Abstract
Introduction
This was the first study designed to prospectively evaluate treatment patterns in chronic obstructive pulmonary disease (COPD) and the degree of adherence with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy recommendations in routine clinical practice in Bulgaria.
Methods
The study was conducted in an outpatient setting and enrolled patients of both genders, aged >40 years, who were diagnosed with COPD (as per GOLD 2013). Evaluations were performed at baseline and at 6- and 12-month visits.
Results
Of the 811 enrolled patients, 719 were assessed and completed the 12-month observation period. Overall, a substantial number of patients experienced moderate airflow limitation (~49% patients, GOLD 2 as per GOLD 2013; mean postbronchodilator forced expiratory volume in 1 second value was ~50% of the predicted value), belonged to GOLD group D (~51% patients), and had COPD assessment test score ≥10 or modified Medical Research Council score ≥2 (~79% patients), and ≤1 exacerbation in the past 1 year (~80% patients). Short-acting β2-agonists (~63% patients), inhaled corticosteroids/long-acting β2-agonist fixed-dose combination (~62% patients), and long-acting muscarinic antagonists (~59% patients) were the most frequently used medications at all visits, regardless of severity. High levels of deviation from GOLD recommendations were observed in GOLD groups A and B patients. The deviation comprised high use of inhaled corticosteroid-containing regimens in ~45% and 63% of patients in GOLD groups A and B, respectively. Only 25 (3%) of the 796 patients reported at least one adverse event.
Conclusion
The routine clinical practice for COPD in Bulgaria deviates from the GOLD recommendations largely in patients at a low risk (GOLD groups A and B), while the deviation was lesser in those at a higher risk (GOLD groups C and D).
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the major causes of disability and mortality globally and imposes a significant burden on the health care system and economy.Citation1–Citation3 It affects ~64 million individuals worldwide and contributes to ~3 million deaths every year.Citation4 In Europe, the prevalence of COPD is estimated to be ~10%.Citation5 In Bulgaria, the prevalence rate of COPD is on the rise, with a relatively high percentage (45%) of severe COPD patients;Citation6 a Bulgarian epidemiological study showed a COPD prevalence of 14.9% in the Pleven region.Citation7
Commonly prescribed medications for COPD include short-acting β2-agonists (SABAs), short-acting muscarinic antagonists (SAMAs), long-acting β2-agonists (LABAs), long-acting muscarinic antagonists (LAMAs), inhaled corticosteroids (ICS), methyl xanthines (eg, oral theophylline), phosphodiesterase (PDE)-4 inhibitors (eg, roflumilast), and fixed-dose combinations (FDCs) of LABA/LAMA and ICS/LABA.Citation8 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy, revised up to 2016, provides a holistic approach for treating COPD.Citation8 The combined assessment suggested by GOLD 2013 categorizes patients into four groups based on lung function, symptom burden, and exacerbations: group A (low risk and fewer symptoms), group B (low risk and more symptoms), group C (high risk and fewer symptoms), and group D (high risk and more symptoms). As per the GOLD 2013 recommendations, short-acting bronchodilators (SABAs and SAMAs) are the preferred option in group A patients and long-acting bronchodilator monotherapy (LABAs and LAMAs) is the preferred choice in group B patients. For patients who remain symptomatic despite monotherapy, an intensified treatment approach with bronchodilator combination therapy (LABA + LAMA) has been advocated as an alternative for GOLD group B patients. Use of ICS has been recommended only in high-risk patients (groups C and D), where ICS + LABA and/or a LAMA are treatments of first choice.Citation8 The recently published GOLD 2017 strategy acknowledges the limitation of forced expiratory volume in 1 second (FEV1) in predicting COPD prognosis and excludes spirometry values as a criterion for the ABCD grouping, with much emphasis on patient symptoms and exacerbation risk in making diagnostic and therapeutic decisions for an individualized patient care.Citation9
Several studies have shown that a low degree of adherence to GOLD recommendations could potentially lead to under- or overprescription of medications.Citation10,Citation11 Approximately 24% of clinicians have reported unfamiliarity with the GOLD strategy.Citation12 In addition to GOLD, several other guidelines for the diagnosis and management of COPD have been published worldwide.Citation13 A large degree of variation has been noted in the type of guidelines used and the extent to which they are adhered to by physicians in routine clinical practice.Citation13 Implementation of guidelines seems to be a major issue in clinical settings, causing significant negative impact on patient outcomes, health care resource utilization, and cost of care for COPD patients.Citation13
In general, studies assessing adherence to guidelines in COPD are few, with very limited number of reports available on the Bulgarian population. This study was designed to prospectively evaluate the treatment patterns in COPD and degree of adherence with GOLD 2013 recommendations in routine clinical practice in Bulgaria.
Methods
Study design
This prospective observational study in COPD patients was performed at 19 key outpatient and hospital centers through-out Bulgaria. The study centers were selected according to their COPD capacity, availability of COPD patients, technical capability to perform COPD-related tests (eg, spirometry), and capability to follow-up patients in the course of 1 year. Approximately 30 sites in Bulgaria fulfilled these conditions; all were approached with the aim to include 20 sites in the study. Observing physicians were expected to enroll consecutive patients who comply with the inclusion and exclusion criteria. Patient enrollment began in August 2013 and the last patient’s last visit occurred in December 2014. The evaluation period comprised three clinical visits scheduled at an interval of 6 months between each visit, which was consistent with local routine clinical practice (baseline visit, 6-month visit, and 12-month visit). Patients enrolled in the study continued to be on their prior treatments. The observing pulmonologist performed assessments as per usual clinical practice at each visit. Data were collected from medical charts and with the help of questionnaires that patients completed at every visit. Data were recorded in an electronic case report form.Citation14 The complete list of the participating centers and respective contributors is provided in Table S1.
