Abstract
COPD is a significant cause of morbidity and mortality. In some patients with COPD, eosinophils contribute to inflammation that promotes airway obstruction; approximately a third of stable COPD patients have evidence of eosinophilic inflammation. Although the eosinophil threshold associated with clinical relevance in patients with COPD is currently subject to debate, eosinophil counts hold potential as biomarkers to guide therapy. In particular, eosinophil counts may be useful in assessing which patients may benefit from inhaled corticosteroid therapy, particularly regarding exacerbation prevention. In addition, several therapies targeting eosinophilic inflammation are available or in development, including monoclonal antibodies targeting the IL5 ligand, the IL5 receptor, IL4, and IL13. The goal of this review was to describe the biologic characteristics of eosinophils, their role in COPD during exacerbations and stable disease, and their use as biomarkers to aid treatment decisions. We also propose an algorithm for inhaled corticosteroid use, taking into consideration eosinophil counts and pneumonia history, and emerging eosinophil-targeted therapies in COPD.
Introduction
COPD is a major cause of morbidity and mortality, and is predicted to be the third-leading cause of death worldwide by 2020.Citation1 Health-care costs rise significantly with increased severity of COPD symptoms and exacerbations.Citation1 COPD is characterized by persistent, progressive airflow limitation associated with chronic inflammation that is responsible for permanent structural changes to the airway and lungs. These include airway narrowing due to increased wall thickness, mucus occlusion, and destruction of lung parenchyma with loss of lung elasticity.Citation1,Citation2
The underlying pattern of inflammation in COPD can vary; most often it is predominated by neutrophils, cytotoxic CD8+ T cells, and alveolar macrophages.Citation2 Eosinophils may play a significant role in airway inflammation in some patients with COPD.Citation3 While eosinophilic inflammation is most often associated with asthma and has been used to differentiate asthma from COPD,Citation4 some studies indicate that approximately a third of patients with COPD have sputum eosinophilia,Citation5–Citation7 with prevalence dependent on the eosinophil threshold used and the specific patient population studied. In addition, eosinophil counts in sputumCitation7 are increased during exacerbations in some patients with COPD.Citation8 Importantly, sputum and/or blood eosinophilia in COPD may predict response to inhaled corticosteroids (ICSs) for prevention of exacerbationsCitation7,Citation9 and to systemic corticosteroids (CSs) for treatment of exacerbations.Citation10
This review describes the biology of eosinophils and their role in COPD airway inflammation during exacerbations and in stable disease, as well as the potential of eosinophils as biomarkers to inform treatment decisions for COPD patients. An algorithm for the appropriate use of ICS therapy in COPD is proposed, based on the presence of eosinophils and whether or not a history of pneumonia exists,Citation1 and the potential role of emerging eosinophil-targeted therapies in COPD is also discussed.
We used a PubMed search from January 1970 through October 2017, with Boolean search terms “eosinophil OR eosinophilia” AND “chronic obstructive pulmonary disease OR asthma”. This core search phrase was modified to include “AND biologics”, “AND pneumonia”, “AND (sputum OR blood)”, or “AND corticosteroid”. Searches were also conducted on ClinicalTrials.gov for “chronic obstructive pulmonary disease” (or “COPD”), “eosinophil”, “biologic” (or “biologic therapy”), or “asthma”, “eosinophil”, and “biologics” (or “biologic therapy”); the term “phase III” was also used to qualify searches. For web-based searches, terms included “eosinophil”, “blood cell”, and “quantification”.
Eosinophils: characteristics and methods of quantification
Eosinophils are key immunoeffector and inflammatory cells. They have diverse functions, with roles in homeostasis and disease in various tissues, including the lungs.Citation11 Eosinophils are derived from progenitor stem cells in the bone marrow that are released into the blood upon maturity.Citation12,Citation13
Under normal conditions, eosinophils remain quiescent in the blood; upon exposure to proinflammatory mediators (IL3, IL5, and granulocyte-macrophage colony-stimulating factor; see ), they may become partially activated before migrating to sites of inflammation.Citation12,Citation14,Citation15 IL5 is one of the major cytokines involved in eosinophil-mediated inflammation: it promotes the differentiation, survival, trafficking, activation, and effector functions of eosinophils.Citation13,Citation16 Migration of eosinophils to the lungs is controlled by specific chemotactic factors, such as CCL5 (RANTES), CCL7 (MCP3), CCL11 (eotaxin 1), CCL13 (MCP-4), CCL15, CCL24, and CCL26 acting on the CCR3, along with CRTH2, which is expressed on T-helper 2 cells and its ligand, prostaglandin D2 ().Citation12,Citation17,Citation18 Once in the lungs, proinflammatory mediators, including basic proteins (major basic protein, eosinophil cationic protein, eosinophil peroxidase, and eosinophil-derived neurotoxin), cytokines (IL2, IL3, IL4, IL5, IL10, IL12, IL13, IL16, IL25), chemokines (CCL5, CCL11, CCL13), and growth factors (tumor necrosis factor [TNF], transforming growth factor [TGF]α/β), that are released by eosinophilsCitation12,Citation19 contribute to sustained inflammationCitation14 and tissue damage.Citation14,Citation20 In addition, thymic stromal lymphopoietin, an IL7-like proallergic cytokine associated with chronic airway-disease inflammation,Citation21 can act on eosinophils to upregulate inflammatory cytokine expression.Citation21,Citation22
Figure 1 Eosinophil trafficking from bone marrow to airway.
Abbreviation: GM-CSF, granulocyte-macrophage colony-stimulating factor.
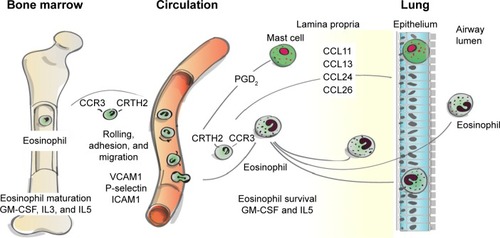
Blood eosinophil concentration is reported as an absolute count (cells/µL) or as a percentage of total leukocytes; studies show that these two quantification methods are concordant.Citation8,Citation9 Eosinophil concentrations in the lungs can also be measured using induced sputum or bronchoalveolar lavage (BAL) fluid, or by quantifying eosinophils in a biopsy of lung tissue.Citation12 Counts are reported as the percentage of eosinophils of total leukocytes in sputumCitation6,Citation23 and BAL fluid,Citation24 whereas the quantification for eosinophils in biopsy specimens is expressed per specified area of tissue.Citation23,Citation25,Citation26 Of note, these eosinophil counts reflect overall levels, and do not consider eosinophil activation.
