Abstract
Background
The objective of the FAVOR study was to evaluate the effect of indacaterol/glycopyrronium (IND/GLY) versus tiotropium on peak forced expiratory volume in 1 s (FEV1) and also to investigate patient satisfaction and treatment preference.
Methods
Patients with moderate-to-severe airflow limitation (FEV1/forced vital capacity ratio of <0.70), those with a COPD assessment test score of ≥10, and those who were maintained on tiotropium HandiHaler® therapy prior to enrollment were recruited for the study, and randomized (1:1) to receive either 4 weeks open-label IND/GLY (110/50 μg) once daily followed by 4 weeks of tiotropium (18 μg) once daily or vice versa. The primary endpoint was FEV1 1 h post-inhalation after 4 weeks of treatment. Other endpoints included patient’s and physician’s preference for treatment, patient’s satisfaction evaluated using a study-specific questionnaire and the abbreviated Treatment Satisfaction Questionnaire for Medication, and safety and tolerability.
Results
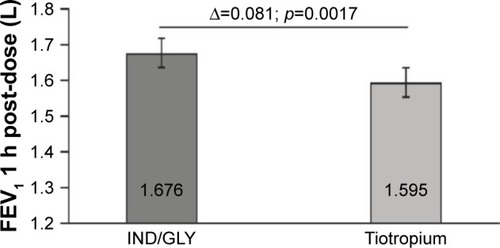
Eighty-seven out of 88 randomized patients completed the study and showed significantly higher FEV1 1 h post-inhalation after 4 weeks of treatment with IND/GLY versus tiotropium (treatment difference =0.081 L; p=0.0017). IND/GLY was preferred over tiotropium among the patients (69.4% versus 30.6%, p=0.0004) and the physicians (81.6% versus 18.4%, p<0.0001). A higher proportion of the patients stated they were very satisfied or satisfied with IND/GLY versus tiotropium with regard to dyspnea reduction (79.3% versus 58.0%, respectively) and reduction of dyspnea on exertion (72.4% versus 43.2%, respectively). Patients treated with IND/GLY showed significant improvement in Treatment Satisfaction Questionnaire for Medication domain scores versus tiotropium. IND/GLY demonstrated a good safety and tolerability profile.
Conclusion
This study indicated that, beyond FEV1, important patient-reported outcomes improved with the open-label dual bronchodilator IND/GLY when compared with tiotropium. This study suggests that individual patients felt the lung function benefits with IND/GLY compared with tiotropium, which, in turn, may also have contributed to the preference for IND/GLY.
Introduction
COPD, a debilitating and progressive lung disease, is one of the major causes of morbidity and mortality worldwide and is expected to become the third leading cause of death by 2030.Citation1,Citation2 In Germany, the overall COPD prevalence is about 13% in the population >40 years of age, with an increase of about 40% observed in men >70 years old.Citation3
Long-acting bronchodilators are the mainstay for maintenance therapy of COPD and include 2 categories of drugs: muscarinic antagonists (anticholinergics) and β2 agonists.Citation4 For patients who continue to have severe symptoms on long-acting β2 agonist (LABA) or long-acting muscarinic antagonist (LAMA) monotherapy, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy recommends combining a LABA along with a LAMA, 2 bronchodilators with synergistic mechanisms, for achieving better symptomatic relief.Citation4–Citation6 Indacaterol/glycopyrronium (IND [LABA]/GLY [LAMA]) 110/50 μg once daily (o.d.) is an approved inhaled fixed-dose combination that demonstrates a significant and clinically important improvement in lung functionCitation7,Citation8 and a reduction in the use of rescue medicationsCitation8,Citation9 versus LAMA monotherapy, along with a favorable long-term (52 weeks) safety profile.Citation10 However, according to GOLD 2013, the strategy valid at the time of starting recruitment for the study and data available for different LABA/LAMA combinations on patient-related outcomes (PROs), that is, dyspnea and quality of life, were less consistent.Citation11 It also remains unclear as to what is the minimal clinical important increase in FEV1 that would have an impact on short-term PROs.
