Abstract
Objective
Endoscopic valve therapy aims at target lobe volume reduction (TLVR) that is associated with improved lung function, exercise tolerance and quality of life in emphysema patients. So far, a TLVR of >350 mL was considered to be indicative of a positive response to treatment. However, it is not really known what amount of TLVR is crucial following valve implantation.
Patients and methods
TLVR, forced expiratory volume in 1 second (FEV1), residual volume (RV) and 6-minute walk distance (6-MWD) were assessed before and 3 months after valve implantation in 119 patients. TLVR was calculated based on computed tomography (CT) scan analysis using imaging software (Apollo; VIDA Diagnostics). Minimal important difference estimates were calculated by anchor-based and distribution-based methods.
Results
Patients treated with valves experienced a mean change of 0.11 L in FEV1, −0.51 L in RV, 44 m in 6-MWD and a TLVR of 945 mL. Using a linear regression and receiver operating characteristic analysis based on two of three anchors (ΔFEV1, ΔRV), the estimated minimal important difference for TLVR was between 890 and 1,070 mL (ie, 49%–54% of the baseline TLV).
Conclusion
In future, a TLVR between 49% and 54% of the baseline TLV, should be used when interpreting the clinical relevance.
Introduction
Lung hyperinflation impairs respiratory muscle function, negatively affects exercise capacity and predicts mortality in patients with COPD and emphysema.Citation1 In the recent decade, endoscopic valve therapy that minimizes hyperinflation has emerged as a substantial therapy option in the treatment of severe emphysema.
Several randomized controlled trials (RCTs) confirmed the efficacy of valve placement in a well-selected population of patients with advanced emphysema and absent interlobar collateral ventilation.Citation2–Citation6 Efficacy was assessed by changes in lung function parameters, exercise tests and health-related quality-of-life questionnaires. In various trials, the target lobe volume reduction (TLVR) was calculated based on quantitative multidetector computer tomography (MDCT) scan analysis as an efficacy parameter. In these trials, a TLVR of 350 mL was assumed to be clinically significant. This threshold derives from the results of the first and biggest RCT, known as VENT (“Endobronchial Valve for Emphysema Palliation Trial”), as patients treated with valves experienced a TLVR of 378 mL at 6 months following valve implantation. Thus, a threshold of 350 mL was used as an indication of a positive response to valve treatment in subsequent trials.Citation6–Citation8 In the European cohort of VENT, a TLVR of 55% was used as the threshold for success, since it was the median shown by patients with absent collateral ventilation treated by valves.Citation3
However, it is not really known what size of TLVR can be considered as being crucial following valve implantation. So far, the minimal important difference (MID) for TLVR – based on valid MID calculations and statistical analysis – has not been solidly established. The knowledge of the threshold value for a meaningful change of TLVR helps to interpret the clinical relevance of the results following valve placement.
The aim of this study was to establish the MID for TLVR in emphysema patients treated by endoscopic valve therapy.
Patients and methods
In this retrospective analysis, clinical and MDCT scan data of emphysema patients treated by valves were examined to determine the MID for TLVR. The protocol of this retrospective analysis was approved by the local Ethics Committee of Heidelberg (S-609/2012). All patients gave consent for the scientific use of the data acquired during hospitalization. Furthermore, the majority of the patients were treated within different prospective trials for endoscopic lung volume reduction after obtaining additional written consent. As the data in this analysis were retrospectively analyzed, no further patient consent was required.