In the general population, a wide range of eosinophil counts in blood has been reported.Citation27–Citation32 Recent studies have reported a median blood eosinophil count of 2.4% (of total leukocytes) in a general unselected populationCitation33 and absolute blood eosinophil cell counts of 100–200 cells/µL in healthy individuals.Citation34,Citation35 In induced sputum, mean percentages of 0.3%–1.4% have been reported for healthy nonsmokersCitation36,Citation37; in BAL fluid, the mean proportions of eosinophils are similar in current smokers (0.2%–0.6%) and former smokers (0.5%) and generally lower in healthy never-smokers, with a majority of reports in the range 0.07%–0.3% (but some studies reporting 0.7%–0.8% in this population).Citation36 In patients with COPD, median blood eosinophil counts of 2.41%–2.70%Citation33,Citation38 and 180–230 cells/µL,Citation33,Citation38,Citation39 geometric mean percentages of eosinophils in induced sputum of 0.8%–6.2%,Citation10,Citation40–Citation42 and a mean percentage in BAL fluid of approximately 1.0% have been reported.Citation43 In both the general population and patients with COPD, higher eosinophil levels have been found in men versus women.Citation33,Citation44
Thresholds for defining high and low eosinophils
There is a lack of consensus on the appropriate thresholds used to define eosinophilic inflammation in COPD. Because eosinophil numbers differ during stable disease, exacerbations, and following treatment,Citation12 the threshold used may depend on the study design and outcomes. In addition, blood eosinophil counts in individuals fluctuate during a 24-hour period.Citation45,Citation46 Therefore, it is unlikely that a single threshold will be recommended for guiding all treatment decisions. In sputum, studies have used a threshold of 1%,Citation47 2%,Citation8 or 3%Citation7,Citation10,Citation48 for defining eosinophilia in COPD, whereas studies using blood have mostly used a threshold of 2%.Citation9,Citation10,Citation42,Citation49,Citation50 However, some of these thresholds (eg, 2% and 3%) were adopted from those established in studies of eosinophilia in asthma, and thus may not necessarily be the optimal threshold for COPD.Citation51,Citation52 In a post hoc analysis of the Withdrawal of Inhaled Steroids during Optimized Bronchodilator Management (WISDOM) study, the effect of ICS withdrawal on COPD-exacerbation rate was analyzed according to various blood eosinophil thresholds in the ranges of 150–400 cells/µL and 2%–6%. The most significant deleterious effects of ICS withdrawal on exacerbation risk were observed at thresholds of ≥4% or ≥300 cells/µL.Citation53 summarizes recent COPD trials, highlighting eosinophil thresholds used and clinical findings.Citation8,Citation49,Citation53–Citation57
Table 1 Eosinophil thresholds and findings in recent COPD clinical trials
Concordance between eosinophil counts in blood and lungs (sputum, BAL, tissue)
Blood eosinophil concentrations are generally thought to be reasonably good predictors of eosinophil concentrations in the airways. A study of 491 patients with asthma indicated that those with high blood eosinophilia (defined as ≥300 cells/µL) had significantly greater mean concentrations of eosinophil counts in induced sputa versus patients with mean blood eosinophil counts <300 cells/µL (11.7% vs 0.4% of leukocytes, respectively; P<0.001).Citation58 Several recent COPD studies have yielded moderate to strong correlations between eosinophil counts in blood and those in sputum, with a positive predictive value (area under the curve [95% CI]) of 0.85 [0.78–0.93] (during an exacerbation) and 0.76 [0.67–0.84], depending on use of blood eosinophil thresholds of 2% or ≥3%, respectively.Citation10,Citation38 Blood eosinophil percentages also correlate with eosinophil counts in bronchial submucosal samples (r=0.57).Citation59 Post hoc analysis from the Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) study also yielded a positive correlation between blood and sputum eosinophil counts in 138 patients with COPD who had both blood and sputum measurements performed over ≥3 visits.Citation8 However, the correlation coefficients (0.24–0.40) were relatively weak.Citation8 Hastie et al showed that a blood eosinophil threshold of 250 cells/µL predicted a sputum eosinophil concentration of ≥2% with a false positive rate of 50%, even with a statistically significant positive correlation between the two values.Citation60 In addition, a recent study demonstrated that patients with COPD and high blood eosinophil concentrations (>250 cells/µL) had significantly more eosinophils in sputum, bronchial mucosa, and BAL fluid than those having low blood eosinophil concentrations (<150 cells/µL).Citation61 Moreover, BAL aliquots from patients with high blood eosinophil concentrations, compared to those with low blood eosinophils, exhibited more markers indicative of airway inflammation and remodeling.Citation61 These results suggest that blood eosinophil counts may be a simple, albeit somewhat inconsistent, predictor of increased lung-resident eosinophils. As blood is easier to obtain than airway samples, blood eosinophil counts may be more readily used in clinical practice.Citation9,Citation38
In contrast to these findings, Turato et al found no correlation between tissue eosinophils assessed in resected lung tissue and blood eosinophils in patients with COPD.Citation62 Interestingly, the authors also reported that low eosinophils were, if anything, associated with worse outcomes in COPD patients than high eosinophils, possibly due to a beneficial effect of eosinophils on host defense (eosinophils have been shown to contribute to the immune response against infection via their antimicrobial function).Citation63
The prevalence of eosinophilia in COPD patients varies depending on the threshold used for evaluation. In a meta-analysis of ten trials, 63% of patients (n=10,861) had blood eosinophil counts ≥2%.Citation50 In the WISDOM trial, 53% of patients had ≥150 eosinophils/µL, 20% had ≥300 eosinophils/µL, and 11% had ≥400 eosinophils/µL (total number of patients with eosinophil-count data 2,420).Citation53 In a population-based matched-cohort study, 34.9% of 39,824 COPD patients had blood eosinophil counts of ≥340 cells/µL compared with 25.8% of matched non-COPD controls (n=90,772, P<0.001); 36.4% of the COPD patients in this study had blood eosinophil counts of <2%, whereas 38.3%, 16.3%, and 9.2% had elevated blood eosinophil counts based on thresholds of 2.0%–3.9%, 4.0%–5.9%, and ≥6.0%, respectively.Citation64
Persistence of eosinophilia in COPD
The detection of elevated eosinophil concentrations and eosinophil-associated proinflammatory factors in the airways and blood of patients with COPDCitation65–Citation67 suggests that eosinophils actively contribute to inflammatory processes in these patients. However, evidence suggests that eosinophilic inflammation may not persist over time. A post hoc analysis of the ECLIPSE study found that 37.4% of patients with COPD had persistently elevated blood eosinophil counts (≥2% at baseline and years 1, 2, and 3), whereas 49% had intermittent elevation of eosinophils.Citation8 In that analysis, the group with persistently elevated blood eosinophils was slightly older, had a higher proportion of males, and had a smaller percentage of current smokers compared with the other groups.Citation8
In an 8-year follow-up of patients with COPD and matched non-COPD controls, the percentage of patients with COPD and persistently high eosinophil counts (≥340 cells/µL) was 80% at 6 months and 30% at 4 years, declining to 18% at 8 years.Citation64 Over the entire follow-up period, the stability of eosinophil counts was significantly lower in patients with COPD versus controls and in patients with COPD with higher versus lower eosinophil counts (≥340 vs <340 cells/µL, respectively). The authors suggested that the decreased eosinophil-count stability seen in patients with COPD may be related to variations in disease stability (eg, exacerbations) or pharmacologic therapy (eg, oral CS courses) that would not be seen in the non-COPD controls.Citation64
A secondary analysis of the Acute Exacerbation and Respiratory InfectionS in COPD (AERIS) cohort examined the stability of eosinophilic inflammation in COPD, and found that blood eosinophil counts ≥2% were stable (≤1 visit with count <2% allowed) over a year in 58% of patients and predicted an overall lower risk of bacterial presence at exacerbation.Citation68 Furthermore, the subset of patients who underwent bronchoscopy in the Subpopulations And Intermediate Outcomes in COPD Study (SPIROMICS; NCT1969344)Citation69 showed significantly increased BAL eosinophils for current smokers with COPD compared with former smokers with and without COPD, as well as current smokers without COPD.Citation70 Other large observational studies – Genetic Epidemiology of COPD (COPDGene; NCT0608764)Citation71 and The Novel Observational Longitudinal Study (NOVELTY; NCT2760329)Citation72 – that are currently under way will further characterize patients with COPD and healthy volunteers and provide more insight into the eosinophil profiles of these populations.