Taking patient’s treatment preferences into consideration may help improve treatment adherence and consequently the treatment outcomes,Citation12 and it is also recommended by GOLD.Citation5 We hypothesize that treatment improvement could influence patient’s preference for medication. Hence, we compared IND/GLY and tiotropium, the 2 single-dose, o.d. bronchodilator treatments based on their potential for lung function improvement and the subsequent impact on patients’ overall treatment preferences. Each treatment was delivered by a comparable single-dose dry powder inhaler, thus minimizing the impact of the device on patient preference.
FAVOR is the first study to compare IND/GLY (110/50 μg o.d.; administered via Breezhaler® inhaler) versus tiotropium HandiHaler® (18 μg o.d.) in German COPD patients who were being treated with tiotropium monotherapy before entering the study and reported significant complaints regarding their symptoms. The primary objective of the study was to assess improvement in lung function with IND/GLY treatment versus tiotropium (18 μg o.d.). Furthermore, because it is unclear as to what is the minimal clinical important increase in FEV1 that would translate into important PROs, the study investigated both FEV1 and patient satisfaction and preference with the fixed-dose combination of IND/GLY compared with tiotropium.
Methods
Study design
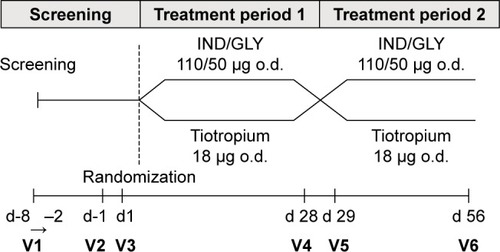
This randomized, open-label, multicenter, crossover study was conducted at 18 centers in Germany (Clinical Trial registration: NCT2125734; ClinicalTrials.gov). The first patient was enrolled on April 24, 2014, and the last patient’s last visit occurred on January 12, 2015. The study consisted of a 7-day screening period and two 4-week treatment periods (). The patients either continued to receive prior tiotropium monotherapy or underwent treatment escalation from tiotropium monotherapy to IND/GLY combination. This is in line with the GOLD strategy document, which recommends an escalation of therapy for the patients who need an intensified therapeutic approach.Citation5,Citation11 The patients were randomized (1:1 ratio) into 2 sequences: sequence 1 (IND/GLY – tiotropium), open-label IND/GLY 110/50 μg o.d. on days 1–28 (treatment period 1) followed by tiotropium 18 μg o.d. on days 29–56 (treatment period 2), or sequence 2 (tiotropium – IND/GLY), the same regimens in reverse order. There was no washout period between the 2 treatment periods. Patients were instructed to inhale the study medications between 7 and 11 AM: Breezhaler® inhaler was used for IND/GLY, and HandiHaler® device was used for tiotropium. All patients received training before taking study drugs to familiarize them with both devices in order to minimize the potential impact of device change even though both devices were similar according to the optimum inhalation technique.
Figure 1 Study design.
Abbreviations: IND/GLY, indacaterol/glycopyrronium; o.d., once daily; V, visit.
Patients
Patients of either gender, aged ≥40 years, diagnosed with stable COPD, and who had a COPD assessment test (CAT) score of ≥10 while taking tiotropium, and thus belonged to high-symptom groups, Group B or D (as per GOLD 2013Citation11), were recruited in the study. Patients were included if they had a post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity ratio of <0.70, a post-bronchodilator FEV1 of ≥30% and <80% of predicted normal values, and a smoking history of at least 10 pack-years at screening. Patients were required to be on stable tiotropium (as maintenance COPD monotherapy) for at least 8 weeks before screening. Inhaled corticosteroids were allowed to be continued without dose adaptations. Salbutamol (100 μg/puff) was the only rescue medication that was allowed during the study; however, its use was restricted to within 6 h of the start of each visit unless absolutely necessary.
Exclusion criteria
COPD exacerbation that required treatment with antibiotics and/or systemic steroids (oral or intravenous) and/or hospitalization in the 6 weeks prior to or during screening. Other inclusion and exclusion criteria, and the list of prohibited medications are provided in online supplementary materials (Tables S1 and S2, respectively).
