Abstract
Background
Pulmonary tuberculosis (PTB) is a risk factor for COPD, but the clinical characteristics and the chest imaging features (emphysema and bronchiectasis) of COPD with previous PTB have not been studied well.
Methods
The presence, distribution, and severity of emphysema and bronchiectasis in COPD patients with and without previous PTB were evaluated by high-resolution computed tomography (HRCT) and compared. Demographic data, respiratory symptoms, lung function, and sputum culture of Pseudomonas aeruginosa were also compared between patients with and without previous PTB.
Results
A total of 231 COPD patients (82.2% ex- or current smokers, 67.5% male) were consecutively enrolled. Patients with previous PTB (45.0%) had more severe (p=0.045) and longer history (p=0.008) of dyspnea, more exacerbations in the previous year (p=0.011), and more positive culture of P. aeruginosa (p=0.001), compared with those without PTB. Patients with previous PTB showed a higher prevalence of bronchiectasis (p<0.001), which was more significant in lungs with tuberculosis (TB) lesions, and a higher percentage of more severe bronchiectasis (Bhalla score ≥2, p=0.031), compared with those without previous PTB. The overall prevalence of emphysema was not different between patients with and without previous PTB, but in those with previous PTB, a higher number of subjects with middle (p=0.001) and lower (p=0.019) lobe emphysema, higher severity score (p=0.028), higher prevalence of panlobular emphysema (p=0.013), and more extensive centrilobular emphysema (p=0.039) were observed. Notably, in patients with TB lesions localized in a single lung, no difference was found in the occurrence and severity of emphysema between the 2 lungs.
Conclusion
COPD patients with previous PTB had unique features of bronchiectasis and emphysema on HRCT, which were associated with significant dyspnea and higher frequency of severe exacerbations. While PTB may have a local effect on bronchiectasis, its involvement in airspace damage in COPD may be extensive, probably through interactions with cigarette smoke.
Background
Both COPD and tuberculosis (TB) are leading causes of morbidity and mortality in developing countries.Citation1,Citation2 The two diseases not only share common risk factors such as smoking and poor socioeconomic status, but also coexist and interact in disease development and manifestation.Citation1–Citation3 Although pulmonary TB (PTB) has been identified as a risk factor for COPD,Citation4 and several studies have found an association between previous PTB and higher COPD prevalence,Citation5–Citation7 few studies have explored the clinical and radiological features of COPD in patients with previous PTB.
The chronic inflammatory response and long-term anatomic alterations induced by PTB were believed to be the main pathological basis for the impairment of lung function and poor prognosis.Citation8 TB-associated structural alterations, such as scar formation, bronchial stenosis, and bronchiectasis,Citation9 affect both the development of COPD and disease manifestation, which may have unique features compared with COPD without previous PTB. Recently, radiological phenotypes of COPD have received much attention, and the presence of pulmonary emphysema, bronchial wall thickening, and bronchiectasis have been proposed as the 3 main morphological findings likely to provide relevant information about different phenotypes of COPD.Citation10 Interestingly, both emphysema and bronchiectasis were associated with PTB.
Therefore, we set out to explore whether the prevalence, distribution, and severity of emphysema and bronchiectasis are different, and associated with clinical features in COPD patients with radiological signs of previous PTB. Our results showed that compared with those without PTB, COPD patients with previous PTB demonstrated more prevalent and severe bronchiectasis, and the unique distribution of emphysema, which was associated with significant dyspnea and higher frequency of severe exacerbations.
