Abstract
Introduction
This research project sets out to design an integrated disease management model for patients with COPD who were referred to a secondary care setting and who qualified for pharmacological and nonpharmacological intervention options.
Theory and methods
The integrated disease management model was designed according to the guidelines of the European Pathway Association and the content founded on the Chronic Care Model, principles of integrated disease management, and knowledge of quality management systems.
Results
An integrated disease management model was created, and comprises 1) a diagnostic trajectory in a secondary care setting, 2) a nonmedical intervention program in a primary care setting, and 3) a pulmonary rehabilitation service in a tertiary care setting. The model also includes a quality management system and regional agreements about exacerbation management and palliative care.
Discussion
In the next phase of the project, the COPDnet model will be implemented in at least two different regions, in order to assess the added value of the entire model and its components, in terms of feasibility, health status benefits, and costs of care.
Conclusion
Based on scientific theories and models, a new integrated disease management model was developed for COPD patients, named COPDnet. Once the model is stable, it will be evaluated for its feasibility, health status benefits, and costs.
Introduction
COPD is a highly prevalent disease and often puts a high burden of disease on those affected, even when they are in a relatively stable phase of their disease or only have mild-to-moderate airway obstruction.Citation1 Moreover, the impact of COPD places an inordinate burden on health care resources given the significant direct and indirect costs of care.Citation2 Projections on the future suggest a further rise in the prevalence of COPD patients, especially of patients with severe or very severe disease.Citation3
Given this high prevalence, the expected rise, and the significant impact on the individual and on society, it is important to establish a care process that maximizes outcomes in relation to the efforts and costs made.Citation4
Surprisingly, little scientific data are available on the outcome of “real-life” care in these patients in the chronic phase of their illness, that is to say, outcomes of care outside the remit of treatment of exacerbations.Citation5 The first publications on the outcomes of real-life chronic care in COPD are available and suggest room for improvement for the organization of care, as well as for the content of care and the cost-effectiveness of care.Citation6–Citation9
Better outcomes of care for patients with chronic conditions, such as type 2 diabetes, are to be expected from the widespread use of integrated disease management programs.Citation10 This also counts for patients with COPD.Citation11 According to the definition of integrated disease management by Peytremann-Bridevaux and Burnand,Citation12 such programs should address simultaneously both the content of care and the organization of care, that is to say, to provide 1) a patient-centered, holistic care based on the patient’s individual needs, captured through a thorough assessment in 2) a synchronized manner with the coordination of services and therapies across health care settings and health care providers.Citation13
The most recent systematic review confirmed the evidence for the efficacy of integrated disease management interventions in people with COPD of at least 3 months duration on disease-specific quality of life and exercise tolerance up to 12 months of follow-up and demonstrated a reduction in respiratory-related hospital admissions and hospital days per person.Citation14 However, when taking a closer look at the studies, it appeared that only five of the 26 included studies described an integrated disease management program within a combination of primary and secondary health care settings.Citation15–Citation19 In addition, interventions were directed toward either the content of careCitation15,Citation16,Citation19 or the organization of careCitation17,Citation18 but never addressed them together. A recent publication on the effects of the German disease management program for COPD, predominantly directed at primary care, also lends support to the effectiveness of an integrated approach for COPD.Citation20
Finally, our impression is that integrated care models are, as yet, only in limited use in our present care delivery pathways. This was confirmed in a recently performed survey in five European Union countries, including the Netherlands. In this article, the authors concluded that COPD health care pathways are fragmented and care is not integrated properly. In order to succeed in providing integrated chronic disease management care, knowledge from controlled studies should be translated into practical applications.Citation21
This article describes the results of a research project, which was set out to design an integrated disease management model for patients with COPD, named the COPDnet integrated care model. This model may serve as a blueprint for the establishment of regular care for COPD patients across all health care settings, and it will address both the content and the organizational aspects of care. The COPDnet integrated care model was specifically designed for patients with moderate or severe burden of disease, who, according to the Dutch Standard of Care for COPD, meet the criteria for care in a primary, secondary, or tertiary care setting and qualify for both pharmacological and nonpharmacological intervention options.Citation22
Description of the care practice
Description of the development process
The COPDnet integrated care model was designed according to the guidelines of the European Pathway Association (EPA) in which the following seven phases are distinguished: 1) a screening phase, 2) a project management phase, 3) a diagnostic phase (baseline measurements, mapping existing pathways), 4) a design and plan phase (development of care pathway), 5) an implementation phase, 6) an evaluation phase, and 7) a continuous follow-up phase (making it clinical routine and ongoing review).Citation23 We designed this model because it seemed particularly applicable to in-hospital, primary care, and cross-boundary projects. In Phase II and III, an analysis and baseline measurements were carried out and we found that the process of care at that time did not sufficiently comply with the principles of integrated disease management care. During the course of Phase IV, the designing process, general practitioners (GPs), respiratory nurses, pulmonologists, representatives of the Dutch Lung Foundation, and medical advisors of health insurance companies externally reviewed the COPDnet integrated care model.