Study population and clinical measurements
Clinical measures (forced expiratory volume in 1 second [FEV1], residual volume [RV] and 6-minute walk distance [6-MWD]) of 119 patients treated consecutively by endoscopic valve placement in the Thoraxklinik at the University of Heidelberg from 2012 to 2013 were assessed before and around 3 months after valve therapy. In this time period, it was already known that fissure completeness that is a surrogate for absent collateral ventilation is crucial for a good clinical outcome. Before intervention, thin-section computed tomography (CT) fissure analysis was performed in all patients and was supplemented by catheter-based measurement in some subjects to evaluate the presence of interlobar collateral ventilation.Citation9
TLVR assessment
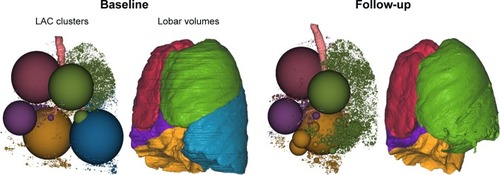
TLVR representing the volumetric change between the baseline and 90-day scan in the treated lobe was calculated based on thin-section MDCT scan analysis using quantitative imaging software (Apollo; VIDA Diagnostics, Coralville, IA, USA) by a core radiology laboratory that was blinded to all clinical details ().
Figure 1 Calculation of the TLVR based on thin-section MDCT scan analysis using quantitative imaging software (Apollo; VIDA Diagnostics, Coralville, IA, USA).
Abbreviations: LAC, low attenuation cluster; LLL, left lower lobe; MDCT, multidetector computed tomography; TLVR, target lobe volume reduction.
MID calculation and statistical analysis
As MID estimates should be based on multiple approaches, different anchor-based as well as distribution-based methods were used for the investigation of MID for TLVR in milliliters and percentage.Citation10
Anchor-based methods for the determination of a MID use a measure for which a MID has been established earlier (the anchor) to estimate the MID for the parameter of interest. In this investigation, FEV1 (MID 100 mL), RV (MID −0.31 to −0.43l) and 6-MWD (26±2 m) were used as anchors.Citation11–Citation13 As a strong association between the variable of interest and an anchor is required, Pearson’s correlation coefficients between the different anchors and TLVR were derived. Statistically significant (p<0.05) correlation coefficients of ≥0.3 are recommended as appreciable. As a first anchor-based method, a linear regression model for each of the anchors was used to specify the linear relationship of the anchor variable to the parameter of interest. The MID of TLVR is then derived by entering the MID of the anchor to the corresponding linear regression equations. Furthermore, the receiver operating characteristic (ROC) methodology was investigated. Grids of potential MID values between 0 and 2,800 in steps of 10 mL and between 0 and 100 in steps of 1% were chosen for the derivation of the MID.
Distribution-based analyses are a more statistical approach that interprets the magnitude of change relative to the variation. The standard deviation (SD) and Cohen’s effect size were implemented to determine the MID. Thereby, a “small” effect size of 0.2 was used to derive a distribution-based MID for TLVR in this investigation.Citation14 All statistical analyses were performed using the open-source R software version 3.2.2.
Results
In this analysis, 119 patients (54% female, mean age 64 years) underwent endoscopic valve therapy. The baseline mean FEV1 was 0.8±0.21 (30%±7% predicted), the mean RV was 5.8±1.31 (271%±55% predicted), and the mean 6-MWD was 281±100 m (). Before valve therapy, all 119 patients underwent CT fissure analysis. According to visual fissure assessment showing incompleteness of up to 10%, in 37 patients, additional catheter-based measurement of collateral ventilation was performed. In 99% (118/119) of the patients, absent collateral ventilation was confirmed by either CT fissure analysis and/or catheter-based measurement of collateral ventilation. All the patients received a complete occlusion of the target lobe by endobronchial valves (n=77; Pulmonx, Inc., Neuchâtel, Switzerland) or intrabronchial valves (n=35; Olympus Corporation, Tokyo, Japan) or a combination of both types of valves (n=7). Fifty-nine patients (50%) were treated with complete occlusion of the left lower lobe, 23 (19%) of the left upper lobe, 21 (18%) of the right lower lobe, 15 (13%) of the right upper lobe and 1 (1%) of the right lower and middle lobes.