Materials and methods
Study subjects
Patients with stable COPD visiting Beijing Tongren Hospital, Capital Medical University from July 2008 to July 2016 were evaluated. The diagnosis of COPD was based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria,Citation4 as used in our previous study. These were as follows:Citation11 1) age >40 years, 2) history of smoking (smoking index >10 pack-year) and/or exposure to biomass fuel (mostly coal or wood burning stoves)/chemical agents/noxious particles for >10 years, 3) chronic cough with or without wheeze for >3 months in each year for 2 consecutive years, and 4) irreversible obstructive dysfunction defined as postbronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <70% on spirometry. Patients were excluded if they met any of the following conditions: receiving therapy of systemic corticosteroids or other immunosuppressive agents in the preceding 8 weeks; with allergic bronchopulmonary mycosis, interstitial lung diseases, active PTB, autoimmune diseases, and severe heart failure; doctor-diagnosed asthma or bronchiectasis before diagnosis of COPD; and previous PTB resulting in severe lung damage (“destroyed lung”), since “destroyed lung” per se could lead to severe impairment of lung functions, restrictive and/or obstructive, which could not be differentiated from COPD.
Definition of previous PTB
Previous PTB was defined as present if discrete linear or reticular fibrotic scars, or dense nodules with distinct margins were identified within the upper lobes and/or superior segment of lower lobes on computed tomography (CT) scan, with or without calcification of the lesions and/or local lymph nodes.Citation11–Citation13 A distinct history of TB was not necessary for the determination.
Lung high-resolution computed tomography (HRCT) and evaluation of bronchiectasis and emphysema
Chest HRCT was performed with a 64-row, multiple detector CT scanner (Philips Company, Amsterdam, the Netherlands). The presence, extent (Smith score), severity (Bhalla score) and types (classified into cylindric, cystic, or mixed) of bronchiectasis, as well as the presence, severity (Goddard score) and types (classified into centrilobular, panlobular, paraseptal, and bullae) of emphysema were evaluated by 2 radiologists experienced in the interpretation of HRCT and blinded to the patients’ clinical data. The radiologists finished the evaluations independently and differences in the readings were resolved by their final consensus.Citation11
Bronchiectasis was determined to be present if HRCT showed bronchial wall thickening with the ratio of the diameter of bronchus to that of the accompanying pulmonary artery being >1.1 (signet ring sign) or the lack of tapering of bronchi (tramline sign).Citation14 The extent of bronchiectasis was scored for each pulmonary lobe, with the lingula as a separate lobe. The grading system proposed by Smith et alCitation15 was adopted in our study. According to this system, the absence of bronchiectasis was scored as 0, bronchiectasis in <25% of bronchi as 1, in 25% to 49% of bronchi as 2, in 50% to 74% as 3, and in ≥75% as 4. The total score ranged from 0 to 24 points. Patients with a score ≤1 were considered as normal, since mild bronchiectasis only visible in a single pulmonary segment may exist in a significant percentage of healthy population.Citation11,Citation16 The type of bronchiectasis was identified as cylindric, cystic, or mixed (varicose) according to the morphological characteristics of the dilated bronchi.Citation14 The severity of bronchiectasis in each lobe was graded by the Bhalla scoring system on a scale of 0 to 3,Citation17,Citation18 with 0 for no involvement; 1 for mild, with luminal diameter slightly greater than that of adjacent blood vessel; 2 for moderate, with lumen 2–3 times that of adjacent vessel; and 3 for severe, with lumen 3 times that of adjacent vessel.
The presence and the type of emphysema were visually assessed for each lobe, with the lingula as a separate lobe. Emphysema was classified into centrilobular, panlobular (panacinar), paraseptal, and bullous as previously reported.Citation14 With the presence scored as 1 and the absence scored as 0, a semi-quantitative method, with scores of both lungs ranging from 0 to 6, was adopted to show the extent of a certain type of emphysema. The severity of emphysema was visually evaluated with the modified Goddard scoring system.Citation19,Citation20 Six images were analyzed in 3 slices (including the aortic arch, carina, and 1–2 cm above the highest hemidiaphragm) of both lungs, and a total score of all images was considered as a representative value of the severity of emphysema for each patient. Each image was classified as normal (score 0), 5% affected (score 0.5), 25% affected (score 1), 50% affected (score 2), 75% affected (score 3), and >75% affected (score 4); therefore, the total score of each patient ranged from 0 to 24 points.