The content of the COPDnet model
The definition of chronic disease management was used as a starting point for the development of the COPDnet integrated care model.Citation12 In order to operationalize this definition in the designing process, we have used the Chronic Care Model (CCM) as a guideline.Citation24 The CCM sets out to transform the daily care for patients with chronic illnesses from acute and reactive to proactive, planned, and population based.Citation25 Moreover, application of the principles of the CCM in the context of COPD has shown added value and highlighted the need for implementing multiple components of the CCM to prevent complications and improve outcomes in patients with COPD.Citation26 Therefore, the following four elements of the health care system, as identified in the CCM, were used in our COPDnet integrated care model: 1) self-management support, 2) decision support, 3) delivery system design, and 4) clinical information systems. The CCM is not an explanatory theory, yet the model is more like a flexible evidence-based guideline.Citation27 We have added specific diagnostic procedures to our model in order to better address the complexity and heterogeneity of COPD and thereby to provide the best personalized treatment of a given patient.Citation28
represents a graphical overview of all the elements of the COPDnet integrated care model, that is, 1) a diagnostic trajectory carried out in a secondary care setting, 2) a nonmedical intervention program in a primary care setting, and 3) a pulmonary rehabilitation service in a tertiary care setting. The model also includes a Quality Management System (QMS) and a set of regional network agreements about exacerbation management and palliative care. The COPDnet model uses the diagnostic trajectory of which one of the authors (AJvtH) was cocreator and is based on the Delphi Panel Study.Citation29 Novelties in this diagnostic trajectory are a measurement of dynamic hyperinflation, an objectivation of physical activity measured with the DynaPort MoveMonitor (McRoberts, The Hague, the Netherlands), and a systematic evaluation of the burden of disease measured with the Nijmegen Clinical Screening Instrument (NCSI). The identification of comorbidities is explicitly part of the diagnostic workup in the COPDnet model and is acted upon if and when this is deemed appropriate by the pulmonologist.Citation30 Details regarding the content of the diagnostic trajectory are published elsewhere.Citation31
Figure 1 The COPDnet integrated care model.
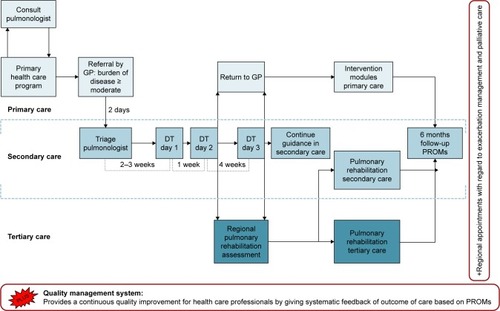
We describe how the four CCM components have been integrated in the COPDnet model in detail below.
Self-management support
More and more, the reinforcement of self-management skills is found to be an important aspect in the care of patients with a chronic health condition. An effective self-management strategy should include the initiation of behavioral change, be tailored individually, take the patient’s perspective into account, and be adapted to the course of the patient’s disease and comorbidities.Citation32 Recently, international consensus has been reached regarding a conceptual definition of what a COPD self-management intervention should comprise.Citation33 Subsequently, we have included the following strategies for self-management support in our COPDnet model: 1) Patient Activation Measurement (PAM) and Motivational Interviewing, 2) Capabilities Opportunities Motivation-Behavior (COM-B) model, 3) shared decision making, and 4) an individual care plan.