Table 1 Patient characteristics
At 3 months following valve placement, patients experienced a mean TLVR of 945±718 mL (54%±40%; n=119) that was associated with a mean improvement in FEV1 of 0.11±0.181 (n=119), in RV of −0.51±1.031 (n=118; RV missing before valve therapy [n=1] due to massive hyperinflation) and in 6-MWD of 44±60 m (n=98; 6-MWD missing before [n=6], following [n=11] valve therapy or both [n=4]). Regarding the health-related quality of life, patients developed a mean decrease in modified Medical Research Council score of −0.7±1.3 pts. shows the 3-month follow-up changes from baseline. Forty-four percent of the patients met the efficacy threshold of >100 mL improvement in FEV1, 57% of the patients developed a >0.31 L reduction in RV and 61% of the patients experienced a >24 m improvement on the 6-MWD.
Table 2 Descriptive statistics for changes from baseline to 3 months after valve implantation
Twenty-two patients (18.5%) experienced a pneumothorax as an anticipated complication following valve placement.
MID estimates according to the anchor-based methods
The highest correlation was present with ΔFEV1 with a Pearson correlation coefficient of 0.59 for TLVR (mL) and 0.56 for TLVR (%), followed by correlation coefficients of 0.42 (mL) and 0.43 (%) for ΔRV. Both parameters had an appropriate correlation with TLVR and can be used as an anchor for the determination of TLVR MID. For Δ6-MWD, however, the Pearson correlation coefficients were 0.09 (m) and 0.14 (%). Although no meaningful correlation could be found, 6-MWD was also considered for the determination of MID for TLVR. show the Pearson correlation coefficients between TLVR and ΔFEV1, ΔRV and Δ6-MWD.
Figure 2 (A and B) Scatter plots.
Abbreviations: 6-MWD, 6-minute walk distance; FEV1, forced expiratory volume in 1 second; RV, residual volume; TLVR, target lobe volume reduction.
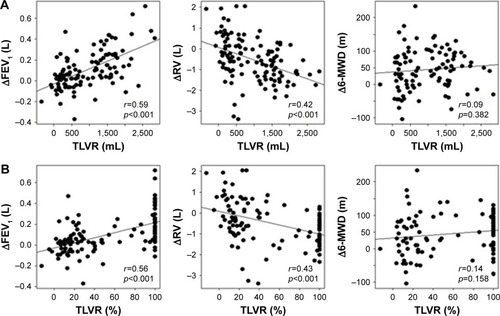
The MID estimates for TLVR (mL) were between 891 mL (ΔRV with a MID of −0.31 L) and 944 mL (Δ6-MWD with a MID of 28 m), and those for TLVR (%) were between 51% (ΔRV with a MID of −0.31 L) and 54% (ΔFEV1 and Δ6-MWD with MIDs of 26 and 28 m, respectively). and present the MID estimates derived from the linear regression equations with use of the anchor MIDs for ΔFEV1, ΔRV and Δ6-MWD.
Table 3 MID estimates for TLVR (mL)
Table 4 MID estimates for TLVR (%)
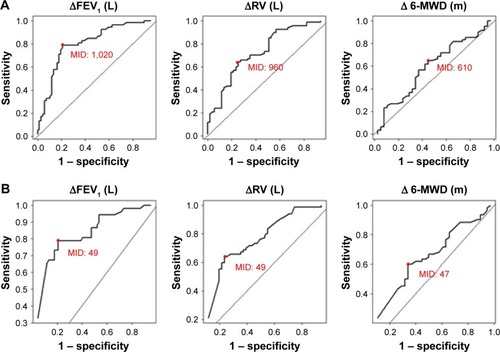
The MID estimates derived using the ROC method based on MIDs for ΔFEV1, ΔRV and Δ6-MWD are also presented in (TLVR mL) and (TLVR%). The MID estimates for TLVR (mL) were 1,020–1,070 mL for ΔFEV1, 960–1,010 mL for ΔRV and 610–620 mL for 6-MWD.
The MID estimates for TLVR (%) were 49%–54% for ΔFEV1 and ΔRV. The MID estimates based on the ROC method using Δ6-MWD were 41%–47%. The ROC curves are presented in .