Pulmonary function tests
Spirometry (JAEGER, MasterScreen-body + diffusion + APS, Hoechberg, Germany) was performed to determine the lung function measurements and bronchodilator reversibility as we previously reported.Citation11 Post-bronchodilator FEV1/FVC% and FEV1 were measured 15 minutes after the inhalation of 400 µg salbutamol.
Definition of respiratory symptoms and exacerbations
Chronic cough and expectoration were considered to be present if the symptoms lasted for more than 3 consecutive months. Exertional dyspnea was identified as present if the mMRC (modified Medical Research Council Questionnaire) score was ≥1. The duration of a symptom such as dyspnea was defined as the length of time from its onset to enrollment of the patient in the study, as we previously described.Citation11 Frequency of acute exacerbation (AE) was defined based on the number of times the patient was hospitalized or presented for emergency visits because of AE during the year before enrollment.
Bacterial culture of sputum samples
Results of sputum cultures were reviewed retrospectively and positive results for bacteria, especially Pseudomonas aeruginosa, were compared between groups as described previously.Citation11 The sputum samples were obtained when the patients experienced a severe exacerbation. Sputum samples were acceptable if they contained fewer than 10 squamous epithelial cells and more than 25 leukocytes per low-powered field.Citation11,Citation17
Analysis
The prevalence, distribution, and severity of emphysema and bronchiectasis on HRCT, and the clinical characteristics were compared between COPD patients with and without previous PTB. To explore whether the potential effect of previous PTB on emphysema and bronchiectasis was localized or extensive, we also compared the presence and the severity of emphysema and bronchiectasis between the lung with TB sequelae and the contralateral lung in patients with TB sequelae in a single lung.
The statistical package SPSS version 17.0 (SPSS, Chicago, IL, USA) was used for statistical analysis. Data were expressed as mean ± SD. Independent-samples t-Test (for normal distribution parameters) and Mann–Whitney U-test (for abnormal distribution parameters) were adopted for comparisons of continuous data between the 2 groups. Comparisons of continuous data among 3 groups were performed by analysis of variance test (for normal distribution) or Kruskal–Wallis test (for abnormal distribution). Categorical variables between different groups were analyzed by χ2 test. p-values <0.05 were considered as statistically significant. As for multiple comparisons among 3 or 4 groups (including χ2 test or Kruskal–Wallis test), p-values <0.017 or p-values <0.008, respectively, were taken as statistically significant.
Results
Clinical characteristics and chest CT features of subjects with COPD
During the study period, 231 patients with COPD were consecutively enrolled. These included 156 males (67.5%) and 75 females (32.5%) with a mean age of 76.8 years, and a majority of them were smokers (82.2%). The GOLD spirometry classification ranged from 2 to 4, with 83 patients (35.9%) in GOLD 2, 109 (47.2%) in GOLD 3, and 39 (16.9%) in GOLD 4. Data are shown in .
Table 1 Baseline and clinical characteristics of subjects with COPD, with and without PTB
Signs of previous PTB were found by chest HRCT in 104 patients (104/231, 45.0%), of whom only 42 (42/231, 18.2%) had a definite history of PTB and had received anti-TB therapy. TB sequelae in the upper lobes were found in most of the patients (99/104, 95.2%). As for the symptoms, the average time course of chronic cough/expectoration and that of exertional dyspnea were 19.1 and 6.28 years, respectively. P. aeruginosa was found positive in the sputum of 15 patients with COPD, and 13 of them had coexistent bronchiectasis on CT ().
Bronchiectasis on HRCT was found in 50.6% (117/231) of the COPD patients. With regard to the sites of bronchiectasis, the middle lobes/lingular segments (64.1%, p=0.018), and the lower lobes (68.3%, p=0.002) were involved more often compared with the upper lobes (48.7%). No statistical difference was found in the Smith score among upper lobes, middle lobes/lingular segments, and lower lobes (3.45, 3.96, and 4.02, respectively, p=0.321). Among COPD patients with comorbid bronchiectasis (COPD-Bx, n=117), most showed mild bronchiectasis (Bhalla score =1), and only 6.8% (8/117) of them had a Bhalla score ≥2. The majority (115/117, 98.3%) of the COPD-Bx subjects demonstrated cylindric bronchiectasis, and mixed (varicose) type was found in only 4 patients (4/117, 3.4%), with no patients showing typical cystic bronchiectasis ().