PAM and Motivational Interviewing
An understanding of the level of activation for self-management, defined as “patients’ knowledge, skills, and self-efficacy regarding self-management” is important because it gives clues as to how self-management may be improved by the individual patient.Citation34 The level of activation for self-management can be determined with the PAM.Citation34,Citation35 In the COPDnet integrated care model, we use the shortened 13-item version of the PAM.Citation34 This measures patients’ activation levels for self-management and classifies patients into the following four different levels: 1) believing in the importance of their own role, 2) having the confidence and knowledge required to take action, 3) actually taking action to maintain and improve health, and 4) staying the course even under stress. With the outcome of the PAM, the stages of change in health behavior can be monitored.Citation36 Based on these outcomes, health care professionals apply motivational interviewing techniques to improve patients’ self-management skills. Motivational interviewing is a communication technique, which focuses on helping patients to change their behaviors, by exploring their personal perspectives as well as their perceived barriers.Citation37
COM-B model
The COM-B model is a theoretical model, which suggests that there are three ways in which human behavior (B) results from the interaction between: psychological capabilities (C), social and environmental opportunities (O), and motivation (M). This model helps to identify which dimension in this COM-B model should be addressed to encourage behavioral change in patients.Citation38
Shared decision making
Shared decision making is used in our COPDnet model. Although the principles of shared decision making are well documented, we have described a comprehensive practical approach to patient-centered care. Achieving shared decision making relies on a good relationship in the clinical encounter so that information is shared and patients are supported to deliberate and express their preferences and views during the decision-making process.Citation39 Shared decision making is based on introducing a choice (choice talk), describing options (option talk), and helping patients to explore preferences and to make informed decisions (decision talk).Citation39 In our COPDnet model, the choice talk takes place during day 1, the option talk takes place during day 2, and the decision talk takes place during day 3 of the diagnostic trajectory.
Individual care plan
Based on the various talks between the health care professionals and the patient, the patient is asked to construct an individual care plan – including the patient’s personal objective(s) – between visits 2 and 3. During visit 3, this individual care plan will be further explored with the respiratory nurse and developed into informed preferences regarding treatment options.
Decision support
Guidelines on decision making
Based on state-of-the-art insights, practical guidelines on decision making were introduced in our model for 1) additional diagnostics, 2) classification of the burden of disease, and 3) choices between care settings for personalized interventions.
Additional diagnostic tests
The COPDnet diagnostic trajectory is designed to provide an optimal diagnosis with minimal measurements, as adequately as possible. The model creates an overview of the individual traits of each patient. In some patients, a further understanding of the pathophysiology is necessary in order to come to a proper diagnosis or to set an appropriate indication for the best interventions. For these patients, additional diagnostics may be required after day 1 of the diagnostic trajectory. In patients to whom exercise training is offered as intervention, a cardiopulmonary exercise test is carried out. The performance on a maximal exercise test is used to set individual training parameters.Citation40 Further diagnostic tests may be requested ().
Table 1 Additional diagnostics
Classification of burden of disease
The diagnostic trajectory of the COPDnet model aims to provide a classification of the burden of disease on the patient. Subsequently, this classification is used for the allocation of patients to the appropriate care setting, which means either a referral back to a primary care setting or a referral to a tertiary care setting for a pulmonary rehabilitation assessment.
Whereas the severity of COPD is defined by the pathophysiological impairment (airway obstruction), the burden of disease is based on the perceived health problems by the individual patient.Citation41 This allows tailoring treatment to the patient, based on a comprehensive assessment of the individual causes of the burden of disease. The severity of the burden of disease is classified into mild, moderate, and severe (). Choices with respect to the indices of and cutoff values for the burden of disease in our model are partially based on the existing literature and the expert opinion based on such thresholds, for instance, to classify the 6-minute walk distance.
Table 2 Classification of burden of disease
Choices between care settings for personalized interventions
During the diagnostic trajectory in a secondary care setting, 1) the medical diagnosis is confirmed, 2) classification of the burden of disease is made, and 3) the number and complexity of individual traits are determined. The classification of the burden of disease leads to the next phase, that is, referral to a primary, secondary, or tertiary care setting for tailor-made interventions. An important aim of the COPDnet model is the provision of appropriate care of patients as close as possible to their home environment, that is, preferably in a primary care setting and, only if necessary, in a secondary or tertiary care setting.
In principle, patients with mild or moderate burden of disease are (re)referred to a primary care setting and, according to their individual care plan, will be offered one or more nonmedical intervention module(s) provided by allied health care professionals. In order to enable referral of the right patients to the appropriate nonmedical intervention module(s), a guideline on decision making was developed for the allocation of COPD patients to modules provided by dieticians, occupational therapists, and physiotherapists ().
Figure 2 Guideline on decision making for nonmedical intervention modules in a primary care setting.
Abbreviations: 6MWD, 6-minute walk distance; BIC, blood isotope clearance; BMI, body mass index; CAT, COPD assessment test; CCQ, Clinical COPD Questionnaire; FFMI, Fat-Free Mass Index; IC, inspiratory capacity; MPT, manually paced tachypnea; NCSI, Nijmegen Clinical Screening Instrument; PAL, physical activity level; pCO2, partial pressure of carbon dioxide.