The study protocol was approved by the local health authority and ethics committees of each participating center (list provided in Table S3). The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All participants provided written informed consent.
Assessments
Efficacy
The primary endpoint was FEV1 1 h post-inhalation after 4 weeks of treatment with IND/GLY versus tiotropium. Patients underwent spirometry testing (as per American Thoracic Society/European Respiratory Society standards) on days 28 and 56 after administration of the study drugs. Other endpoints included the following: patients’ and physicians’ preferences for either treatment; patients’ subjective reason for preference (Table S4); physicians’ rational for decision for future treatment, patients’ satisfaction using the Treatment Satisfaction Questionnaire for Medication (TSQM-9; scores range: 0–100, with higher scores indicating higher satisfaction);Citation13 and patient reported satisfaction on symptom relief using a study-specific questionnaire (a new non-validated tool tested for the first time for feasibility) (Table S5). The use of rescue medication was assessed throughout the study.
A post hoc analysis of the data was carried out to evaluate whether the improvement in FEV1 levels had any influence on the treatment choices made by the patients. The FEV1 1 h post-inhalation was determined separately in the subgroups of patients who preferred IND/GLY or tiotropium as a treatment choice.
Safety assessments
Treatment-emergent adverse events (TEAEs) were recorded as per system organ class from Medical Dictionary for Regulatory Activities (version 17.1). Occurrence of COPD exacerbations was noted during the study. Additionally, clinical laboratory tests, electrocardiograms, vital sign measurements, physical examination, and urine analysis were performed.
Statistical analysis
Based on a previous study, an effect size in relation to FEV1 of about 0.441 was observed.Citation8 Considering a 20% dropout rate, ~88 patients were to be recruited to achieve 90% power on a 2-sided, 5% significance level to reject the null hypothesis of equal efficacy in a crossover, within patient comparison. Power analysis was not required for other parameters.
An analysis of variance model was used for between-group comparison (IND/GLY versus tiotropium) of the improvements in FEV1 1 h post bronchodilation, with center, period, patient within center and treatment as factors (5% level of significance [2-sided], 2-tailed 95% CI). Adjusted (least square) means are provided as point estimates for the pair-wise treatment contrast. Other endpoints were reported as frequency and percentage, and were assessed using Fisher’s exact test (2-tailed, 5% level of significance).
Results
Patient disposition and baseline characteristics
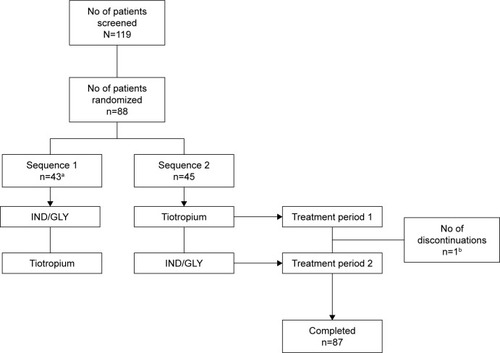
Of the 119 patients screened, 88 were randomized, with 43 patients randomized to sequence 1 (IND/GLY – tiotropium) and 45 patients randomized to sequence 2 (tiotropium – IND/GLY) (). Overall, the study drug exposure was similar across the 2 treatment groups (~28–29 days), and the treatment compliance was almost 100% with both treatments.
Figure 2 Patient disposition (full analysis set).
Abbreviation: IND/GLY, indacaterol/glycopyrronium.
The demographic and baseline characteristics are shown in . A majority of the patients were men (64.8%) and Caucasian (98.9%; ). The mean age across both groups was 65 years. Approximately 64% and 36% of all patients were classified as GOLD B and GOLD D (as per GOLD 2013 criteria), respectively. The mean post-bronchodilator FEV1% was ~58% across both treatment groups (). Hypertension (52.3%, n=46) was the most prevalent comorbidity across both the groups, followed by hyperlipidemia (11.4%, n=10), hypercholesterolemia (10.2%, n=9), coronary artery disease (10.2%, n=9), diabetes mellitus (9.1%, n=8), and hypothyroidism (9.1%, n=8).