Table 2 CT characteristics of bronchiectasis and emphysema of subjects with COPD, with and without PTB
Emphysema on HRCT was found in 62.3% (144/231) of COPD patients. With regard to the sites of emphysema, the upper lobes (92.4%) were involved more often compared with the middle lobes/lingular segments (68.7%), and the lower lobes (63.1%); both p<0.001. In addition, a significant difference was found in the severity of emphysema (Goddard score) among the upper, middle, and lower lobes (overall p=0.015). The Goddard score of the upper lobes was found to be significantly higher than that of the lower lobes (p=0.006). With regard to the types of emphysema, centrilobular emphysema was the most common (42%, 97/231), followed by panlobular (34.6%, 80/231), bullous (26.8%, 62/231), and paraseptal emphysema (19.9%, 46/231). According to the semi-quantitative evaluation, the distribution of centrilobular or panlobular emphysema was found to be more extensive than that of paraseptal or bullae emphysema (2.76 vs 1.71 and 2.76 vs 1.90, 2.93 vs 1.71 and 2.93 vs 1.90, respectively, all the p-values <0.001) ().
Clinical characteristics of COPD patients with previous PTB
No statistical difference was found in age, gender, smoking status, body mass index, maintenance medication, FEV1% predicted, and GOLD grading between COPD patients with and without previous infection of PTB. However, patients with previous PTB showed a longer history of dyspnea (7.31 vs 5.44 years), more severe dyspnea (mMRC: 2.63 vs 2.33; mMRC ≥2: 85.6% vs 74.0%), a higher frequency of exacerbation in the previous year (1.56 vs 1.09), and a higher positive rate of P. aeruginosa by sputum culture (12.5% vs 1.6%) ().
Characteristics of bronchiectasis in COPD patients with previous PTB
The percentage of patients with CT bronchiectasis was higher in those with previous PTB (64.4%) compared with those without PTB (39.4%, p<0.001). The difference was more significant when the upper lobes were compared (62.7% vs 30.0%, p<0.001) (, and ). Coexisting upper lobe bronchiectasis was found in 42.4% (42/99) of patients with PTB sequelae in upper lobes, but in only 11.8% (15/127) of patients without PTB sequelae (p<0.001). For patients with bronchiectasis, the Smith score tended to be higher in those with previous PTB (n=67) compared with those without (n=50), though the difference was not significant. The severity of bronchiectasis was identified as mild (Bhalla score =1) and cylindric for most of the patients in the 2 groups, but more severe (Bhalla score ≥2) or varicose bronchiectasis was found only in COPD patients with previous PTB ().
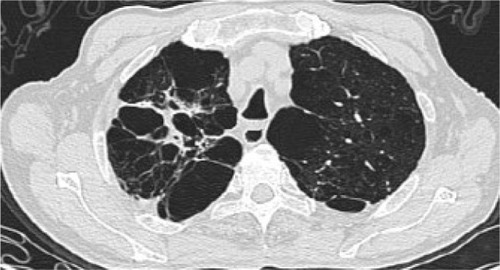
Figure 1 Bronchiectasis in case 1 with TB sequelae in RUL (HRCT).
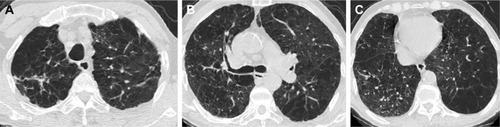
Presence and characteristics of bronchiectasis were also compared between the lung with TB sequelae and the contralateral lung in patients with TB sequelae limited to a single lung (n=65). The prevalence of bronchiectasis in the TB-involved lung, especially the upper lobe, was significantly higher (p<0.01) compared with the contralateral lung (, and ), but no difference was found in the severity of bronchiectasis ().