Figure 3 (A and B) ROC curves.
Abbreviations: 6-MWD, 6-minute walk distance; FEV1, forced expiratory volume in 1 second; MID, minimal important difference; ROC, receiver operating characteristic; RV, residual volume; TLVR, target lobe volume reduction.
MID estimates according to the distribution-based methods
The MID estimates calculated with the distribution-based methods were 228 mL (½ SD) and 91 mL (Cohen’s effect size), as shown in .
Discussion
The aim of endoscopic valve therapy is to achieve a volume reduction of the most emphysematous lung lobe. Therefore, it seems obvious that the higher the TLVR achieved, the greater the success of valve therapy. A consistent relationship between TLVR and clinical and functional parameters has already been confirmed.Citation15 Patients with TLVR >50% at 6 months following valve placement demonstrated greater improvements in lung function, exercise capacity, quality of life, dyspnea and BODE (body mass index, obstruction, dyspnea score, exercise capacity) index, compared to those with TLVR <50%. As observed before, the TLVR and thus the clinical success are greater in patients with absent collateral ventilation and complete occlusion of the target lobe by valves.Citation3
This analysis describes the MID estimates for TLVR following endoscopic valve therapy in patients with advanced emphysema and absent interlobar collateral ventilation. In this study cohort, the patients were treated with the latest scientific developments and current expertise. The response rates to clinical outcome as well as complications rates were very similar to those observed in the last published RCTs.Citation4–Citation6 To date, it is not really known what amount of TLVR can be considered as being clinically relevant following valve implantation.
In different trials, a TLVR of 350 mL or 55% was assumed to be indicative of a positive response to valve treatment, but the MID for TLVR has not been solidly established.Citation2,Citation3 In VENT, patients treated by valves developed a TLVR of 378 mL at 6 months; hence, the threshold of 350 mL was used as an indication of positive response to valve treatment in several trials.Citation2,Citation7,Citation8 It has to be taken into consideration, however, that in VENT patients with interlobar collateral ventilation were also treated by valves as the impact of collateral ventilation was not yet known at that time. In Euro-VENT, the patients who underwent valve treatment experienced a median TLVR of 55% that was used as threshold for success following valve placement.Citation3 The calculation of the TLVR in Euro-VENT is based only on the MDCT scans of patients with absent collateral ventilation as a predictive factor for the success of valve implantation. As only patients with absence of collateral ventilation are treated by valves nowadays, the patient population in Euro-VENT reflects better the current patient cohort that undergoes valve placement.
The MID estimates for TLVR varied from 91 to 1,070 mL depending on the statistical method. Using a linear regression analysis based on ΔFEV1, ΔRV and Δ6-MWD as anchors, and using ROC analysis based on ΔFEV1 and ΔRV, MID estimates for TLVR were between 890 and 1,070 mL and between 49% and 54%, respectively. The MID estimate using ROC analysis based on Δ6-MWD was much lower with 610 mL, ie, 41%. However, Pearson’s correlation coefficient was <0.3, and thus, the estimate of 610 mL cannot be considered as reliable. One explanation for the low correlation between TLVR and change in 6-MWD may be the advent of pneumothorax that occurred as an anticipated complication in 18.5% of the patients and may lead to deterioration of exercise capacity despite significant TLVR. A retrospective analysis demonstrated that nearly 30% of the patients with pneumothorax following valve placement will experience a clinically significant worsening of 6-MWD – frequently despite improvement of lung function parameters – that may result from the prolonged immobilization and subsequent muscle wasting.Citation16,Citation17
For the distribution-based methods, ½ SD and Cohen’s effect size of 0.2 were considered for the determination of MID, resulting in estimates of 228 and 91 mL, which are much lower than the estimates resulting from the anchor-based methods and the values assumed as clinically significant for TLVR in earlier publications.Citation2 The main limitations of the distribution-based methods are that the variability of the change from baseline is not taken into account and that the characteristics of the baseline distribution of the parameter have a strong influence on the individual effect sizes. The estimates of variability will differ from study to study. Furthermore, distribution-based methods do not address the question of clinical importance and do not comply with the primary aim of the MID concept to distinctly separate clinical importance from statistical significance. Distribution-based methods provide no direct information about the MID, but are only a way of expressing the observed change in a standardized metric. Therefore, anchor-based methods are the only way to estimate MID directly.Citation1
To conclude, the results indicate the MID estimates for TLVR after valve implantation of 890–1,070 mL or 49%–54%, respectively, in patients with severe emphysema. These ranges are supported by two different anchor-based methods (linear regression and ROC analysis), using two different anchors (FEV1 and RV) that are highly correlated to TLVR. As the lobar volume is dependent on different factors (eg, body height, severity of the emphysematous destruction and hyperinflation), the relative change of TLVR and thus the MID estimate for TLVR in % may be more meaningful compared to the absolute change of TLVR.