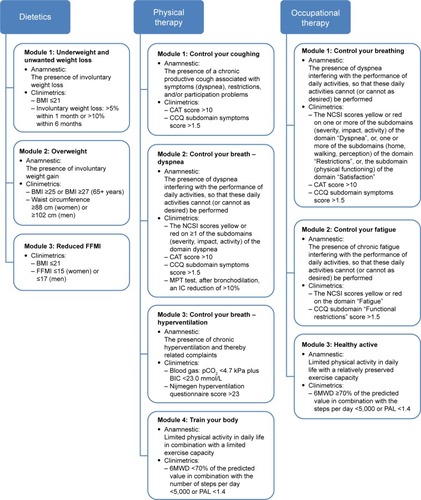
Patients with a severe burden of disease may be referred to a pulmonary rehabilitation setting, where an additional prerehabilitation assessment is carried out and the indication for pulmonary rehabilitation is re-established. Again, personal goals are set and the components of the rehabilitation program are determined, which means that either an inpatient-based rehabilitation program or an outpatient-based rehabilitation program is indicated.Citation42
In the case of such an indication, patients continue under supervision for some time in a secondary care setting by the pulmonologist and/or respiratory nurse. Typically, this applies to patients who were not on the appropriate inhalation medication yet, as recommended by current guidelines. Also, when more time is required to reach an agreement with the patient on the individual care plan.
Multilevel outcome measurement
Most importantly, we deliberately set out to include a systematic registration of the outcome of care at multiple levels in the COPDnet model, using Patient-Reported Outcome Measurements (PROMs): 1) NCSI, 2) Clinical COPD Questionnaire (CCQ), and 3) 13-PAM. Data from the NCSI and CCQ are collected on day 1 of the diagnostic trajectory and on 6 months follow-up. The outcomes of the 13-PAM are also collected on day 1 of the diagnostic trajectory, the last day of the diagnostic trajectory (day 2 or 3), and also 6 months follow-up.
NCSI
The NCSI enables the clinician to obtain a valid, reliable, and detailed picture of a patient’s health status by measuring multiple sub-domains covering the following four domains: physiological functioning, symptoms, functional impairment, and quality of life.Citation43 In combination with the automated monitoring system of the Patient Profile Chart, the NCSI can easily be used in routine care as a guide to patient-tailored treatment.Citation43 The Patient Profile Chart offers a visual and, therefore, easily interpretable picture of the integral health status of an individual patient for the pulmonologist, respiratory nurse, and the patient.Citation44
CCQ
The CCQ is a self-administered 10-item questionnaire specially developed to measure clinical control in patients with COPD. Data support the validity, reliability, and responsiveness of this questionnaire.Citation45
13-PAM
The 13-PAM is a valid and reliable 13-item Guttman-like scale and assesses the level of activation for self-management.Citation34,Citation35
Delivery system design
Cohesive, transmural model
The COPDnet model also aims to improve the transmural, organizational aspects of care. In order to do this, we opera-tionalized the following in our model: 1) standardization of (electronic) referral procedures for the GP to a secondary care setting, 2) proactive management of the patient’s expectations by the GP, 3) preparation of patients for the setting of goals after the diagnostic trajectory (information provided by an information flyer), 4) standardization of reporting by the pulmonologist and respiratory nurse, and 5) agreements as to what information is provided to allied health care providers when referring a patient for an intervention module.
Finally, every 3 months, consultation takes place between representatives of health care providers from primary and secondary care settings and between representatives of secondary care and the regional tertiary care rehabilitation center to discuss organizational aspects of care.
QMS
Based on the knowledge of quality management models, and in collaboration with the participating health care professionals, we developed a QMS primarily focused on the diagnostic trajectory in secondary care settings. The QMS aims to provide a continuous quality improvement for pulmonary specialists and respiratory nurse specialists participating in the COPDnet integrated care model, by giving systematic feedback on outcomes of care based on PROMs. The COP-Dnet QMS includes 1) case presentation and discussion, 2) audit, and 3) education and training.
Case presentation and discussion
Every 3 months, one to two case histories are presented by the pulmonologist and respiratory nurse and then discussed with a psychologist and an independent chairman.Citation46 Discussions may cover the interpretation of health status measurements, interviewing techniques, decisions on additional diagnostic tests and classification of the burden of disease, or choices with regard to the individual care plan. Mirroring the COPDnet guidelines, decision making is an important element in this process.
Audit
Audits are regularly performed between health care professionals from different hospitals working with the COPDnet model to evaluate and to discuss the aspects of the organization of the care process.Citation47 Sharing experiences between users are thought to be helpful to further optimize the model.