Table 3 Bronchiectasis and emphysema of 2 lungs in 65 patients with TB sequelae in a single lung
Characteristics of emphysema in COPD patients with previous PTB
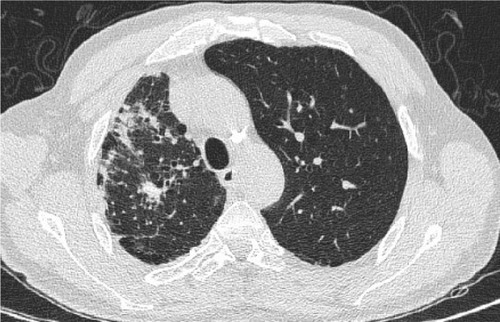
The prevalence of emphysema between patients with and without previous PTB showed no significant difference (67.3% vs 58.2%). Interestingly, in patients with previous PTB, more had emphysema of the middle lobes/lingular segments (81.4% vs 56.7%, p=0.001) and of the lower lobes (72.8% vs 54.1%, p=0.019), compared with those without PTB, but no difference was found in the upper lobe emphysema. The severity score of emphysema (Goddard score) in patients with previous PTB was higher than that in patients without PTB (7.88 vs 6.17, p=0.028). Patients with previous PTB also demonstrated a statistically higher prevalence of panlobular emphysema (43.2% vs 27.5%, p=0.013) and bullae (32.7% vs 22%, p=0.069). The distribution of centrilobular emphysema in patients with PTB was more extensive than that in patients without PTB (3.12 vs 2.40, p=0.039) ().
Presence and characteristics of emphysema were also compared between the lung with TB sequelae and the contralateral lung in patients with TB sequelae limited to a single lung (n=65). Interestingly, no significant difference was seen in the prevalence, severity, and type of emphysema between the 2 lungs of the same patients. (, and ).
Discussion
In this cross-sectional study of the chest imaging (bronchiectasis and emphysema) and clinical features of COPD with previous PTB, the most important finding was that, compared with those without PTB, COPD patients with previous PTB showed higher prevalence and more severe bronchiectasis, increased prevalence of lower lung emphysema, and more extensive emphysema, which were evident in lobes near the local sequelae of PTB and both the lungs, and this could not be explained only by the local effects of TB. Studies have shown that even localized PTB might be a risk factor for COPD,Citation9,Citation14,Citation21 suggesting a role beyond its local effects. Our results provide new data to support the implication of PTB in the development and/or manifestation of COPD associated with cigarette smoking or biomass fuel exposure.
CT bronchiectasis, mostly mild-to-moderate in degree, and found in as high as 57.2% of COPD patients, was associated with exacerbations, poor lung function, and even mortality from the disease.Citation17 The potential mechanisms underlying the development of bronchiectasis in COPD are poorly understood. Our recent study showed that previous PTB was an independent risk factor for coexistent bronchiectasis in COPD,Citation11 suggesting that PTB might be a cause of bronchiectasis in these patients. In the current study, we found that previous PTB was associated with more prevalent and severe bronchiectasis, which was more evident in the TB-involved lung, indicating local airway damage induced by TB infection.
Another interesting finding of our study was the association of previous PTB with higher prevalence of lower lung emphysema and more severe emphysema of both lungs, characterized by more extensive centrilobular emphysema, and higher prevalence of panlobular emphysema and bullae. The association of PTB with emphysema was observed as early as the 1950s. These earlier studies found increased incidence of obstructive ventilatory dysfunction by lung function testing,Citation22,Citation23 and of emphysema by chest X-ray,Citation24,Citation25 although the latter was not consistently confirmed by pathological studies.Citation26 The advent of HRCT made the observation and classification of emphysema easier and more accurate, but few studies have been carried out to re-examine the association of PTB with emphysema using HRCT in cross-sectional, or ideally, longitudinal studies. Our results warrant further studies to elucidate the effect of PTB on the development of emphysema, particularly in the context of cigarette smoke or biomass fuel exposure.