Eosinophilic inflammation in COPD
Relationship to exacerbations of COPD
A growing body of evidence supports an association between high eosinophil concentrations and exacerbations of COPD.Citation3,Citation10,Citation33,Citation55,Citation73 Some studies have noted greater eosinophil counts during exacerbations compared with periods of stable disease,Citation3 while others have reported an association between greater eosinophil counts and an increased exacerbation risk.Citation33,Citation55,Citation73 In a 1-year observational study in which 86 patients had 182 COPD exacerbations, four distinct clusters of exacerbation type were identified: bacterial, viral, eosinophilic, and pauci-inflammatory.Citation10 Overall, 55% of exacerbations were bacteria-associated, 29% were virus-associated, and 28% were associated with sputum eosinophilia (descriptions were not mutually exclusive; the pauci-inflammatory cluster was defined by limited changes in inflammatory profile). The inflammatory profile associated with the eosinophilic cluster in this study included sputum CCL17, CCL13, and IL5 and serum CCL17, CXCL11, and IL5.Citation10 As noted earlier, eosinophils migrate to the lungs under the influence of cytokines (IL5 in particular) and specific chemotactic factors (such as eotaxin 1 and RANTES), along with CRTH2 and prostaglandin D2Citation12,Citation17,Citation18 (). Once in the lungs, eosinophil-derived, proinflammatory mediators, including basic proteins, cytokines, and growth factors, promote persistent inflammation,Citation12,Citation14 which contributes to exacerbations. Viral and/or bacterial infections are found in a majority of exacerbations.Citation74
Eosinophil counts vary between stable and exacerbated COPD states. In a study by Fujimoto et al, sputa from healthy nonsmokers, patients with stable COPD (no exacerbation during the 2- to 3-year study period), and patients with unstable COPD (≥1 exacerbations during the study) were examined. Among unstable COPD patients, sputa were examined during stable (defined as stable condition for ≥3 months with normal day-to-day variations and without signs of airway infection) and exacerbation phases.Citation75 Significant increases in eosinophil concentrations were found in both stable- and unstable-COPD cohorts compared with healthy nonsmokers. Greater concentrations of eosinophil chemotactic factors were found in sputa from patients with unstable COPD during an exacerbation compared with a stable phase and compared with patients with stable COPD and healthy nonsmokers.Citation75 In another study of patients who had an acute COPD exacerbation, those with eosinophilia (≥2% or ≥300 cells/µL) had a significantly shorter length of stay (5 days) and reduced early treatment failure rate (10.3%) compared with patients who did not have elevated eosinophils (7 days and 27.4%, respectively); however, relapse was significantly more frequent in the eosinophilic group (72% vs 42.2%).Citation76
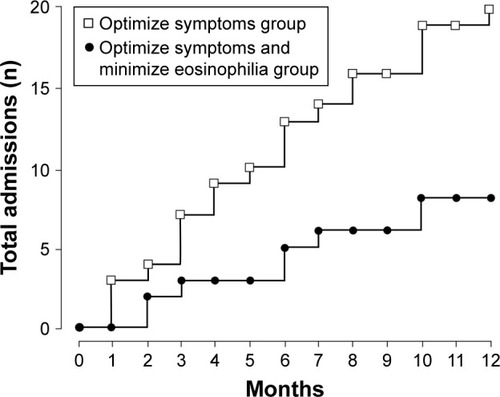
Evaluation of ICS efficacy for treating exacerbations in COPD patients with elevated eosinophil counts supports an association between eosinophils and exacerbations, although results between studies are conflicting. Cheng et al found in a post hoc analysis of 248 patients with COPD treated with medium-or high-dose ICS in combination with a LABA. that a higher percentage of patients with baseline (prerandomization) blood eosinophil counts ≥3% had a history of acute exacerbations with ≥1 hospitalizations in the prior year (before study start) compared with patient baseline blood eosinophil counts <3% (27.1% vs 7.4%, respectively).Citation77 In a study of 82 patients with COPD, Siva et al found that treating patients both to minimize eosinophilic airway inflammation based on induced sputum eosinophil count (ie, adjusting ICS therapy based on predetermined eosinophil thresholds) and optimize symptom control was associated with a greater reduction in severe exacerbations compared with treating patients only to minimize symptomsCitation78 (). Conversely, Barnes et al found in the Inhaled Steroids in Obstructive Lung Disease in Europe (ISOLDE) study of patients with moderate to severe COPD who received ICS treatment, that the percent reduction in the overall exacerbation rate for fluticasone propionate versus placebo was higher in patients with blood eosinophil levels <2% versus ≥2%.Citation57 The difference between the results of these two studies might be attributable to differing methodologies: the Siva et al studyCitation78 optimized anti-inflammatory treatment based on the level of sputum eosinophils, whereas a fixed dose of ICS plus as-needed short-acting bronchodilator therapy was used in the study reported by Barnes et al.Citation57 Kolsum et al recently demonstrated clear differences in markers for airway inflammation in 20 COPD patients with high blood eosinophil concentrations (>250 cells/µL) compared with 21 patients with blood eosinophil concentrations of <150 cells/µL. Although the study involved a small number of patients (n=41), it provides insight into how inflammatory processes in the lung, driven in part by eosinophils, could result in differential response in patients to ICS therapy.Citation61
Figure 2 Hospital admissions due to exacerbations in COPD patients treated to optimize symptoms alone or in combination with minimizing eosinophil counts.
Abbreviations: CS, corticosteroid; ICS, inhaled corticosteroid; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist.
Relationship to pneumonia
A few studies have examined the effect of blood eosinophil count on the incidence of pneumonia in patients with COPD.Citation50,Citation77 Cheng and Lin found that patients with COPD and high eosinophil levels (≥3%) responded to ICS/LABA therapy better (greater improvement in percentage-predicted forced expiratory volume in 1 second (FEV1), quality of life, frequency of annual exacerbations) than those with concentrations <3%; however, no difference in pneumonia incidence in response to ICS/LABA treatment was observed based on high and low eosinophil concentrations.Citation77 Results from a much larger retrospective study showed that patients with COPD had slightly fewer episodes of pneumonia if they had a blood eosinophil count of ≥2% versus <2%, regardless of whether their medication regimen included an ICS.Citation50 The authors speculated that patients with eosinophil counts ≥2% may have better capacity for clearing the lungs of infections before progressing to pneumonia than patients with <2% eosinophil levels, due to the antimicrobial defense role of eosinophils. Consistent with this proposal, an inverse relationship between bacterial infection and blood eosinophil counts has been observed in patients with COPD in an exacerbated state. This further suggests that the ICS responsiveness observed with higher eosinophil counts may be affected by bacterial levels, with lower levels of infection associated with ICS responsiveness.Citation79 Indeed, in a recent clinical study, an increase in bacterial load was observed in patients with stable, moderate COPD who received LABA/ICS therapy and had low blood or sputum eosinophils (≤2%) at baseline. Increases were not observed in LABA + ICS-treated patients with higher eosinophil levels at baseline or those treated with LABA alone, regardless of eosinophil level at baseline.Citation80
Relationship to lung function
The exact nature of the relationship between eosinophilic inflammation and decrements or rates of decline in lung function in patients with COPD remains unclear.Citation3 In a small sample of patients with moderate–severe COPD (n=10), lower FEV1 values were associated with greater sputum concentrations of both eosinophils and eosinophil cationic protein (a biomarker of eosinophilic inflammation), potentially suggesting a link between airway eosinophilia and airflow obstruction.Citation66 Similarly, in comparison with asymptomatic smokers, COPD and chronic bronchitis, defined by an FEV1 <80% (predicted) in addition to symptoms of chronic bronchitis, has been associated with an increased ratio of activated eosinophils to total eosinophils infiltrating large-airway subepithelia.Citation81 In COPD patients with emphysema (n=16) versus current smokers without emphysema (n=8), significantly higher levels of eosinophil markers (eosinophil cationic protein and eotaxin 1) were observed in BAL fluid.Citation82 In a subgroup analysis of the Feasibility of Retinoids for the Treatment of Emphysema (FORTE) study in patients with COPD with emphysema (n=27), high eotaxin 1 concentrations in BAL fluid (>50 pg/mL) and plasma (>1,300 pg/mL) were predictive of a rapid decline in lung function over 6 months (≥10% decrease in percentage-predicted FEV1).Citation83 In the COPD cohort (n=7,225) of the Copenhagen General Population Study, individuals with elevated blood eosinophil counts (≥340 cells/µL [threshold associated with an increased risk of exacerbations]) had slightly lower percentage-predicted FEV1 values than individuals with lower eosinophil counts.Citation33 The elevated blood eosinophil subgroup included a greater percentage of men, heavier smoking history, greater likelihood of experiencing wheezing during a cold, and greater probability of producing sputum for at least 3 months.Citation33 In contrast, in the ECLIPSE study (n=1,483), a subset of patients with blood eosinophil concentrations persistently ≥2% (n=554) had a higher mean FEV1 percentage predicted than patients with blood eosinophil concentrations persistently <2% (n=201, 51% vs 48%; P=0.009). They also tended to be slightly older and male.Citation8
ICS treatment may have a differential impact on lung function in patients with COPD, depending on whether eosinophil concentrations are normal or elevated. A post hoc analysis from the ISOLDE trial, evaluating fluticasone propionate versus placebo in 751 patients with moderate–severe COPD, reported that ICS-treated patients with elevated blood eosinophils (≥2%), but not those with blood eosinophils <2%, experienced a significantly lower rate of decline in FEV1 versus patients treated with placebo (P=0.003).Citation57 These results suggest that lung function in patients with high eosinophil levels is more responsive to ICS therapy.