Education and training
Education and training sessions are offered depending on the specific needs indicated by the health care professionals. The topics of the education and training may vary, but they are always related to the COPDnet model.
Exacerbation management and palliative care
In the COPDnet model, regional action plans with regard to exacerbation management and palliative care were agreed.Citation48,Citation49
Clinical information systems
Electronic Health Record (EHR)
An EHR is used to register key administrative and clinical data of patients during the diagnostic trajectory of the COPD-net model. Relevant clinical parameters for the evaluation of the individual health status at baseline, as well as the change of health status over time, systematically registered in the EHR. The data may be used to support the clinical decision-making process at the individual level and may also be used in aggregated (anonymized) data at the population level for quality purposes, as well as for scientific research purposes. Also, important features with regard to the care process are periodically analyzed.
Electronic communication platform
To facilitate digital communication between health care professionals and the patient in the COPDnet model, a patient portal is used, for instance, to exchange information or to enable the administration of questionnaires at home at regular intervals. This portal is considered an important digital add-on contributing to the effectiveness of the care model but also to the perceived quality of care by patients.
Discussion
With the current project, we wish to present an evidence-based comprehensive integrated disease management model for patients with COPD with moderate or severe burden of disease, who, according to the Dutch Standards of Care for COPD, meet the criteria for shared care between primary and secondary care settings.Citation22
The idea behind this project arose from the awareness of the poor and fragmented use of the principles of integrated care in COPD patients in real-life care in various health care settings.Citation21 This is not in keeping with the scientific evidence of the added value of an integrated approach, in terms of improving the quality and the efficiency of care and reducing health care costs.Citation50 In addition, the first observational studies on the outcome of real-life COPD care, recently carried out in Germany, demonstrated extensive room for improvement.Citation6–Citation9 More studies on the outcome of real-life COPD care are expected in due time.Citation51–Citation53 Last but not least, the poor outcome observations are in line with a recently performed survey in five European Union countries, including the Netherlands, in which the authors concluded that COPD health care pathways are fragmented and care is not integrated properly.Citation21
We assume that the availability of a scientifically documented care pathway, based on principles of integrated disease management and founded on the CCM, may facilitate the wider use of the principles of integrated disease management care in real-life clinical practice and that it will boost the clinical effectiveness of care in COPD patients. Evidence in support or this assumption is the outcome of a study on the effects of the introduction of a QMS-targeting patients treated on an outpatient base in hospitals in Denmark. In this study, it was found that with the implementation of a nationwide registration, the care provided was more in line with principles of integrated care.Citation53
Although the added value of our model has to be empirically determined, we believe that it has a strong basis. During the development of our integrated disease management pathway, we used a robust and scientifically based method, that is, the seven-phase model of the EPA.Citation23 With this method, several other care pathways have been successfully developed and implemented, including a pathway for acutely ill patients with COPD who were in need of hospitalization.Citation54–Citation57 In the seven-phase model, co-creation in the designing process is acknowledged to be crucial in establishing a supported innovative care model.Citation58 Therefore, early on in the designing process, we consulted different stakeholders, both with respect to their opinions regarding the care process and their views on the content of the integrated disease management pathway.
Notably, the content of our model addresses disease-specific aspects based on knowledge regarding its complexity and heterogeneity,Citation1 but it also includes features relating to the more general needs of patients with a chronic condition. We found inspiration for the latter in the CCM.Citation24 The assumption of the CCM is that better outcomes of care in patients with chronic conditions, such as COPD, are to be expected as a result of the productive interaction between proactive health care professionals and an activated patient. To enable and support this productive interaction, several features of the health care system should be reconsidered and improved by united efforts. These improvements concern self-management strategies, decision support, delivery system design, and clinical information systems.Citation26 In our COPDnet model, all these four elements are explicitly addressed. With the incorporation of the systematic measurement on outcomes (using PROMs) of the integrated care pathway, as a basis for the QMS, we supported the creation of a continuously learning organization. This will enable us to keep on introducing new improvements to our COPDnet model.
Despite our positive expectations with regard to the added value of our care pathway, we acknowledge some challenges in its use. Care according to the COPDnet model starts at the moment a patient with COPD is referred from primary care (GP) to secondary care (pulmonologist) because of persistent burden of disease. Hence, proper working of the COPDnet model presupposes timely and adequate medical diagnosis and a correct determination of the burden of disease by the GP. Under- or misdiagnosis of COPD and a wrong estimate of the burden of disease in primary care would result in absent, late, or improper referrals to secondary care and challenge the proper application of the COPDnet model. In addition, during the diagnostic workup of the pulmonologist, the medical diagnosis is verified and adjusted if indicated.
