao et al, sputum BPIFB1 levels were also significantly negatively correlated with FEV1% predicted and FEV1/FVC%, at both the baseline and after a 4-year follow-up period.Citation13
As it has been shown previously that both BPIFA1 and BPIFB1 are expressed in nonciliated cells,Citation9,Citation25,Citation26 we performed colocalization staining of the two proteins with known cellular markers of two types of nonciliated cells, MUC5AC and P63. MUC5AC is an abundantly expressed mucin localized to goblet cells, and P63 is a marker for basal cells. Our data show strongly positive double staining for MUC5AC and BPIFB1 () and suggest a limited colocalization of MUC5AC and BPIFA1 (). In addition, we measured the number of goblet cells by means of PAS staining. As expected, the number of PAS-positive cells was significantly increased in COPD patients compared with patients without COPD. The data show that BPIFB1 levels were strongly positively correlated with the number of goblet cells, confirming a strong relationship between BPIFB1 and goblet cells as visualized with immunofluorescence staining. A previous work on late-stage CF lung tissue has also shown that both proteins are increased in the remodeled epithelium.Citation10
In this study, the two proteins continued to be expressed in different cell types, with BPIFA1 being found in a serous cell population that was distinct from the BPIFB1-expressing goblet cells.Citation10 The same mutually exclusive localization is also seen in the murine CF model.Citation10 Our data suggest that in COPD patients BPIFA1 can also be expressed in some goblet cells. In addition, we performed double staining for P63 (a marker for basal cells) and BPIFA1 or BPIFB1. As expected, we did not see any BPIFA1 or BPIFB1 staining in P63+ basal cells. This corresponds to the paper by Mulay et al, which describes that BPIFA1 staining is absent in basal cells of mouse middle ear epithelial cells in air–liquid interface culture.Citation26 BPIFA1 is an important player in airway protection, and given its expression in the upper airways, it is ideally located to exert this function. One major component of BPIFA1’s protective role seems to be mediated by its antimicrobial actions.Citation27 In addition, BPIFA1 plays a role in ion transport and regulation of the airway surface liquid. Abnormal mucus rheology, caused for example by enhanced epithelial sodium channel (ENaC) activity, results in airway obstruction and mucostasis, which in turn might lead to bacterial infections and subsequent increased inflammation in patients with COPD. This could contribute to a faster decline in lung function.Citation3,Citation28,Citation29 BPIFA1 acts as a negative regulator of ENaC and might in this way be able to positively influence the airway surface liquid height and, concurrent with this, protect epithelial homeostasis.Citation30,Citation31 Consistent with this observation, BPIFA1 silencing using small interfering RNA technology in a model of nontypeable Haemophilus influenzae otitis media resulted in an impaired mucus transport and mucociliary clearance.Citation32 In addition, genetic polymorphisms associated with lower BPIFA1 levels have also been associated with decreased lung function.Citation24 Interestingly, BPIFA1 levels are significantly higher in patients with CF compared with subjects without CF.Citation33 Likewise, data from our study and other studies also indicate higher levels of BPIFA1 in COPD patients compared with non-COPD patients.Citation14,Citation15 This might suggest that BPIFA1 has a protective function in the airways and that patients with inflammatory diseases (CF and COPD) are in need of higher levels of this protein to conserve airway epithelial homeostasis in their inflamed lungs. Studies investigating the acute effects of inflammation and infection describe a fast release of the stored protein, followed by a temporarily decreased BPIFA1 expression.Citation34 In addition, neutrophil elastase has been reported to degrade BPIFA1 in a dose-dependent manner.Citation18 In COPD patients, where bacterial infections are common and neutrophil elastase is present in high amounts, the need for secreted BPIFA1 is elevated. Our hypothesis is that the airway epithelium responds to this need by increasing the production and storage of BPIFA1; hence, the elevated levels of airway epithelial BPIFA1 protein are found in our study. However, further studies investigating the function of BPIFA1 in the diseased lung are necessary.