ICS-treatment considerations in COPD
Role of eosinophils and pneumonia history
The current thinking on the role of ICS in COPD therapy has evolved, in part because of evidence suggesting an increased risk of pneumonia with ICS treatment in some patients,Citation84–Citation89 in addition to evidence of superior efficacy of a LABA–long-acting muscarinic antagonist (LAMA) combination compared to LABA–ICS in reducing exacerbations.Citation56 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 strategy recommends use of ICS in combination with a LABA in patients with COPD with exacerbations who have a history of asthma or findings suggestive of asthma–COPD overlap (ACO), or in combination with a LAMA and a LABA in those with further exacerbations despite treatment with dual long acting-bronchodilator therapy.Citation1 High eosinophil counts may also support use of ICS (in combination with bronchodilator therapy) in COPD; however, the GOLD statement notes the relationship is still under debate, and no specific recommendations for thresholds indicating eosinophilia are provided.Citation1
Impact of eosinophils on responses to ICS therapy
Across several studies, elevated eosinophil concentrations have been found to predict response to ICS in patients with COPD.Citation9,Citation55,Citation57,Citation90 In patients with blood eosinophil counts of ≥2%, ICS–LABA has significantly reduced exacerbation rates compared with placebo (P<0.001; Trial of Inhaled Steroids And Long-Acting β2 Agonists [TRISTAN] study) and with tiotropium (P=0.006; Investigating New Standards For Prophylaxis in Reducing Exacerbations [INSPIRE] study).Citation49,Citation52 In contrast, there were no significant differences in exacerbation rates between treatments in patients with blood eosinophil counts <2% in either study.Citation49 In addition, a post hoc analysis of the WISDOM trial showed that patients with high blood eosinophil counts tended to be more sensitive to withdrawal of ICS from a LABA–LAMA–ICS combination and had an increase in exacerbations when ICS therapy was stopped.Citation53 Exacerbation-rate differences between patients who were maintained on ICS therapy and those who stopped ICS therapy increased with increasing blood eosinophil thresholds; a significant treatment-by-subgroup interaction was seen at the 4% and 5% and 300 and 400 cells/µL thresholds only. The authors suggested that a threshold of ≥4% or ≥300 cells/µL may identify patients in whom ICS withdrawal from a triple combination may be detrimental.Citation53
In contrast, the Effect of Indacaterol Glycopyrronium vs Fluticasone Salmeterol on COPD Exacerbations (FLAME) study found no association between baseline eosinophil concentration and response to ICS treatment.Citation56 In this study, the annual rate of exacerbations was lower with indacaterol–glycopyrronium (LABA–LAMA) versus salmeterol–fluticasone (LABA–ICS), independently of baseline blood eosinophil concentration (<2% vs ≥2%).Citation56 Subsequent analyses of this study did not indicate a significant difference in the rate of exacerbations between the LABA–LAMA and LABA–ICS groups at higher baseline eosinophil thresholds (ie, ≥3%, ≥5%, or ≥300 cells/µL).Citation91 It would be of interest to investigate differences in treatment regimens based on high eosinophil levels in patients with a frequent-exacerbator phenotype (ie, ≥2 moderate or severe exacerbations or ≥1 severe [ie, hospitalized] exacerbations in the previous year). Because data analysis by eosinophil threshold in the FLAME study was presented for all exacerbations or combined moderate and severe exacerbations, it would be interesting to examine the results for moderate and for severe exacerbations separately. In that regard, the Copenhagen General Population study reported a significantly greater risk of severe exacerbations, but not of moderate exacerbations, in patients with COPD and a blood eosinophil count of ≥3.3% versus <3.3%.Citation33
Patients with ACO
Patients with COPD who also have clinical features associated with asthma have been described as having ACO. The overlap in clinical features varies, with some patients having more asthma symptoms, including evidence of TH2-mediated inflammation and increased eosinophils and/or mast cells, whereas others have more COPD symptoms and neutrophilic inflammation.Citation92–Citation94 ACO remains a controversial topic, and some experts consider it an oversimplification, with the entity more likely to comprise a spectrum of diseases and phenotypes than a single disorder.Citation95
At present, there is no clear definition of ACO. The 2017 Global Initiative for Asthma (GINA) strategy report described ACO as
characterized by persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.Citation96
The most current update (2017) to the Spanish COPD guidelines include specific criteria for identification of ACO.Citation101 To establish a diagnosis, a patient should fulfill the criteria for COPD (35 years of age or older, a smoking history, and a postbronchodilator FEV1/forced vital capacity <70%) and have an asthma diagnosis. In cases in which a diagnosis of asthma cannot be established, a marked response to a bronchodilator (FEV1 ≥15% and ≥400 mL) or an elevated blood eosinophil count of ≥300 cells/µL is diagnostic of ACO.Citation101 The most recent Czech Republic COPD guidelinesCitation98 and guidelines recently proposed by Cosío et alCitation99 also include specific diagnostic criteria for ACO similar to those in the Spanish guidelines, albeit with the inclusion of a recommended sputum eosinophil threshold concentration ≥3%Citation98 and blood eosinophil threshold concentrations >5%Citation99 in the Czech Republic and Cosío et al guidelines, respectively. Similarly to the 2017 Spanish guidelines, a recently proposed consensus definition included an eosinophil concentration ≥300 cells/µL as one possible minor criterion that can be used with major criteria (based on lung function, smoking history, or a history of asthma) to identify ACO.Citation95
Assessing risk with ICS: focus on pneumonia
ICS use is associated with certain risks that should be taken into account when assessing the benefit:risk ratio of ICS therapy. In particular, studies have reported that patients with COPD receiving ICS are at increased risk of developing pneumonia.Citation84–Citation89,Citation102 In addition, potential differences in risk for pneumonia may be affected by the specific ICS used.Citation86,Citation103,Citation104 Therefore, considering a patient’s prior history of pneumonia may be important when choosing ICS therapy as part of the COPD-treatment regimen.
Notably, the 2017 GOLD statement bases COPD treatment recommendations predominantly on the basis of burden of respiratory symptoms and history of exacerbations, while neither pneumonia history nor the presence of eosinophilia is factored into the COPD-treatment algorithm.Citation1 Therefore, we propose a new algorithm for optimizing treatment of COPD patients that takes these factors into consideration (). For COPD patients with high eosinophil counts and no history of pneumonia, ICS is recommended as part of therapy, as treatment benefits (improved lung function and reduced symptoms and rate of exacerbations)Citation1 are high and risk of pneumonia comparatively low. Whether or not ICS treatment should be recommended in patients with COPD with low eosinophil counts with or without a history of pneumonia is still not clear. In the situation of a patient with high eosinophil counts plus a history of pneumonia, the benefits and risks must be evaluated on a case-by-case basis. For example, an ICS may be considered in patients with high eosinophil counts and a history of pneumonia who continue to have exacerbations, particularly those leading to hospitalization, while on dual-bronchodilator therapy, assuming the benefit of decreasing future exacerbations outweighs the potential risks.