A MID estimate for TLVR of 890–1,070 mL is fundamentally different from the previously assumed value of 350 mL for clinical relevant TLVR. This is what we expected, as the TLVR threshold of 350 mL is based on the average TLVR of patients who underwent valve placement irrespective of collateral ventilation. The MID estimate of 49%–54%, however, is similar to the TLVR threshold of 55% that was used in previous trials, as this TLVR threshold of 55% is based on the average TLVR of patients with absent collateral ventilation.
These MID estimates for TLVR may also be applicable to other interventions that focus on targeted lung volume reduction (eg, bronchoscopic thermal vapor ablation). However, this hypothesis has to be evaluated in further trials.
In general, however, it should be kept in mind that not only one parameter, for example, TLVR, should be used to determine the clinical importance of endoscopic lung volume reduction technologies, but a combination of various parameters (eg, TLVR, FEV1, RV, 6-MWD, health-related quality-of-life questionnaires) that reflect the clinical relevance of this endoscopic therapeutic approach. In previous trials as well as in this analysis, a great variability in the clinical outcome could be observed also among patients with a TLVR of 100%.Citation18 Different variables, for example, emphysema index, baseline vital capacity, 6-MWD or RV, have impact on the clinical outcome and lead to interindividual variability in clinical success despite high TLVR.
Conclusion
In summary, the MID for TLVR of 890–1,070 mL or 49%–54%, respectively, following valve placement should be validated in a prospective trial and should be used in future studies – preferably in combination with other outcome parameters – when interpreting the clinical relevance.
Disclosure
DG obtained lecture and travel fees from Pulmonx, Olympus, Chiesi, Boehringer Ingelheim, Novartis, Astra Zeneca, Munidpharma, Berlin Chemie and Grifols. MS obtained fees for lectures and advisory boards from the following companies: Olympus, Pulmonx, Astra Zeneca, Novartis, Teva, GSK, PneumRx and Boston Scientific. RE obtained lecture and travel fees from Olympus, Pulmonx and Uptake Medical. CPH has stock ownership in medical industry: Stada, GSK; Patents: Method and Device for Representing the Microstructure of the Lungs. IPC8 Class: AA61B5055FI, PAN: 20080208038, Inventors: W Schreiber, U Wolf, AW Scholz and CP Heussel; consultation or other fees from Schering-Plough (2009, 2010), Pfizer (2008–2014), Basilea (2008–2010), Boehringer Ingelheim (2010–2014), Novartis (2010 and 2012), Roche (2010), Astellas (2011 and 2012), Gilead (2011–2014), MSD (2011–2013), Lilly (2011), Intermune (2013 and 2014) and Fresenius (2013 and 2014); expert testimony no; research funding from Siemens (2012–2014), Pfizer (2012–2014), MeVis (2012 and 2013) and Boehringer Ingelheim (2015); lecture fees from Gilead (2008–2014), Essex (2008–2010), Schering-Plough (2008–2010), AstraZeneca (2008–2012), Lilly (2008, 2009 and 2012), Roche (2008 and 2009), MSD (2009–2014), Pfizer (2010–2014), Bracco (2010 and 2011), MEDA Pharma (2011), Intermune (2011–2014), Chiesi (2012), Siemens (2012), Covidien (2012), Pierre Fabre (2012), Boehringer Ingelheim (2012–2014), Grifols (2012) and Novartis (2013 and 2014). FH obtained fees for lectures and advisory boards from Astra, Allmirall, Berlin Chemie, Boehringer, Roche, GSK, Pulmonx, BTG, Olypmus, PneumRx, Boston Scientific, Medupdate, Grifols, CSL Behring, Omniamed, Lilly, Novartis, Teva and Uptake and Vital Air. The authors report no other conflicts of interest in this work.