The role of BPIFB1 has been less extensively studied. Due to its structural similarity with proteins involved in protection against Gram-negative bacteria (lipopolysaccharide-binding and BPIs), it has been suggested that BPIFB1 might also play a role in innate immune defense,Citation35,Citation36 although compelling functional data are lacking. As mentioned before, BPIFB1 levels are elevated in the airways of patients with CF and also in βENaC-Tg mice, a murine model for CF.Citation10 BPIFB1 staining in this model was associated with mucus obstruction, goblet cell metaplasia, and inflammation in the airways, suggesting that the increased BPIFB1 levels in the CF lung are a consequence of this epithelial remodeling.Citation10 Also in COPD patients, extended epithelial remodeling with goblet cell metaplasia and inflammation takes place, and the observed increase in BPIFB1 levels in COPD patients could be a direct consequence of this. This idea is strengthened by the observation that the number of goblet cells correlates very strongly with the BPIFB1 protein levels in our study. It is possible that BPIFB1, similarly to its family member BPIFA1, also plays a role in airway protection, but the way that BPIFB1 is able to perform this function requires additional functional analysis.
We quantified BPIFA1 levels in a large COPD patient cohort. In addition, we report quantitative data on the expression of BPIFB1 in airway epithelium, which is a valuable addition to the data on BPIFB1 levels in induced sputum.Citation13,Citation20 Moreover, we showed data on expressions of BPIFA1 and BPIFB1 at both the mRNA and protein levels and related these to different lung function measurements. By using DLCO and KCO values, we were able to correlate BPIFA1 and BPIFB1 expressions with an adequate measurement for the degree of emphysema. A limitation of our study is that we have data only on the BPIFA1 and BPIFB1 protein expressions in the airway epithelium and not on the level of secreted proteins. We are also not able to comment on the role that submucosal gland secretion of these two proteins may play in modulating levels of the proteins in COPD. Both proteins are known to be present in submucosal glandsCitation7,Citation9,Citation14 and have been identified in gland mucus.Citation37
Conclusion
We demonstrated that the mRNA and protein expressions of both BPIFA1 and BPIFB1 are increased in patients with COPD compared with those without COPD. Moreover, we showed that BPIFA1 and BPIFB1 expressions are correlated with goblet cell metaplasia and inversely correlated with lung function parameters of airway obstruction (FEV1 and FEV1/FVC). Future experiments are necessary to elucidate the function of BPIFA1 and BPIFB1, not only in the context of airway protection toward infectious agents, but also in a setting of exaggerated and imbalanced airway inflammation in COPD patients.
Author contributions
KRB, GGB, CDB, and LJMS conceived the project and designed the experiments; KRB, LJMS, FMV, and EGDS conducted the experiments; KRB, GGB, FMV, LJMS, BMV, and EGDS contributed to the data analysis and interpretation; LJMS and EGDS wrote the manuscript; and all authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank Greet Barbier, Indra De Borle, Katleen De Saedeleer, Anouck Goethals, Marie-Rose Mouton, and Ann Neesen from the Laboratory for Translational Research in Obstructive Pulmonary Diseases, Department of Respiratory Medicine (Ghent University Hospital, Ghent, Belgium), for their excellent technical assistance. They would also like to thank Prof Wim Janssens and Dr Stijn Verleden (Department of Pneumology, Leuven, Belgium) for providing the explant lungs of patients with severe COPD. The BPIFA1 and BPIFB1 antibodies were produced and validated using funding from the Wellcome Trust (076491/Z/05/Z). This research was supported by the Concerted Research Action of the Ghent University (BOF/GOA, 01G01009), by the Fund for Scientific Research in Flanders (FWO Vlaanderen, G.0195.09 and G.0194.10), and by the Interuniversity Attraction Poles program (IUAP, P7/30).
Supplementary materials
Figure S1 Correlation of BPIFA1 and BPIFB1 mRNA levels with different lung function parameters.
Notes: Spearman correlation analysis of BPIFA1 and BPIFB1 mRNA levels with post-bronchodilator values of FEV1 (A, B), and the ratio of FEV1 to FVC (FEV1/FVC) (C, D), DLCO (E, F), and the ratio of DLCO to alveolar volume (KCO) (G, H). R2= determination coefficient.
Abbreviations: BPIF protein, bactericidal/permeability-increasing fold-containing protein; DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; KCO, the ratio of DLCO to alveolar volume.