Table 1 Demographic and baseline characteristics (full analysis set)
Efficacy
Lung function
On the basis of the combined data for treatment periods 1 and 2, after 4 weeks of treatment, there was a significant increase observed in FEV1 1 h post-inhalation with IND/GLY compared with tiotropium (treatment difference [Δ] =0.081 L; p=0.0017) ().
Treatment preference
Almost all patients in both the treatment sequences completed the questionnaires (). Overall, the proportion of patients who preferred IND/GLY (69%) was greater than those who preferred tiotropium (31%). The preference rate for IND/GLY was notably greater when used first in the treatment sequence (). Physicians’ preference for treatment was in line with the patients’ treatment choice ().
Table 2 Treatment preference after receiving both the treatments (full analysis set)
Subjective reasons of preference
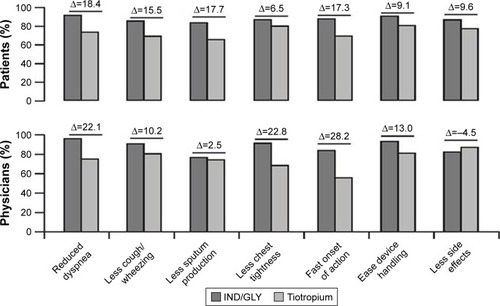
Across all frequently cited very important/important reasons for preferring one treatment or the other, a higher percentage of patients and physicians preferred IND/GLY compared with tiotropium (). Reduction in dyspnea was the most frequently cited reason by patients (91.5%) and the physicians (97.2%) for preferring IND/GLY.
Figure 4 Very important/important subjective reasons cited by patients and physicians for a preference for IND/GLY or tiotropium (full analysis set).
Abbreviation: IND/GLY, indacaterol/glycopyrronium.
Symptom relief and use of rescue medication
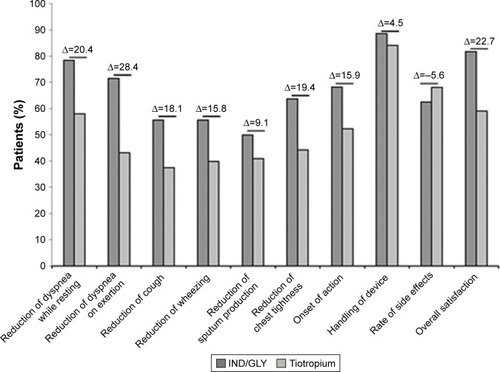
Overall, a higher number of patients experienced symptom relief while receiving IND/GLY treatment than tiotropium, indicating greater treatment satisfaction with IND/GLY ( and Table S6). A higher proportion of patients stated they were very satisfied or satisfied with IND/GLY (79.3%) compared with tiotropium (58.0%) with regard to dyspnea reduction after 4 weeks. The difference was even higher when considering shortness of breath on exertion (IND/GLY 72.4% versus tiotropium 43.2%) ().
Figure 5 Patients very satisfied/satisfied with their treatment (full analysis set).
Abbreviation: IND/GLY, indacaterol/glycopyrronium.
Rescue medication use was higher in patients when on tiotropium treatment than IND/GLY (mean [SD]: 25.88 [36.77] days versus 17.87 [31.17] days; p=0.0023). The number of days with rescue medications was numerically higher during treatment with tiotropium (635 days) than with IND/GLY (449 days), indicating better symptom control with IND/GLY.
Treatment satisfaction, effectiveness, and convenience
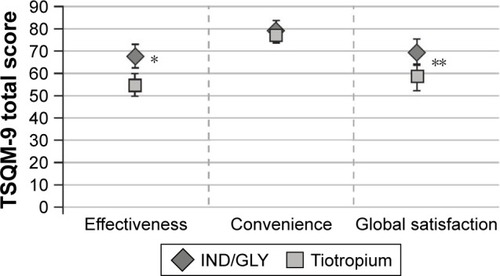
Overall, the mean TSQM-9 scores for the domains “effectiveness” and “global satisfaction” were higher with IND/GLY compared with tiotropium (least square mean difference [IND/GLY versus tiotropium]: 13.02 [p=0.0001] and 11.17 [p=0.0035], respectively) ().