Our COPDnet model strongly relies on the reinforcement of self-management strategies and seeks to initiate behavioral change in patients.Citation33,Citation59 However, much is still unclear in this domain and requires further development.Citation60 Also, our model requires a change in attitude on the part of the health care professionals. The one-dimensional medical perspective is abandoned to make way for a multidimensional biopsychosocial approach to patients, which is not an easy task for health care providers.Citation61,Citation62 Furthermore, our COPDnet model relies on an adequate interaction between health care professionals working in different settings within the health care system, which are primary, secondary, and tertiary care settings. This means that although communications can be easily digitally supported, in real life, communication appears to be not as easy as that.Citation63–Citation65
Finally, although our integrated disease management pathway has been established in a scientific manner and the content is in line with current insights, we acknowledge that the COPDnet model is complex. Significant investments may be needed to use the full model. These investments must focus on clear agreements on effective communications between health care professionals in order to facilitate the transfer of patients across the COPDnet model. In addition, education and training of health care professionals in the use of the different components will be required and are therefore integrated in the QMS. Further studies on the feasibility, health status benefits, and costs of the model will provide answers as to the added value of the COPDnet model.
Conclusion
A new integrated disease management pathway in patients with COPD, named COPDnet, has been designed according to current knowledge on important disease-specific aspects as well as on insights regarding effective care in patients with a chronic condition. The model provides the application of principles of a learning organization, through a continuous evaluation of the results. This in turn may lead to future adaptations of the model. Once the model is stable, it will be evaluated for its feasibility, health status benefits, and costs of care.
Acknowledgments
Heleen van der Niet, Jeanine Antons, and Jan Vercoulen are thanked for their valuable contributions during the designing process of the COPDnet integrated care model. Jeanine Antons is also thanked for her contributions during the revision of the manuscript, and Renata Straver is thanked for her linguistic support. This study was funded by unrestricted grants from AstraZeneca, Glaxo Smith Klein, Novartis, Chiesi, Takeda, Mundipharma, Teva, and Boehringer-Ingelheim.
Disclosure
The authors report no conflicts of interest in this work.
References
- AgustiACalverleyPMCelliBCharacterisation of COPD heterogeneity in the ECLIPSE cohortRespir Res20101112220831787
- WoutersEFEconomic analysis of the Confronting COPD survey: an overview of resultsRespir Med200397suppl CS3S14
- ManninoDMBuistASGlobal burden of COPD: risk factors, prevalence, and future trendsLancet2007370958976577317765526
- PorterMEWhat is value in health care?N Engl J Med2010363262477248121142528
- PorterMELarssonSLeeTHStandardizing patient outcomes measurementN Engl J Med2016374650450626863351
- WorthHBuhlRCrieeCPKardosPMailanderCVogelmeierCThe ‘real-life’ COPD patient in Germany: the DACCORD studyRespir Med2016111647126775251
- BuhlRCrieeCPKardosPA year in the life of German patients with COPD: the DACCORD observational studyInt J Chron Obstruct Pulmon Dis2016111639164627499620
- HaughneyJGruffydd-JonesKRobertsJLeeAJHardwellAMcGarveyLThe distribution of COPD in UK general practice using the new GOLD classificationEur Respir J2014434993100224176990
- KeXMarvelJYuTCImpact of lung function on exacerbations, health care utilization, and costs among patients with COPDInt J Chron Obstruct Pulmon Dis2016111689170327555759
- FuchsSHenschkeCBlumelMBusseRDisease management programs for type 2 diabetes in Germany: a systematic literature review evaluating effectivenessDtsch Arztebl Int20141112645346325019922
- LemmensKMNieboerAPHuijsmanRA systematic review of integrated use of disease-management interventions in asthma and COPDRespir Med2009103567069119155168
- Peytremann-BridevauxIBurnandBDisease management: a proposal for a new definitionInt J Integr Care20099e1619513182
- NiciLZuWallackRAmerican Thoracic Society Subcommittee on Integrated Care of the CPAn official American Thoracic Society workshop report: the Integrated Care of The COPD PatientProc Am Thorac Soc20129191822421582