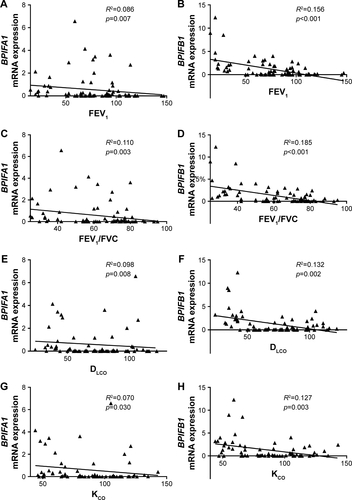
Figure S2 Correlation of BPIFA1 and BPIFB1 protein levels with parameters of lung diffusion capacity.
Notes: Spearman correlation analysis of BPIFA1 and BPIFB1 protein levels with DLCO (A, C) and KCO (B, D). R2= determination coefficient.
Abbreviations: BPIF protein, bactericidal/permeability-increasing fold-containing protein; DLCO, diffusing capacity of the lung for carbon monoxide; KCO, the ratio of DLCO to alveolar volume.
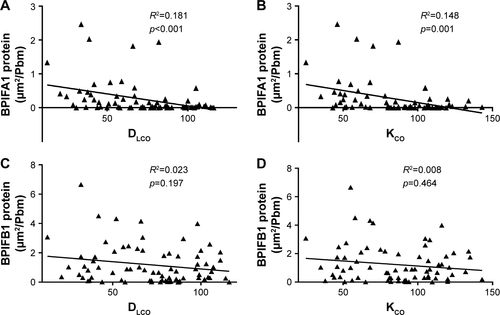
Table S1 General linear model for BPIFA1 mRNA expression
Table S2 General linear model for BPIFB1 mRNA expression
Table S3 General linear model for BPIFA1 protein expression
Table S4 General linear model for BPIFB1 protein expression
Disclosure
The authors report no conflicts of interest in this work.
References
- VogelmeierCFCrinerGJMartinezFJGlobal strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summaryEur Respir J2017493 pii: 1700214
- RostronBLChangCMPechacekTFEstimation of cigarette smoking-attributable morbidity in the United StatesJAMA Intern Med2014174121922192825317719
- HoggJCTimensWThe pathology of chronic obstructive pulmonary diseaseAnnu Rev Pathol2009443545918954287
- RahmanIAdcockIMOxidative stress and redox regulation of lung inflammation in COPDEur Respir J200628121924216816350
- BingleCDCravenCJPLUNC: a novel family of candidate host defence proteins expressed in the upper airways and nasopharynxHum Mol Genet200211893794311971875
- BingleCDSealRLCravenCJSystematic nomenclature for the PLUNC/PSP/BSP30/SMGB proteins as a subfamily of the BPI fold-containing superfamilyBiochem Soc Trans201139497798321787333
- BingleLCrossSSHighASSPLUNC1 (PLUNC) is expressed in glandular tissues of the respiratory tract and in lung tumours with a glandular phenotypeJ Pathol2005205449149715685591
- CamposMAAbreuARNlendMCCobasMAConnerGEWhitneyPLPurification and characterization of PLUNC from human tracheobronchial secretionsAm J Respir Cell Mol Biol200430218419212920053
- BingleCDWilsonKLunnHHuman LPLUNC1 is a secreted product of goblet cells and minor glands of the respiratory and upper aerodigestive tractsHistochem Cell Biol2010133550551520237794
- BingleLWilsonKMusaMBPIFB1 (LPLUNC1) is upregulated in cystic fibrosis lung diseaseHistochem Cell Biol2012138574975822767025
- RossAJDaileyLABrightonLEDevlinRBTranscriptional profiling of mucociliary differentiation in human airway epithelial cellsAm J Respir Cell Mol Biol200737216918517413031
- Martinez-AntonASokolowskaMKernSChanges in microRNA and mRNA expression with differentiation of human bronchial epithelial cellsAm J Respir Cell Mol Biol201349338439523590309
- GaoJOhlmeierSNieminenPElevated sputum BPIFB1 levels in smokers with chronic obstructive pulmonary disease: a longitudinal studyAm J Physiol Lung Cell Mol Physiol20153091L17L2625979078
- DiYPHarperRZhaoYPahlavanNFinkbeinerWWuRMolecular cloning and characterization of spurt, a human novel gene that is retinoic acid-inducible and encodes a secretory protein specific in upper respiratory tractsJ Biol Chem200327821165117312409287
- NicholasBLSkippPBartonSIdentification of lipocalin and apolipoprotein A1 as biomarkers of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2010181101049106020110559