Figure 6 TSQM-9 questionnaire results by domain (full analysis set).
Abbreviations: IND/GLY, indacaterol/glycopyrronium; TSQM-9, Treatment Satisfaction Questionnaire for Medication.
Post hoc analysis
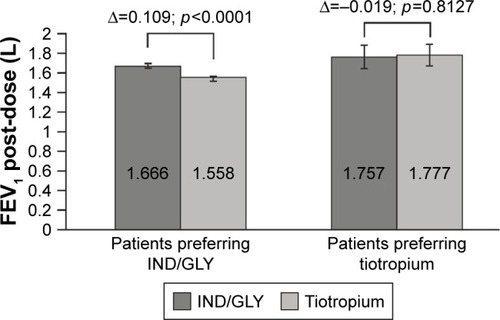
In contrast to the primary outcome (post-bronchodilation FEV1 in both groups), in patients who preferred tiotropium, the FEV1 1 h post treatment levels did not differ significantly between the 2 treatments, with levels in IND/GLY group slightly lower than tiotropium (Δ=−0.019 L; p=0.8127) irrespective of the treatment sequence (). However, in patients who preferred IND/GLY, the FEV1 1 h posttreatment levels significantly improved with IND/GLY versus tiotropium (Δ=0.109 L; p<0.0001) (). The analysis showed among others that patients preferring IND/GLY had slightly more severe COPD than those preferring tiotropium (severity according to GOLD 2010: Stage II 55.9% versus 65% and Stage III 40.7% versus 30.8%; group classification according to GOLD 2015: Group B 59.3% versus 69.2% and Group D 40.7% versus 30.8%) (Table S7).
Safety
The incidence of TEAEs was higher with IND/GLY (31.8%) versus tiotropium (23.9%); almost all TEAEs were of mild severity (). The most common drug-related TEAE was cough, which was observed after administration of IND/GLY (13.6% of patients in IND/GLY group and 4.5% in tiotropium group). “Cough” persisted for a transient time and resolved after 1 day in the majority of the patients (60%). One patient reported serious TEAE that occurred, while on IND/GLY treatment, this patient discontinued prematurely. There were no deaths reported in the study.
Table 3 Incidences of TEAEs reported by ≥2.5% of patients in either group (safety analysis set)
Discussion
In the FAVOR study, the open-label IND/GLY o.d. was compared with tiotropium o.d. monotherapy in similar single-dose dry powder inhalers in COPD patients with moderate-to-severe airflow limitation and a CAT score ≥10. The patients were on tiotropium treatment prior to enrollment, and according to randomization, they either continued to receive tiotropium or were directly shifted to IND/GLY without any washout period, reflecting what would occur in clinical practice. This kind of treatment escalation is in line with the GOLD strategy document.Citation5 In this study, the primary objective was achieved by demonstrating superiority of IND/GLY over tiotropium after 4 weeks of treatment in terms of lung function, assessed by FEV1 1 h post-inhalation. It was observed that both patients’ and physicians’ preferences for IND/GLY were higher compared with those of tiotropium.
Consistent with other clinical trials, irrespective of treatment sequence, in the FAVOR study, IND/GLY showed statistically significant improvement in FEV1 1 h post-inhalation levels compared with tiotropium. Improvement in lung function is inversely correlated with the use of rescue medications.Citation14,Citation15 This was also evident in our study: the patients on IND/GLY showed less need for rescue medications than patients on tiotropium. Consistently, more patients showed signs of symptom relief when treated with IND/GLY than with tiotropium, indicating greater treatment effect and satisfaction with IND/GLY.