Table 2 Consideration for inclusion/exclusion of ICS as part of therapy
Emerging treatments for COPD with eosinophilic inflammation
Several therapeutics targeting eosinophils are in development ().Citation54,Citation105–Citation113 As previously discussed, IL5 is the principal eosinophilic regulatory cytokine that promotes the differentiation, survival, and activation of eosinophils.Citation13,Citation16 Treatments successfully targeting the IL5 pathway to reduce eosinophilic inflammation in asthma are now under investigation in COPD.
Table 3 Recent and ongoing trials for monoclonal antibody treatments targeting eosinophils in COPD
Mepolizumab is a humanized monoclonal antibody that targets the IL5 ligand and inhibits IL5-receptor signaling in eosinophils. Mepolizumab was approved by the US Food and Drug Administration (FDA) in 2015 as adjunct treatment in patients with severe asthma and eosinophilic inflammation.Citation114,Citation115 Clinical benefits of mepolizumab in patients with eosinophilic asthma include reductions in exacerbation rate and asthma symptoms,Citation116–Citation118 improvement in FEV1,Citation117 and reduced oral CS use.Citation116 Although an initial pilot studyCitation119 did not show significant improvement with mepolizumab in lung function or reductions in exacerbations in patients with COPD, results from two Phase III studies showed some improvements in exacerbation frequency in mepolizumab-treated patients who had an eosinophilic phenotype ().Citation105,Citation106,Citation113 In both of these Phase III studies, patients had COPD and had experienced moderate or severe exacerbations while using inhaled glucocorticoid-based triple therapy. The primary end point for both studies was the annual rate of exacerbations considered moderate (defined as leading to use of systemic glucocorticoids and/or antibiotics) or severe (defined as leading to hospitalization or death). In METREX,Citation113 patients were divided into an eosinophilic group (≥150 cells/µL at screening or ≥300 cells/µL any-time during the prior year) and a noneosinophilic group. Among patients with an eosinophilic phenotype, those treated with mepolizumab 100 mg had a significantly lower annual moderate/severe exacerbation rate compared with placebo-treated patients (1.40 vs 1.71; adjusted P=0.04); no treatment difference was observed in the total population. In METREO,Citation113 all patients were required to have an eosinophilic phenotype (as defined in METREX). Despite this, the annual moderate/severe exacerbation rate was not significantly different for the mepolizumab 100 mg, mepolizumab 300 mg, and placebo-treatment groups (1.19, 1.27, and 1.49, respectively). Together, results from the METREX and METREO studies indicate a role for eosinophils in COPD, but suggest that more study is needed to understand the mechanisms underlying COPD.
Reslizumab is a humanized anti-IL5 monoclonal antibody that was approved by the FDA in 2016 as an add-on maintenance treatment in adult patients with severe asthma and an eosinophilic phenotype.Citation120 Similarly to mepolizumab, it targets the IL5 ligand.Citation121 Two 52-week Phase III studies showed that reslizumab significantly reduced asthma exacerbations and increased FEV1 in patients with eosinophilic asthma (≥400 cells/µL).Citation122 Reslizumab has yet to be formally evaluated in clinical trials for COPD treatment.
Benralizumab is a humanized, afucosylated, monoclonal antibody targeting the IL5-receptor α-chain.Citation123 Benralizumab depletes eosinophils through antibody-dependent, cell-mediated cytotoxicity via apoptosis of eosinophils induced by activated natural-killer cells. In Phase III clinical studies in severe eosinophilic asthma (blood eosinophil counts of ≥300 cells/µLCitation123,Citation124 or ≥150 to <300 cells/µLCitation125), benralizumab significantly reduced the annual rate of exacerbations,Citation123 increased FEV1,Citation123,Citation124 reduced asthma symptoms,Citation123,Citation124 and had significant oral CS-sparing effects.Citation125 In a Phase IIA trial in patients with COPD with sputum eosinophilia (≥3%), benralizumab treatment did not significantly reduce the annual rate of moderate or severe acute exacerbations. However, significant improvements in FEV1 were observed in the benralizumab group, and the results of prespecified subgroup analyses by baseline blood eosinophil count with benralizumab versus placebo supported further examination of benralizumab in patients with COPD.Citation54 Ongoing Phase III trials evaluating benralizumab in COPD are described in .
Although IL13 has a more limited role in its scope of effects on eosinophils than IL5, it is a major factor mediating eosinophil recruitment to the lungs.Citation126,Citation127 Subcutaneous administration of the anti-IL13 monoclonal antibody lebrikizumab significantly increased mean FEV1 in asthma patients with high periostin blood concentrations versus placebo in a Phase II study.Citation128 However, inconsistent results of lebrikizumab were seen in two recent Phase III studies evaluating its effects on asthma exacerbations (primary end point) in patients with elevated blood biomarkers (≥50 ng/mL periostin or ≥300 eosinophils/µL), with one study (LAVOLTA I) yielding significant reductions in exacerbations and the other (LAVOLTA II) nonsignificant reductions.Citation129 A Phase II trial of lebrikizumab has been completed in patients with COPD ().Citation109 The anti-IL13 antibody tralokinumab (300 mg subcutaneously every 2 weeks [Q2W] to week 50 or Q2W to week 12 followed by Q4W to week 48) was investigated in a Phase IIB study (NCT1402986) in patients with severe uncontrolled asthma.Citation130 Significant improvements in FEV1 were observed with the Q2W regimen versus placebo, and post hoc analyses suggested possible benefits of tralokinumab treatment in a defined patient subpopulation (including those with elevated serum dipeptidyl peptidase 4 and/or periostin concentrations).Citation130 The potential utility of other antieosinophil targets for treatment of COPD (eg, anti-IL4-receptor monoclonal antibody [dupilumab],Citation131 CRTH2 antagonists [OC000459,Citation132–Citation135 fevipiprantCitation136]) are yet to be determined, as these agents are not currently being evaluated in COPD.
Conclusion
Eosinophils are mediators of inflammation in many patients with COPD. Although a widely accepted threshold to define eosinophilic inflammation in the context of COPD has not been firmly established, elevated eosinophil counts have been associated with reduced lung function and increased risk of exacerbations in patients with COPD. Moreover, a reduction in eosinophilic inflammation is associated with reduced exacerbation frequency. Patients with COPD who have evidence of eosinophilic inflammation generally respond better to ICS treatment. Increased eosinophil counts in the blood may be an acceptable surrogate for airway eosinophilia and may serve as a readily accessible biomarker for ICS-treatment response in COPD. Emerging therapies targeting eosinophil chemotactic and survival factors, such as monoclonal antibodies targeting the IL5 ligand, the IL5 receptor, IL4 receptor, and the IL13 ligand, have been studied in asthma, and some are beginning to be studied in COPD, where they may prove to be effective treatments.
Author contributions
Both authors contributed to the development and critical revision of this manuscript, approved the final version to be published, and agree to be accountable for all aspects of the information contained in this review.
Acknowledgments
We thank Beverly E Barton, PhD, Devin S Gary, PhD, and Stella Chow, PhD of Scientific Connexions, an Ashfield company, part of UDG Healthcare PLC (Lyndhurst, NJ, USA), and Courtney St Amour, PhD, of MedErgy Health-Group Inc (Yardley, PA, USA) for medical writing support funded by AstraZeneca LP (Wilmington, DE, USA).