- SteilingKKadarAYBergeratAComparison of proteomic and transcriptomic profiles in the bronchial airway epithelium of current and never smokersPLoS One200944e504319357784
- GhafouriBKihlstromEStahlbomBTagessonCLindahlMPLUNC (palate, lung and nasal epithelial clone) proteins in human nasal lavage fluidBiochem Soc Trans200331Pt 481081412887311
- JiangDWenzelSEWuQBowlerRPSchnellCChuHWHuman neutrophil elastase degrades SPLUNC1 and impairs airway epithelial defense against bacteriaPLoS One201385e6468923741370
- WuJKobayashiMSousaEADifferential proteomic analysis of bronchoalveolar lavage fluid in asthmatics following segmental antigen challengeMol Cell Proteomics2005491251126415951573
- TitzBSewerASchneiderTAlterations in the sputum proteome and transcriptome in smokers and early-stage COPD subjectsJ Proteomics201512830632026306861
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- VandesompeleJDe PreterKPattynFAccurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genesGenome Biol200237RESEARCH003412184808
- LiuYLatocheJPilewskiJMPeter DiYPDifferential expression of SPLUNC1 and LPLUNC1 in lungs of healthy human subjects and COPD patientsAm J Respir Crit Care Med2010181A2915
- SaferaliAObeidatMBerubeJCPolymorphisms associated with expression of BPIFA1/BPIFB1 and lung disease severity in cystic fibrosisAm J Respir Cell Mol Biol201553560761425574903
- AkramKMMoyoNALeemingGHAn innate defense peptide BPIFA1/SPLUNC1 restricts influenza A virus infectionMucosal Immunol Epub2017517
- MulayAAkramKMWilliamsDAn in vitro model of murine middle ear epitheliumDis Model Mech20169111405141727660200
- BrittoCJCohnLBactericidal/permeability-increasing protein fold-containing family member A1 in airway host protection and respiratory diseaseAm J Respir Cell Mol Biol201552552553425265466
- HoggJCChuFSTanWCSurvival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathologyAm J Respir Crit Care Med2007176545445917556723
- VestboJEpidemiological studies in mucus hypersecretionNovartis Found Symp2002248312 discussion 12–19, 277–28212568485
- Garcia-CaballeroARasmussenJEGaillardESPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavageProc Natl Acad Sci U S A200910627114121141719541605
- RollinsBMGarcia-CaballeroAStuttsMJTarranRSPLUNC1 expression reduces surface levels of the epithelial sodium channel (ENaC) in Xenopus laevis oocytesChannels (Austin)20104425525920519934
- McGillivaryGBakaletzLOThe multifunctional host defense peptide SPLUNC1 is critical for homeostasis of the mammalian upper airwayPLoS One2010510e1322420949060
- BingleLBarnesFACrossSSDifferential epithelial expression of the putative innate immune molecule SPLUNC1 in cystic fibrosisRespir Res200787917988392
- BrittoCJLiuQCurranDRPathamBDela CruzCSCohnLShort palate, lung, and nasal epithelial clone-1 is a tightly regulated airway sensor in innate and adaptive immunityAm J Respir Cell Mol Biol201348671772423470624
- BingleCDGorrSUHost defense in oral and airway epithelia: chromosome 20 contributes a new protein familyInt J Biochem Cell Biol200436112144215215313462
- DiYPFunctional roles of SPLUNC1 in the innate immune response against Gram-negative bacteriaBiochem Soc Trans20113941051105521787346
- JooNSEvansIAChoHJParkIHEngelhardtJFWineJJProteomic analysis of pure human airway gland mucus reveals a large component of protective proteinsPLoS One2015102e011675625706550