The potential effect of TB on the development of COPD was believed to be mediated by mechanisms of chronic inflammation.Citation27–Citation29 Chronic inflammatory response may persist in patients with TB even after the microbiological cure.Citation30 Radovic et alCitation31 demonstrated that after 6 months of anti-TB treatment, serum markers of systemic inflammation such as erythrocyte sedimentation rate and fibrinogen decreased significantly, but were still abnormally high.Citation31 As early as 1960s, Hennes et alCitation32 showed that antibodies reacting with extracts of human lungs were present in the serum of many patients with PTB and idiopathic obstructive emphysema, suggesting that tubercle bacilli might serve as an “endogenous Freund adjuvant” stimulating antibody response to damaged tissue.Citation32 Recently, Tang et alCitation33 found that serum concentrations of cytokines such as soluble interleukin-2 receptor (sIL-2R), IL-6 and tumor necrosis factor (TNF-α) in COPD patients with TB were higher than those without TB or those with TB but without COPD,Citation33 suggesting that COPD combined with TB may result in excessive inflammation. Studies on airway or lung inflammation, such as with bronchoalveolar lavage (BAL), in patients with PTB showed that expressions of matrix metalloproteinases (MMPs) such as matrix metalloproteinase-1, -3 and -9 (MMP-1, MMP-3 and MMP-9), which played important roles in tissue destruction, were significantly upregulated when compared with normal or symptomatic controls.Citation34,Citation35 BAL concentrations of proinflammatory cytokines such as TNF-α, IL-6 and IL-8, were also found to be correlated with the HRCT score in active pulmonary TB.Citation36 However, the features of airway/lung inflammation in COPD patients with previous PTB need to be clarified further.
Previous PTB in COPD patients was also clinically relevant as these patients complained of more significant dyspnea and had more severe exacerbations. In our study, no significant difference was found in spirometry measurements (FEV1/FVC and FEV1% predicted) between COPD patients with or without PTB, which was similar to the results of Yakar et al.Citation37 Given that FEV1 correlated poorly with symptoms, impairment of a patient’s quality of life, and exacerbations,Citation4 this result could be explained, at least partially, by the presence of more prevalent and severe bronchiectasis and emphysema.Citation11,Citation18,Citation38 Other results of clinical relevance include the finding that positive culture of P. aeruginosa in sputum was seen mostly in COPD patients with prior PTB, which could be explained by the presence of more severe bronchiectasis in this group of patients. Therefore, in COPD patients with prior PTB, especially those with coexistent bronchiectasis, regular sputum surveillance for P. aeruginosa, and strategies for eradication of the pathogen may be useful for prevention of COPD exacerbations. Because previous PTB was also associated with unique features of emphysema, interventional treatments such as volume reduction may need special consideration; for example, in volume reduction surgery, COPD patients with prior PTB may experience less post-treatment pneumothorax due to pleural adhesions.
It should be noted that, in our study, typical TB sequelae on chest CT was the only criterion for the diagnosis of prior PTB in the majority of our patients. This diagnostic criterion has been used in several studies,Citation11–Citation13 especially in high TB-burden countries.Citation9,Citation37,Citation39 However, prior PTB cannot be completely excluded if no TB sequelae are found by chest X-ray or CT. Our study also has several limitations. Because of the cross-sectional design of the study, the time sequence of the occurrence of PTB and emphysema and/or bronchiectasis cannot be determined, and therefore, a cause–effect relationship cannot be obtained. No evaluation of the interaction between PTB and cigarette smoke was attempted, though the smoking status of the TB group and the non-TB group of our patients was similar. We believe that this interaction would be very important for understanding the potential effect of PTB in COPD.