- KruisALSmidtNAssendelftWJIntegrated disease management interventions for patients with chronic obstructive pulmonary diseaseCochrane Database Syst Rev201310CD009437
- Mendes de OliveiraJCStudart Leitão FilhoFSMalosa SampaioLMOutpatient vs. home-based pulmonary rehabilitation in COPD: a randomized controlled trialMultidiscip Respir Med20105640140822958267
- ReaHMcAuleySStewartALamontCRosemanPDidsburyPA chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary diseaseIntern Med J2004341160861415546454
- SmithBJAppletonSLBennettPWThe effect of a respiratory home nurse intervention in patients with chronic obstructive pulmonary disease (COPD)Aust N Z J Med199929571872510630654
- SridharMTaylorRDawsonSRobertsNJPartridgeMRA nurse led intermediate care package in patients who have been hospitalised with an acute exacerbation of chronic obstructive pulmonary diseaseThorax200863319420017901162
- StrijbosJHPostmaDSvan AltenaRGimenoFKoeterGHA comparison between an outpatient hospital-based pulmonary rehabilitation program and a home-care pulmonary rehabilitation program in patients with COPD. A follow-up of 18 monthsChest199610923663728620707
- AchelrodDWelteTSchreyoggJStargardtTCosts and outcomes of the German disease management programme (DMP) for chronic obstructive pulmonary disease (COPD)-A large population-based cohort studyHealth Policy201612091029103927552849
- KayyaliROdehBFrerichsICOPD care delivery pathways in five European Union countries: mapping and health care professionals’ perceptionsInt J Chron Obstruct Pulmon Dis2016112831283827881915
- Long Alliantie Nederland (LAN) [Lung Alliance Netherlands]Zorg-standaard COPD [Care Standard COPD]2016 Available from: http://www.longalliantie.nl/zorgstandaard-copdAccessed March 16, 2018 Dutch
- VanhaechtKVan GervenEDeneckereS7-fasenmodel voor de ontwikkeling, implementatie, evaluatie en continue opvolging van zorgpaden [The 7 phase model to develop, implement, evaluate and continuously follow up care pathways]Tijdschr Geneeskd2011108 Dutch
- WagnerEHAustinBTVon KorffMImproving outcomes in chronic illnessManag Care Q1996421225
- ColemanKAustinBTBrachCWagnerEHEvidence on the Chronic Care Model in the new millenniumHealth Aff20092817585
- AdamsSGSmithPKAllanPFAnzuetoAPughJACornellJESystematic review of the chronic care model in chronic obstructive pulmonary disease prevention and managementArch Intern Med2007167655156117389286
- WagnerEHAustinBTDavisCHindmarshMSchaeferJBonomiAImproving chronic illness care: translating evidence into actionHealth Aff20012066478
- AgustiAMacNeeWThe COPD control panel: towards personalised medicine in COPDThorax201368768769023117977
- van den AkkerEFVan’t HulAJBirnieEChavannesNHRuttenvan MolkenMPIn’t VeenJCComprehensive diagnostic assessment of health status of patients with asthma or COPD: a Delphi Panel Study among Dutch ExpertsCOPD201714219019928026983
- HillasGPerlikosFTsiligianniITzanakisNManaging comorbidities in COPDInt J Chron Obstruct Pulmon Dis2015109510925609943
- van den AkkerEFvan’t HulAJChavannesNHDevelopment of an integral assessment approach of health status in patients with obstructive airway diseases: the CORONA studyInt J Chron Obstruct Pulmon Dis2015102413242226609228
- EffingTWBourbeauJVercoulenJSelf-management programmes for COPD: moving forwardChron Respir Dis201291273522308551
- EffingTWVercoulenJHBourbeauJDefinition of a COPD self-management intervention: International Expert Group consensusEur Respir J2016481465427076595
- HibbardJHMahoneyERStockardJTuslerMDevelopment and testing of a short form of the patient activation measureHealth Serv Res2005406 pt 11918193016336556
- HibbardJHStockardJMahoneyERTuslerMDevelopment of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumersHealth Serv Res2004394 pt 11005102615230939
- ProchaskaJODiClementeCCStages of change in the modification of problem behaviorsProg Behav Modif1992281832181620663
- ElwynGDehlendorfCEpsteinRMMarrinKWhiteJFroschDLShared decision making and motivational interviewing: achieving patient-centered care across the spectrum of health care problemsAnn Fam Med201412327027524821899
- MichieSvan StralenMMWestRThe behaviour change wheel: a new method for characterising and designing behaviour change interventionsImplement Sci201164221513547
- ElwynGFroschDThomsonRShared decision making: a model for clinical practiceJ Gen Intern Med201227101361136722618581
- SpruitMASinghSJGarveyCAn official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitationAm J Respir Crit Care Med20131888e13e6424127811