Importantly, alongside with the improvement in lung function, this study demonstrated that more patients preferred dual therapy with IND/GLY over monotherapy with tiotropium. The treatment choice was influenced by the sequential order of drug administration; patients were more likely to prefer IND/GLY when given first in the sequence. This may be because the perception of more effective medication was perhaps much pronounced when patients were switched from the standard therapy (prior tiotropium treatment) to IND/GLY and then returned back to tiotropium in the last sequence. However, it was an open-label study; thus, patients’ expectations could have influenced drug preference. Efficacy was the main determinant underlying patients’ preference for either of the study medications. The very important/important reasons cited by patient for using both the medications were mainly related to reduced airflow limitation (as shown in the post hoc analysis), improved dyspnea, less cough/wheezing, less sputum production, and less chest tightness. Based on each of these efficacy determinants, IND/GLY was preferred by more patients than tiotropium. More physicians preferred treatment with IND/GLY than with tiotropium primarily due to reduced dyspnea, less chest tightness, and fast onset of action. However, these data should be interpreted with much caution, since the physicians were aware of the potential dual versus mono treatment benefits and may have expected better efficacy. Reduction in dyspnea was the most frequently cited reason for preferring IND/GLY by patients and the physicians. Based on the post hoc analysis, the patients preferring IND/GLY were observed to have better improvement in FEV1 levels with IND/GLY compared with tiotropium (about 100 mL increase in FEV1). This indicated that even an improvement in lung function of about 100 mL is subjectively detectable and is important for the patient. This is in line with the current notion of COPD-related minimal clinically important difference, whereby a decrease of 100 mL in pre- or trough-FEV1 is considered to be of clinical significance. Furthermore, this analysis showed that patients preferring IND/GLY had slightly more severe COPD than those preferring tiotropium (severity according to GOLD 2010: Stage II 55.9% versus 65% and Stage III 40.7% versus 30.8%; group classification according to GOLD 2015: Group B 59.3% versus 69.2% and Group D 40.7% versus 30.8%). However, it is important to note that this does not reflect any bias in the study design as the patients were properly randomized, but rather confirms the hypothesis that 1) patients who experience more symptoms benefit more from dual bronchodilation than from monotherapy and 2) patients who experience a better treatment effect prefer the more effective therapy.
In patients who preferred tiotropium, no statistical difference in FEV1 levels between the 2 treatments was observed. This result suggests that some patients may not experience an increase in lung function with dual bronchodilation therapy when compared with a monotherapy, hence preferring the monotherapy treatment.
Treatment satisfaction may help to gauge a patient’s perception on current treatment.Citation16,Citation17 In this study, the TSQM-9 score was higher with IND/GLY than with tiotropium, particularly for the domains of “Effectiveness” and “Global satisfaction”, which could result in better adherence. In contrast, for “Convenience,” the scores were comparable between treatments. Both the drugs were given o.d. and were inhaled using similar single-dose powder inhaler devices. Therefore, a device effect was unlikely, and the level of convenience while using devices for inhaling IND/GLY or tiotropium perhaps would be similar.
The safety profiles of both the drugs were consistent with the reported safety in global trials,Citation7–Citation10 with no new safety issues reported with IND/GLY or tiotropium in the FAVOR study. A disproportionate ratio of occurrences of cough between the 2 treatments (IND/GLY – 13.6% patients and tiotropium – 4.5% patients) was observed. Apparently, this observation seems to be contrary to the fact that more patients preferred IND/GLY over tiotropium based on the subjective reason “less cough and wheezing” and “less sputum production”. Notably, the TEAE cough persisted for a transient time, and majority of the patients recovered from this event after 1 day. As the treatment progressed, patients experienced fewer symptoms of cough and wheezing as evident based on their reasons for preferring IND/GLY.
As this was an open-label study, the patients’ and doctors’ perceived views about the study medications, be it positive or negative, perhaps had some influence on the study outcomes. IND/GLY was only introduced in the German market at the start of this study, while tiotropium is a well-established drug available commercially for over a decade. This could have raised the expectations for the new drug, which could have influenced patients’ and physicians’ subjective judgment. Nevertheless, the data obtained gives a consistent picture wherein the improvement in lung function experienced by patients (reduction in airflow limitation) with IND/GLY may have led to a consistent improvement in symptoms of COPD like dyspnea. This might have mainly contributed to patients’ feeling of well-being, resulting in a preference for IND/GLY. Indeed, single patients without improvement in FEV1 did not prefer IND/GLY.