Conclusion
We have found in a well-defined cohort of COPD patients that those with previous PTB had more prevalent and severe bronchiectasis and emphysema on HRCT, which were associated with significant dyspnea and higher frequency of severe exacerbations. Although PTB may have a local effect on bronchiectasis, its involvement in airspace damage of COPD may be extensive. Further prospective studies of larger sample size are warranted to clarify the effects and mechanisms of PTB on the development or progression of COPD, particularly in the context of cigarette smoking.
Ethics approval and consent to participate
Written informed consent was obtained from all the patients, and the study was approved by the local Ethics Committee of Beijing Tongren Hospital, Capital Medical University.
Authors’ contribution
All authors contributed toward data analysis, drafting and critically revising the paper, and approved the final version of the manuscript. JJ completed the recruitment of patients, performed the collection and analysis of all data, and was a major contributor to the study design and manuscript. YS was the primary investigator of this study and a major contributor to the study design and manuscript writing. WY and SL were mainly responsible for image evaluation. XL mainly completed the recruitment of patients, and performed the collection of clinical data.
Acknowledgments
The authors thank Lijin Lu, Ying Zhang, Yang Wang, Xiufang Luo, Jie Zhuo, Dongning Chen, Yong Liu, Peng Bai, Ran Li, Yuhong Wang and Haiyan Sheng for their support in screening subjects participating in the study. The authors acknowledge financial support from National Natural Science Foundation of China (81170039, 81470239), High-level Talent Training Foundation of Beijing Health System (2014–3-011), and Beijing Talent Training Foundation (No 2009D003003000002).
Disclosure
The authors report no conflicts of interest in this work.
References
- SunYCA dangerous combination: tuberculosis and chronic obstructive pulmonary diseaseChin Med J (Engl)2013126122203220423786925
- InghammarMEkbomAEngströmGCOPD and the risk of tuberculosis–a population-based cohort studyPLoS One201054e1013820405056
- van Zyl SmitRNPaiMYewWWGlobal lung health: the colliding epidemics of tuberculosis, tobacco smoking, HIV and COPDEur Respir J2010351273320044459
- Global initiative for chronic obstructive lung disease, global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease2017 Available from: http://www.goldcopd.org/2017Accessed November 2016
- MenezesAMHallalPCPerez-PadillaRLatin American Project for the Investigation of Obstructive Lung Disease (PLATINO) TeamTuberculosis and airflow obstruction: evidence from the PLATINO study in Latin AmericaEur Respir J20073061180118517804445
- HnizdoESinghTChurchyardGChronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatmentThorax2000551323810607799
- CaballeroATorres-DuqueCAJaramilloCPrevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL study)Chest2008133234334917951621
- AmaralAFCotonSKatoBBOLD Collaborative Research GroupTuberculosis associates with both airflow obstruction and low lung function: BOLD resultsEur Respir J20154641104111226113680
- JungJWChoiJCShinJWKimJYChoiBWParkIWPulmonary Impairment in Tuberculosis Survivors: the Korean National Health and Nutrition Examination Survey 2008–2012PLoS One20151010e014123026496500
- Martinez-GarciaMAMiravitllesMBronchiectasis in COPD patients: more than a comorbidity?Int J COPD20171214011411
- JinJYuWLiSLuLLiuXSunYFactors associated with bronchiectasis in patients with moderate-severe chronic obstructive pulmonary diseaseMedicine (Baltimore)20169529e421927442646
- LamKBJiangCQJordanREPrior TB, smoking, and airflow obstruction: a cross-sectional analysis of the Guangzhou Biobank Cohort StudyChest2010137359360019820078
- KimHJBaekSKimHJThe impact of smoking on airflow limitation in subjects with history of asthma and inactive tuberculosisPLoS One201510e012502025915938