References
- CasanovaCCoteCde TorresJPInspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2005171659159715591470
- SciurbaFCErnstAHerthFJVENT Study Research GroupA randomized study of endobronchial valves for advanced emphysemaN Engl J Med2010363131233124420860505
- HerthFJNoppenMValipourAInternational VENT Study GroupEfficacy predictors of lung volume reduction with Zephyr valves in a European cohortEur Respir J20123961334134222282552
- DaveyCZoumutZJordanSBronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomized controlled trialLancet201538699981066107326116485
- KloosterKten HackenNHHartmanJEKerstjensHAvan RikxoortEMSlebosDJEndobronchial valves for emphysema without interlobar collateral ventilationN Engl J Med2015373242325233526650153
- ValipourASlebosDJHerthFIMPACT Study TeamEndo-bronchial valve therapy in patients with homogeneous emphysema. Results from the IMPACT StudyAm J Respir Crit Care Med201619491073108227580428
- GompelmannDEberhardtRSlebosDJDiagnostic performance comparison of the chartis system and high-resolution computerized tomography fissure analysis for planning endoscopic lung volume reductionRespirology201419452453024612306
- SchuhmannMRaffyPYinYComputed tomography predictors of response to endobronchial valve lung reduction treatment: comparison with ChartisAm J Respir Crit Care Med2015191776777425635349
- KosterTDvan RikxoortEMHueberRHPredicting lung volume reduction after endobronchial valve therapy is maximized using a combination of diagnostic toolsRespiration201692315015727577190
- RevickiDHaysRDCellaDSloanJRecommended methods for determining responsiveness and minimally important differences for patient-reported outcomesJ Clin Epidemiol2009612102109
- DonohueJFMinimal clinically important differences in COPD lung functionCOPD20052111112417136971
- HartmanJETen HackenNHKloosterKBoezenHMde GreefMHSlebosDJThe minimal important difference for residual volume in patients with severe emphysemaEur Respir J20124051137114122441742
- PuhanMAChandraDMosenifarZNational Emphysema Treatment Trial (NETT) Research GroupThe minimal important difference of exercise tests in severe COPDEur Respir J201137478479020693247
- SamsaGEdelmanDRothmanMLWilliamsGRLipscombJMatcharDDetermining clinically important differences in health status measures. A general approach with illustration to the Health Utility index Mark IIPharmacoeconomics199915214115510351188
- ValipourAHerthFJBurghuberOCVENT Study GroupTarget lobe volume reduction and COPD outcome measures after endobronchial valve therapyEur Respir J201443238739623845721
- GompelmannDBenjaminNKontogianniKClinical and radiological outcome following pneumothorax after endoscopic lung volume reduction with valvesInt J Chron Obstruct Pulmon Dis2016113093309927994448
- LangenRCGoskerHRRemelsAHScholsAMTriggers and mechanisms of skeletal muscle wasting in chronic obstructive pulmonary diseaseInt J Biochem Cell Biol201345102245225623827718
- GompelmannDHofbauerTGerovasiliVPredictors of clinical outcome in emphysema patients with atelectasis following endoscopic valve therapy: a retrospective studyRespirology20162171255126127250924