Disclosure
DPT has served on advisory boards for AstraZeneca, Novartis, and Sunovion, as a speaker for Boehringer-Ingelheim, and as a consultant for Theravance/Innoviva. MEW has served as a consultant for Ambit Bioscience, AstraZeneca, Boehringer-Ingelheim, BSCI, Genentech, Gilacure, GlaxoSmithKline, Meda, Mylan, Neurotronic, Novartis, Regeneron, Sanofi, Sunovion, Teva, Theravance, Tunitas, and Vectura, received research funding from Sanofi, served on an advisory board for Teva, was a DSMB member for Sentien, and received honoraria from BSCI. The authors report no other conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for the Diagnosis, Management, and Prevention of COPD. Bethesda (MD): GOLD2017 Available from: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/Accessed December 14, 2017
- KimVRogersTJCrinerGJNew concepts in the pathobiology of chronic obstructive pulmonary diseaseProc Am Thorac Soc20085447848518453359
- SahaSBrightlingCEEosinophilic airway inflammation in COPDInt J Chron Obstruct Pulmon Dis200611394718046901
- CukicVLovreVDragisicDUstamujicAAsthma and chronic obstructive pulmonary disease (COPD): differences and similaritiesMater Sociomed201224210010523678316
- EltboliOBafadhelMHollinsFCOPD exacerbation severity and frequency is associated with impaired macrophage efferocytosis of eosinophilsBMC Pulm Med20141411225007795
- PizzichiniEPizzichiniMMGibsonPSputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitisAm J Respir Crit Care Med19981585 Pt 1151115179817701
- LeighRPizzichiniMMMorrisMMMaltaisFHargreaveFEPizzichiniEStable COPD: predicting benefit from high-dose inhaled corticosteroid treatmentEur Respir J200627596497116446316
- SinghDKolsumUBrightlingCELocantoreNAgustiATal-SingerREosinophilic inflammation in COPD: prevalence and clinical characteristicsEur Respir J20144461697170025323230
- PascoeSLocantoreNDransfieldMTBarnesNCPavordIDBlood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trialsLancet Respir Med20153643544225878028
- BafadhelMMcKennaSTerrySAcute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkersAm J Respir Crit Care Med2011184666267121680942
- JacobsenEAHelmersRALeeJJLeeNAThe expanding role(s) of eosinophils in health and diseaseBlood2012120193882389022936660
- GeorgeLBrightlingCEEosinophilic airway inflammation: role in asthma and chronic obstructive pulmonary diseaseTher Adv Chronic Dis201671345126770668
- HoganSPRosenbergHFMoqbelREosinophils: biological properties and role in health and diseaseClin Exp Allergy200838570975018384431
- DavoineFLacyPEosinophil cytokines, chemokines, and growth factors: emerging roles in immunityFront Immunol2014557025426119
- LuijkBLindemansCAKantersDGradual increase in priming of human eosinophils during extravasation from peripheral blood to the airways in response to allergen challengeJ Allergy Clin Immunol20051155997100315867857
- MolfinoNAGossageDKolbeckRParkerJMGebaGPMolecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptorClin Exp Allergy201242571273722092535
- ConroyDMWilliamsTJEotaxin and the attraction of eosinophils to the asthmatic lungRespir Res20012315015611686879
- SmitJJLukacsNWA closer look at chemokines and their role in asthmatic responsesEur J Pharmacol20065331–327728816464446
- MoqbelRLevi-SchafferFKayABCytokine generation by eosinophilsJ Allergy Clin Immunol1994946 Pt 2118311887798558
- KitaHThe eosinophil: a cytokine-producing cell?J Allergy Clin Immunol19969748898928655882
- ZieglerSFRoanFBellBDStoklasekTAKitajimaMHanHThe biology of thymic stromal lymphopoietin (TSLP)Adv Pharmacol20136612915523433457
- RedhuNSGounniASFunction and mechanisms of TSLP/TSLPR complex in asthma and COPDClin Exp Allergy2012427994100522168549
- RutgersSRTimensWKaufmannHFvan der MarkTWKoëterGHPostmaDSComparison of induced sputum with bronchial wash, bronchoalveolar lavage and bronchial biopsies in COPDEur Respir J200015110911510678630
- MeyerKCRaghuGBaughmanRPAn official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung diseaseAm J Respir Crit Care Med201218591004101422550210
- BrightlingCEBraddingPSymonFAHolgateSTWardlawAJPavordIDMast-cell infiltration of airway smooth muscle in asthmaN Engl J Med2002346221699170512037149
- GrootendorstDCSontJKWillemsLNComparison of inflammatory cell counts in asthma: induced sputum vs bronchoalveolar lavage and bronchial biopsiesClin Exp Allergy19972777697799249269
- DuhSHCookJDLaboratory reference range values2005 Available from: www.stedmansonline.com/webFiles/Dict-Stedmans28/APP17.pdfAccessed December 6, 2017
- Medical Council of CanadaClinical laboratory tests – normal values Available from: http://mcc.ca/objectives/normal-valuesAccessed December 6, 2017
- Medline PlusBlood differential test Available from: https://medline-plus.gov/ency/article/003657.htmAccessed December 6, 2017
- WinkelPStatlandBESaundersAMOsbornHKuppermanHWithin-day physiologic variation of leukocyte types in healthy subjects as assayed by two automated leukocyte differential analyzersAm J Clin Pathol19817556937007234755
- ZacharskiLRElvebackLRKinmanJWLeukocyte counts in healthy adultsAm J Clin Pathol19715621481505567719
- YoungBLoweJOStevensAHeathJWWheater’s Functional Histology6th edPhiladelphiaChurchill Livingstone2014
- Vedel-KroghSNielsenSFLangePVestboJNordestgaardBGBlood eosinophils and exacerbations in chronic obstructive pulmonary disease: the Copenhagen general population studyAm J Respir Crit Care Med2016193996597426641631
- ChisaleMRKumwendaPNgwiraMM’BayaBChosamataBIMwapasaVA pilot study to determine the normal haematological indices for young Malawian adults in Blantyre, MalawiMalawi Med J20152739610026715954
- WangBYanLYaoZRoskosLKPopulation pharmacokinetics and pharmacodynamics of benralizumab in healthy volunteers and patients with asthmaCPT Pharmacometrics Syst Pharmacol20176424925728109128
- BalbiBPignattiPCorradiMBronchoalveolar lavage, sputum and exhaled clinically relevant inflammatory markers: values in healthy adultsEur Respir J200730476978117906085
- DavidsonWJTheSLeighREstablishing a normal range for induced sputum cell counts in western CanadaCan Respir J201320642442524083301
- NegewoNAMcDonaldVMBainesKJPeripheral blood eosinophils: a surrogate marker for airway eosinophilia in stable COPDInt J Chron Obstruct Pulmon Dis2016111495150427445469
- KreindlerJLWatkinsMLLettisSTal-SingerRLocantoreNEffect of inhaled corticosteroids on blood eosinophil count in steroid-naïve patients with COPDBMJ Open Respir Res201631e000151
- BrightlingCEMonteiroWWardRSputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trialLancet200035692401480148511081531
- RoyKSmithJKolsumUBorrillZVestboJSinghDCOPD phenotype description using principal components analysisRespir Res2009104119480658
- BafadhelMMcKennaSTerrySBlood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trialAm J Respir Crit Care Med20121861485522447964
- LacosteJYBousquetJChanezPEosinophilic and neutrophilic inflammation in asthma, chronic bronchitis, and chronic obstructive pulmonary diseaseJ Allergy Clin Immunol19939245375488409114
- AminiMBashirovaDPrinsBPEosinophil count is a common factor for complex metabolic and pulmonary traits and diseases: the LifeLines Cohort StudyPLoS One20161112e016848027978545
- SennelsHPJørgensenHLHansenALGoetzeJPFahrenkrugJDiurnal variation of hematology parameters in healthy young males: the Bispebjerg study of diurnal variationsScand J Clin Lab Invest201171753254121988588
- SpectorSLTanRAIs a single blood eosinophil count a reliable marker for “eosinophilic asthma”?J Asthma201249880781022900679