- HansellDMBankierAAMacMahonHMcLoudTCMüllerNLRemyJFleischner society: glossary of terms for thoracic imagingRadiology2008246369772218195376
- SmithIEJurriaansEDiederichSAliNShneersonJMFlowerCDChronic sputum production: correlations between clinical features and findings on high resolution computed tomographic scanning of the chestThorax19965199149188984702
- GallegoMPomaresXEspasaMPseudomonas aeruginosa isolates in severe chronic obstructive pulmonary disease: characterization and risk factorsBMC Pulmon Med201414103
- Martínez-GarcíaMAde la Rosa CarrilloDSoler-CataluñaJJPrognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013187882383123392438
- BhallaMTurciosNAponteVCystic fibrosis: scoring system with thin-section CTRadiology199117937837882027992
- GoddardPRNicholsonEMLaszloGWattIComputed tomography in pulmonary emphysemaClin Radiol19823343793877083738
- MakitaHNasuharaYNagaiKHokkaido COPD Cohort Study GroupCharacterisation of phenotypes based on severity of emphysema in chronic obstructive pulmonary diseaseThorax2007621193293717573447
- HarriesADAdeSBurneyPHoaNBSchlugerNWCastroJLSuccessfully treated but not fit for purpose: paying attention to chronic lung impairment after TB treatmentInt J Tuberc Lung Dis20162081010101427393532
- LancasterJFTomashefskiJFTuberculosis–a cause of emphysemaAm Rev Respir Dis19638743543713928393
- AnnoHTomashefskiJFStudies on the impairment of respiratory function in pulmonary tuberculosisAm Rev Tuberc1955713, Part 133334814350194
- SinghDRichardsWFObstructive emphysema in primary pulmonary tuberculosisTubercle195738639740213519512
- MatsaniotisNKattamisCeconomou-MavrouEKyriazakouMBullous emphysema in childhood tuberculosisJ Pediatr19677157037085299123
- MartinCJCochranTHKatsuraSTuberculosis, emphysema, and bronchitisAm Rev Respir Dis1968976108910935649854
- de la MoraILMartínez-OcegueraDLaniado-LaborínRChronic airway obstruction after successful treatment of tuberculosis and its impact on quality of lifeInt J Tuberc Lung Dis201519780881026056106
- HwangYIKimJHLeeCYThe association between airflow obstruction and radiologic change by tuberculosisJ Thorac Dis20146547147624822105
- JordanTSSpencerEMDaviesPTuberculosis, bronchiectasis and chronic airflow obstructionRespirology201015462362820409028
- PasipanodyaJGMillerTLVecinoMPulmonary impairment after tuberculosisChest200713161817182417400690
- RadovicMRisticLCiricZChanges in respiratory function impairment following the treatment of severe pulmonary tuberculosis – limitations for the underlying COPD detectionInt J Chron Obstruct Pulmon Dis2016111307131627366058
- HennesARMooreMZCarpenterRLHammarstenJFAntibodies to human lung in patients with obstructive emphysema and pulmonary tuberculosisAm Rev Respir Dis19618335435813713360
- TangSCuiHYaoLIncreased cytokines response in patients with tuberculosis complicated with chronic obstructive pulmonary diseasePLoS One201384e6238523626814
- ChangJCWysockiATchou-WongKMMoskowitzNZhangYRomWNEffect of Mycobacterium tuberculosis and its components on macrophages and the release of matrix metalloproteinasesThorax19965133063118779137
- ElkingtonPShiomiTBreenRMMP-1 drives immunopathology in human tuberculosis and transgenic miceJ Clin Invest201112151827183321519144
- CasariniMAmeglioFAlemannoLCytokine levels correlate with a radiologic score in active pulmonary tuberculosisAm J Respir Crit Care Med199915911431489872832
- YakarHIGunenHPehlivanEAydoganSThe role of tuberculosis in COPDInt J Chron Obstruct Pulmon Dis20171232332928176901
- AlcaideABSanchez-SalcedoPBastarrikaGClinical features of smokers with radiological emphysema but without airway limitationChest2017151235836527818328
- AllwoodBWGoldinJSaid-HartleyQAssessment of previous tuberculosis status using questionnaires, chest X-rays and computed tomography scansInt J Tuberc Lung Dis201519121435144026614183