- WilsonIBClearyPDLinking clinical variables with health-related quality of life. A conceptual model of patient outcomesJAMA1995273159657996652
- van RanstDOttenHMeijerJWvan’t HulAJOutcome of pulmonary rehabilitation in COPD patients with severely impaired health statusInt J Chron Obstruct Pulmon Dis2011664765722162650
- PetersJBDaudeyLHeijdraYFMolemaJDekhuijzenPNVercoulenJHDevelopment of a battery of instruments for detailed measurement of health status in patients with COPD in routine care: the Nijmegen Clinical Screening InstrumentQual Life Res200918790191219543807
- VercoulenJHA simple method to enable patient-tailored treatment and to motivate the patient to change behaviourChron Respir Dis20129425926823129804
- van der MolenTWillemseBWSchokkerSten HackenNHPostmaDSJuniperEFDevelopment, validity and responsiveness of the Clinical COPD QuestionnaireHealth Qual Life Outcomes200311312773199
- KruisALSoljakMChavannesNHElkinSLCOPD multidisciplinary team meetings in the United Kingdom: health care professionals’ perceptions of aims and structureCOPD201613563964126263193
- IversNJamtvedtGFlottorpSAudit and feedback: effects on professional practice and healthcare outcomesCochrane Database Syst Rev20126CD00025922696318
- RetickerALNiciLZuWallackRPulmonary rehabilitation and palliative care in COPD: two sides of the same coin?Chron Respir Dis20129210711622498494
- TrappenburgJCMonninkhofEMBourbeauJEffect of an action plan with ongoing support by a case manager on exacerbation-related outcome in patients with COPD: a multicentre randomised controlled trialThorax2011661197798421785156
- KruisALSmidtNAssendelftWJCochrane corner: is integrated disease management for patients with COPD effective?Thorax201469111053105524415716
- KardosPVogelmeierCBuhlRCrieeCPWorthHThe prospective non-interventional DACCORD study in the National COPD Registry in Germany: design and methodsBMC Pulm Med201515225578330
- BourbeauJTanWCBenedettiACanadian Cohort Obstructive Lung Disease (CanCOLD): fulfilling the need for longitudinal observational studies in COPDCOPD201411212513222433011
- TottenborgSSThomsenRWNielsenHJohnsenSPFrausing HansenELangePImproving quality of care among COPD outpatients in Denmark 2008–2011Clin Respir J20137431932723163961
- PanellaMMarchisioSBrambillaRVanhaechtKDi StanislaoFA cluster randomized trial to assess the effect of clinical pathways for patients with stroke: results of the clinical pathways for effective and appropriate care studyBMC Med2012107122781160
- PanellaMMarchisioSDemarchiMLManzoliLDi StanislaoFReduced in-hospital mortality for heart failure with clinical pathways: the results of a cluster randomised controlled trialQual Saf Health Care200918536937319812099
- VanhaechtKSermeusWPeersJThe impact of care pathways for patients with proximal femur fracture: rationale and design of a cluster-randomized controlled trialBMC Health Serv Res20121212422640531
- VanhaechtKSermeusWPeersJThe impact of care pathways for exacerbation of Chronic Obstructive Pulmonary Disease: rationale and design of a cluster randomized controlled trialTrials20101111121092098
- GrolRGrolRImproving Patient Care: The Implementation of Change in Health Care2nd edChichester, UK; Hoboken, NJ, USAWiley Blackwell, BMJ/Books2013
- JonkmanNHSchuurmansMJJaarsmaTShortridge-BaggettLMHoesAWTrappenburgJCSelf-management interventions: proposal and validation of a new operational definitionJ Clin Epidemiol201680344227531245
- KapteinAAFischerMJScharlooMSelf-management in patients with COPD: theoretical context, content, outcomes, and integration into clinical careInt J Chron Obstruct Pulmon Dis2014990791725214777
- YoungHMAppsLDHarrisonSLJohnson-WarringtonVLHudsonNSinghSJImportant, misunderstood, and challenging: a qualitative study of nurses’ and allied health professionals’ perceptions of implementing self-management for patients with COPDInt J Chron Obstruct Pulmon Dis2015101043105226082628
- GrolRGrimshawJFrom best evidence to best practice: effective implementation of change in patients’ careLancet200336293911225123014568747
- SampsonRBarbourRWilsonPEmail communication at the medical primary-secondary care interface: a qualitative explorationBr J Gen Pract201666648e467e47327162209
- SampsonRBarbourRWilsonPThe relationship between GPs and hospital consultants and the implications for patient care: a qualitative studyBMC Fam Pract2016174527074867
- SheuLFungKMouradMRanjiSWuEWe need to talk: primary care provider communication at discharge in the era of a shared electronic medical recordJ Hosp Med201510530731025755159