- BasantaMIbrahimBDockryRExhaled volatile organic compounds for phenotyping chronic obstructive pulmonary disease: a cross-sectional studyRespir Res2012137222916684
- BrightlingCEMcKennaSHargadonBSputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary diseaseThorax200560319319815741434
- PavordIDLettisSLocantoreNBlood eosinophils and inhaled corticosteroid/long-acting β-2 agonist efficacy in COPDThorax201671211812526585525
- PavordIDLettisSAnzuetoABarnesNBlood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: a patient-level meta-analysisLancet Respir Med20164973174127460163
- OrtegaHKatzLGunsoyNKeeneOYanceySBlood eosinophil counts predict treatment response in patients with severe eosinophilic asthmaJ Allergy Clin Immunol2015136382582626194540
- SchleichFBrusselleGLouisRHeterogeneity of phenotypes in severe asthmatics: the Belgian Severe Asthma Registry (BSAR)Respir Med2014108121723173225456708
- WatzHTetzlaffKWoutersEFBlood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trialLancet Respir Med20164539039827066739
- BrightlingCEBleeckerERPanettieriRABenralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2A studyLancet Respir Med201421189190125208464
- SiddiquiSHGuasconiAVestboJBlood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2015192452352526051430
- WedzichaJABanerjiDChapmanKRIndacaterol-glycopyrronium versus salmeterol-fluticasone for COPDN Engl J Med2016374232222223427181606
- BarnesNSharmaRLettisSCalvarleyPBlood eosinophils as a marker of response to inhaled corticosteroids in COPDEur Respir J2016471299130327132257
- de GrootJCStormHAmelinkMClinical profile of patients with adult-onset eosinophilic asthmaERJ Open Res20162200100201527730197
- EltboliOMistryVBarkerBBrightlingCERelationship between blood and bronchial submucosal eosinophilia and reticular basement membrane thickening in chronic obstructive pulmonary diseaseRespirology201520466767025645275
- HastieAAlexisNDoerschuckCBlood eosinophils poorly correlate with sputum eosinophils, and have few associations with spirometry, clinical and quantitated computed tomography measures compared to sputum eosinophils in the SPIROMICS cohortAm J Respir Crit Care Med2016139Suppl Available from:http://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2016.193.1_MeetingAbstracts.A6168Accessed December 12, 2017
- KolsumUDameraGPhamTHPulmonary inflammation in patients with chronic obstructive pulmonary disease with higher blood eosinophil countsJ Allergy Clin Immunol201714041181.e71184.e728506852
- TuratoGSemenzatoUBazzanEBlood eosinophilia does not reflect tissue eosinophils nor worsen clinical outcomes in COPDAm J Respir Crit Care Med Epub20171016
- RavinKALoyMThe eosinophil in infectionClin Rev Allergy Immunol201650221422726690368
- OshagbemiOABurdenAMBraekenDCWStability of blood eosinophils in patients with chronic obstructive pulmonary disease and in control subjects, and the impact of sex, age, smoking, and baseline countsAm J Respir Crit Care Med2017195101402140428165763
- MoermansCBonnetCWillemsESputum cytokine levels in patients undergoing hematopoietic SCT and comparison with healthy subjects and COPD: a pilot studyBone Marrow Transplant201449111382138825068427
- BalzanoGStefanelliFIorioCEosinophilic inflammation in stable chronic obstructive pulmonary disease: relationship with neutrophils and airway functionAm J Respir Crit Care Med19991605 Pt 11486149210556110
- MoermansCHeinenVNguyenMLocal and systemic cellular inflammation and cytokine release in chronic obstructive pulmonary diseaseCytokine201156229830421880505
- KimVLCoombsNAStaplesKJImpact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohortEur Respir J2017504
- University of North CarolinaStudy of COPD subgroups and biomarkers (SPIROMICS) Available from: https://clinicaltrials.gov/ct2/show/NCT1969344. NLM identifier: NCT1969344Accessed December 12, 2017
- MartinezCHLiSXHirzelAJAlveolar eosinophilia in current smokers with chronic obstructive pulmonary disease in the SPIROMICS cohortJ Allergy Clin Immunol Epub2017912
- Brigham and Women’s HospitalExamining the genetic factors that may cause chronic obstructive pulmonary disease (COPD) (COPDGene) Available from: https://clinicaltrials.gov/ct2/show/NCT0608764. NLM identifier: NCT0608764Accessed December 12, 2017
- AstraZenecaObservational study of obstructive lung disease (NOVELTY) Available from: https://clinicaltrials.gov/ct2/show/NCT2760329. NLM identifier: NCT2760329Accessed December 12, 2017
- PriceDRigazioAPostmaDBlood eosinophilia and the number of exacerbations in COPD patientsEur Respir J201444Suppl 584416
- PapiABellettatoCMBraccioniFInfections and airway inflammation in chronic obstructive pulmonary disease severe exacerbationsAm J Respir Crit Care Med2006173101114112116484677
- FujimotoKYasuoMUrushibataKHanaokaMKoizumiTKuboKAirway inflammation during stable and acutely exacerbated chronic obstructive pulmonary diseaseEur Respir J200525464064615802337
- PrinsHJDuijkersRLutterRBlood eosinophilia as a marker of early and late treatment failure in severe acute exacerbations of COPDRespir Med201713111812428947018
- ChengSLLinCHEffectiveness using higher inhaled corticosteroid dosage in patients with COPD by different blood eosinophilic countsInt J Chron Obstruct Pulmon Dis2016112341234827703344
- SivaRGreenRHBrightlingCEEosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trialEur Respir J200729590691317301099
- KolsumUDonaldsonGCSinghRBlood and sputum eosinophils in COPD: relationship with bacterial loadRespir Res20171818828482840
- ContoliMPaulettiARossiMRLong-term effects of inhaled corticosteroids on sputum bacterial and viral loads in COPDEur Respir J2017504170045128982774
- LamsBESousaARReesPJLeeTHSubepithelial immunopathology of the large airways in smokers with and without chronic obstructive pulmonary diseaseEur Respir J200015351251610759445
- MillerMRamsdellJFriedmanPJChoJYRenvallMBroideDHComputed tomographic scan-diagnosed chronic obstructive pulmonary disease-emphysema: eotaxin-1 is associated with bronchodilator response and extent of emphysemaJ Allergy Clin Immunol200712051118112517983873
- D’ArmientoJMScharfSMRothMDEosinophil and T cell markers predict functional decline in COPD patientsRespir Res20091011319925666
- JansonCLarssonKLisspersKHPneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting β2 agonist: observational matched cohort study (PATHOS)BMJ2013346f330623719639
- KewKMSeniukovichAInhaled steroids and risk of pneumonia for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20143CD01011524615270
- MorjariaJBRigbyAMoriceAHInhaled corticosteroid use and the risk of pneumonia and COPD exacerbations in the UPLIFT studyLung2017195328128828255905
- SinghSLokeYKRisk of pneumonia associated with long-term use of inhaled corticosteroids in chronic obstructive pulmonary disease: a critical review and updateCurr Opin Pulm Med201016211812219926996
- SuissaSPatenaudeVLapiFErnstPInhaled corticosteroids in COPD and the risk of serious pneumoniaThorax201368111029103624130228
- WangCYLaiCCYangWCThe association between inhaled corticosteroid and pneumonia in COPD patients: the improvement of patients’ life quality with COPD in Taiwan (IMPACT) studyInt J Chron Obstruct Pulmon Dis2016112775278327877031
- HindsDRDiSantostefanoRLLeHVPascoeSIdentification of responders to inhaled corticosteroids in a chronic obstructive pulmonary disease population using cluster analysisBMJ Open201666e010099
- RocheNChapmanKRVogelmeierCFBlood eosinophils and response to maintenance chronic obstructive pulmonary disease treatment: data from the FLAME trialAm J Respir Crit Care Med201719591189119728278391