In conclusion, the results of the FAVOR study demonstrate the benefit of IND/GLY 110/50 μg o.d. combination therapy versus tiotropium 18 μg o.d. monotherapy for treating stable COPD patients with persistent complaints based on both patient-reported outcomes and lung function. Further studies are required to investigate whether the favored treatment option translates into improved adherence and long-term treatment outcomes.
Acknowledgments
The authors thank patients and investigators who participated in the study. They also thank Ananya Chikramane, PhD, and David Bergin, PhD (professional medical writers; Novartis) for assistance in the preparation of this paper. Writing support was funded by Novartis Pharma GmbH, Germany. This study was funded and supported by Novartis Pharma GmbH, Germany.
Disclosure
Dr Peter Kardos reports personal fees and study honoraria for his practice from Novartis during the conduct of the study; personal fees and other from AstraZeneca and Boehringer Ingelheim; and personal fees from Chiesi, GSK, Menarini, and Teva, outside the submitted work. Dr Ina Hagedorn-Peinz is an employee of Novartis Pharma GmbH and was in a medical role during the study conduct and in a commercial role during the manuscript development. The authors report no other conflicts of interest in this work.
References
- TashkinDPLong-acting anticholinergic use in chronic obstructive pulmonary disease: efficacy and safetyCurr Opin Pulm Med20101629710520019615
- World Health OrganizationThe top 10 causes of death. [webpage on the Internet] Available from: http://www.who.int/mediacentre/factsheets/fs310/en/Accessed August 17, 2017
- KardosPVogelmeierCBuhlRCrieeCPWorthHThe Prospective Non-Interventional DACCORD Study in the National COPD Registry in Germany: design and methodsBMC Pulm Med2015151225578330
- TashkinDPFergusonGTCombination bronchodilator therapy in the management of chronic obstructive pulmonary diseaseRespir Res20131414923651244
- GOLDGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease2016 Available from: www.goldcopd.orgAccessed August 17, 2017
- CazzolaMPageCLong-acting bronchodilators in COPD: where are we now and where are we going?Breathe2014102110120
- BatemanEDFergusonGTBarnesNDual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE studyEur Respir J20134261484149423722616
- WedzichaJADecramerMFickerJHAnalysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group studyLancet Respir Med20131319920924429126
- MahlerDAKerwinEAyersTFLIGHT1 and FLIGHT2: efficacy and safety of QVA149 (indacaterol/glycopyrrolate) versus its mono-components and placebo in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201519291068107926177074
- DahlRChapmanKRRudolfMSafety and efficacy of dual bronchodilation with QVA149 in COPD patients: the ENLIGHTEN studyRespir Med2013107101558156723867808
- GOLDGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease2013 Available from: www.goldcopd.orgAccessed August 17, 2017w
- RestrepoRDAlvarezMTWittnebelLDMedication adherence issues in patients treated for COPDInt J Chron Obstruct Pulmon Dis20083337138418990964
- BharmalMPayneKAtkinsonMJDesrosiersMPMoriskyDEGemmenEValidation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medicationsH Health Qual Life Outcomes20097136
- JonesPWDonohueJFNedelmanJPascoeSPinaultGLassenCCorrelating changes in lung function with patient outcomes in chronic obstructive pulmonary disease: a pooled analysisRespir Res201112116122206353
- DonohueJFJonesPBartelsCRelationship between change in trough FEV1 and COPD patient outcomes: pooled analysis of 23 clinical trials in patients with COPDEur Respir Soc201546A1013
- RevickiDAPatient assessment of treatment satisfaction: methods and practical issuesGut20045344044
- RuizMAPardoARejasJSotoJVillasanteFArangurenJLDevelopment and validation of the “Treatment Satisfaction with Medicines Questionnaire” (SATMED-Q)Value Health200811591392618494753