- PostmaDSRabeKFThe asthma-COPD overlap syndromeN Engl J Med2015373131241124926398072
- LouisRECataldoDBuckleyMGEvidence of mast-cell activation in a subset of patients with eosinophilic chronic obstructive pulmonary diseaseEur Respir J200220232533112212963
- SoltaniAEweYPLimZSMast cells in COPD airways: relationship to bronchodilator responsiveness and angiogenesisEur Respir J20123961361136722034650
- SinDDMiravitllesMManninoDMWhat is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussionEur Respir J201648366467327338195
- Global Initiative for Asthma (GINA)Global Strategy for Asthma Management and Prevention2017 Available from: http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/Accessed December 13, 2017
- Soler-CataluñaJJCosíoBIzquierdoJLConsensus document on the overlap phenotype COPD-asthma in COPDArch Bronconeumol201248933133722341911
- KoblizekVChlumskyJZindrVChronic obstructive pulmonary disease: official diagnosis and treatment guidelines of the Czech Pneu-mological and Phthisiological Society; a novel phenotypic approach to COPD with patient-oriented careBiomed Pap Med Fac Univ Palacky Olomouc Czech Repub2013157218920123733084
- CosíoBGSorianoJBLopez-CamposJLDefining the asthma-COPD overlap syndrome in a COPD cohortChest20161491455226291753
- CosíoBGde LlanoLPViñaALTh-2 signature in chronic airway diseases: towards the extinction of asthma-COPD overlap syndrome?Eur Respir J2017495160239728461299
- MiravitllesMSoler-CataluñaJJCalleMSpanish guidelines for management of chronic obstructive pulmonary disease (GesEPOC) 2017: pharmacological treatment of stable phaseArch Bronconeumol201753632433528477954
- WedzichaJACalverleyPMSeemungalTAThe prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromideAm J Respir Crit Care Med20081771192617916806
- HollisSJorupCLythgoeDMartenssonGRegnellPEckerwallGRisk of pneumonia with budesonide-containing treatments in COPD: an individual patient-level pooled analysis of interventional studiesInt J Chron Obstruct Pulmon Dis2017121071108428435240
- SinDDTashkinDZhangXBudesonide and the risk of pneumonia: a meta-analysis of individual patient dataLancet2009374969171271919716963
- GlaxoSmithKlineEfficacy and safety of mepolizumab as an add-on treatment in COPD Available from: https://clinicaltrials.gov/ct2/show/NCT2105961. NLM identifier: NCT2105961Accessed December 14, 2017
- GlaxoSmithKlineStudy to evaluate efficacy and safety of mepolizumab for frequently exacerbating COPD patients Available from: https://clinicaltrials.gov/ct2/show/NCT2105948. NLM identifier: NCT2105948Accessed December 14, 2017
- AstraZenecaEfficacy and safety of benralizumab in patients with moderate to very severe COPD with exacerbation history (TERRANOVA) Available from: https://clinicaltrials.gov/ct2/show/NCT2155660. NLM identifier: NCT2155660Accessed December 14, 2017
- AstraZenecaBenralizumab efficacy in moderate to very severe COPD with exacerbation history (GALATHEA) Available from: https://clinicaltrials.gov/ct2/show/NCT2138916. NLM identifier: NCT2138916Accessed December 14, 2017
- Hoffmann-La RocheA study to evaluate safety and efficacy of lebrikizumab in patients with chronic obstructive pulmonary disease Available from: https://clinicaltrials.gov/ct2/show/NCT2546700. NLM identifier: NCT2546700Accessed December 14, 2017
- CorrenJWeinsteinSJankaLZangrilliJGarinMPhase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil countsChest2016150479981027018175
- DasguptaAKjarsgaardMCapaldiDA pilot randomised clinical trial of mepolizumab in COPD with eosinophilic bronchitisEur Respir J2017493160248628298405
- GlaxoSmithKlineGSK announces headline phase III results of mepolizumab in patients with severe chronic obstructive pulmonary disease [press release]LondonGSK2017 [May 10]. Available from: https://www.gsk.com/en-gb/media/press-releases/gsk-announces-headline-phase-iii-results-of-mepolizumab-in-patients-with-severe-chronic-obstructive-pulmonary-diseaseAccessed December 14, 2017
- PavordIDChanezPCrinerGJMepolizumab for eosinophilic chronic obstructive pulmonary diseaseN Engl J Med2017377171613162928893134
- KeatingGMMepolizumab: First Global ApprovalDrugs201575182163216926603873
- NUCALA [package insert]Research Triangle Park, NCGlaxo-SmithKline122017 Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Nucala/pdf/NUCALA-PI-PIL.pdfAccessed December 14, 2017
- BelEHWenzelSEThompsonPJOral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthmaN Engl J Med2014371131189119725199060
- OrtegaHGLiuMCPavordIDMepolizumab treatment in patients with severe eosinophilic asthmaN Engl J Med2014371131198120725199059
- PavordIDKornSHowarthPMepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trialLancet2012380984265165922901886
- NairPDasguptaAKjarsgaardMMepolizumab in COPD with eosinophilic bronchitis: a randomized clinical trialJ Allergy Clin Immunol20161372AS392
- CINQAIR® [package insert]Frazer, PATeva Respiratory, LLC2016
- CastroMMathurSHargreaveFReslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled studyAm J Respir Crit Care Med2011184101125113221852542
- CastroMZangrilliJWechslerMEReslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trialsLancet Respir Med20153535536625736990
- FitzGeraldJMBleeckerERNairPBenralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trialLancet2016388100562128214127609406
- BleeckerERFitzGeraldJMChanezPEfficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trialLancet2016388100562115212727609408
- NairPWenzelSRabeKFOral glucocorticoid-sparing effect of benralizumab in severe asthmaN Engl J Med2017376252448245828530840
- FajtMLWenzelSEAsthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized careJ Allergy Clin Immunol2015135229931125662302
- PopeSMBrandtEBMishraAIL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanismJ Allergy Clin Immunol2001108459460111590387
- CorrenJLemanskeRFHananiaNALebrikizumab treatment in adults with asthmaN Engl J Med2011365121088109821812663
- HananiaNAKorenblatPChapmanKREfficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trialsLancet Respir Med201641078179627616196
- BrightlingCEChanezPLeighREfficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2B trialLancet Respir Med20153969270126231288
- WenzelSCastroMCorrenJDupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2B dose-ranging trialLancet201638810039314427130691
- BarnesNPavordIChuchalinAA randomized, double-blind, placebo-controlled study of the CRTH2 antagonist OC000459 in moderate persistent asthmaClin Exp Allergy2012421384821762224
- PettipherRHunterMGPerkinsCMHeightened response of eosinophilic asthmatic patients to the CRTH2 antagonist OC000459Allergy20146991223123224866478
- SinghDCaddenPHunterMInhibition of the asthmatic allergen challenge response by the CRTH2 antagonist OC000459Eur Respir J2013411465222496329
- StraumannAHoesliSBussmannCAnti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitisAllergy201368337538523379537
- GonemSBerairRSingapuriAFevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trialLancet Respir Med20164969970727503237
- McMaster UniversityMepolizumab in chronic obstructive pulmonary diseases (COPD) with eosinophilic bronchitis Available from: https://clinicaltrials.gov/ct2/show/NCT1463644. NLM identifier: NCT1463644Accessed December 14, 2017
- MedImmune LLCA study to evaluate the effectiveness of MEDI-563 in subjects with chronic obstructive pulmonary disease (COPD) Available from: https://clinicaltrials.gov/ct2/show/NCT1227278. NLM identifier: NCT1227278Accessed December 14, 2017