Patients
Patients of either gender, aged ≥40 years, and diagnosed with COPD (as per GOLD 2013) were consecutively enrolled in the study. The key exclusion criteria were unwillingness to provide informed consent, participation in a clinical trial, presence of a malignancy, and any contraindication for COPD medications approved by the European Medicines Agency and/or the Bulgarian Drug Agency. All patients were informed about the study procedures and signed a written informed consent. The study was approved by the Ethics Committee for Scientific Research of the Medical University of Sofia, and the Ministry of Health was notified.
Assessments
Efficacy
Data collected by the observing pulmonologist and those related to COPD diagnosis and/or treatment were used for analyses. Data regarding demographic characteristics were collected at baseline: age, gender, smoking history, and duration of disease. Data regarding COPD treatment (medications used), concomitant diseases and their treatment, COPD severity stages according to FEV1 values (using spirometry), COPD symptoms based on COPD assessment test (CAT) and modified Medical Research Council (mMRC) scores, number of exacerbations in the past year, comorbidities, number of hospital admissions and visits to emergency care, general practitioner, and pulmonologist related to COPD in the past year (from medical charts and patient questionnaires) were collected at baseline and at 6- and 12-month visits.
Safety
Safety data were collected at 6- and 12-month visits. All adverse events (AEs) and serious AEs (SAEs) were recorded.
Statistical analysis
The estimated sample size was 384 patients assuming that the ratio of adequate-to-nonadequate therapy according to GOLD guidelines was 50%–50% or P=0.5 and q=0.5, and the probability of error was 5%. However, as the study was descriptive and in order to obtain a better overview of local clinical practice, the number was increased to 800 patients. Descriptive statistics was used to summarize data, including number and frequency for categorical variables and number, mean, standard deviation (SD), and 95% confidence interval (CI) for continuous variables. Statistical evaluation was performed using SPSS 19.0.0. Correlation analyses were performed using Pearson’s correlation coefficient to establish the relation between exacerbation rate and airflow limitation, GOLD group D, and number of hospitalizations and between treatment used and airflow limitation and symptoms. Efficacy analysis set included all enrolled patients with correctly completed inclusion and exclusion criteria and primary end point variables. Safety analysis set included all patients enrolled in the study.
Results
Patient disposition and baseline characteristics
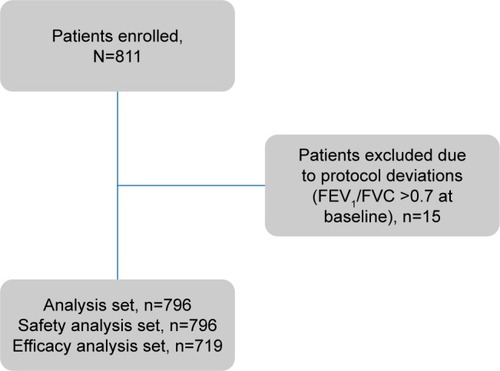
Of the 811 patients enrolled, 719 were assessed and completed the observation period (). A majority of patients were men (64.9%), and the mean patient age was 66.75 years (). Of the total study population, 28 patients (3.9%) were newly diagnosed. The mean time since COPD diagnosis was 7.78 years. None of the enrolled patients had a previous diagnosis of asthma.
Table 1 Baseline demographics and clinical characteristics (efficacy analysis set)
COPD severity classification
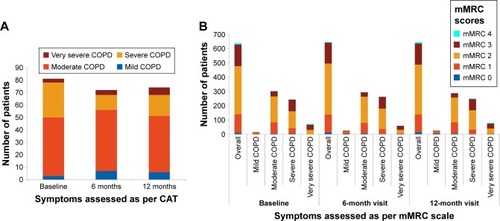
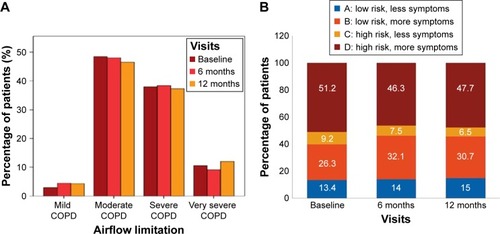
The majority of enrolled patients had moderate airflow limitation (GOLD 2, as per GOLD 2013) at baseline (48.5% patients), 6 months (48.0% patients), and 12 months (46.5% patients) (). The mean percentage predicted postbronchodilator FEV1 values was stable over 1 year (~50% of predicted value) in most patients. Most patients belonged to group D (46%–51%), followed by group B (26%–32%) at all visits (). The overall distribution of patients in GOLD groups did not change significantly over 12 months; however, there was a numerical increase in the number of patients in groups A (by 12 patients; P=0.271) and B (by 32 patients; P=0.008) and a decrease in groups C (by 21 patients; P=0.037) and D (by 25 patients; P=0.068), as shown in .
Figure 2 COPD severity as per GOLD classification based on (A) airflow limitation and (B) GOLD groups (efficacy analysis set).
Health status and dyspnea
The largest proportion of patients had a CAT value of ≥10 or an mMRC value of ≥2 at baseline (77.5%), 6 months (78.4%), and 12 months (78.6%), indicating high level of symptoms in this population (). As expected, these scores were particularly high in patients with moderate and severe airflow limitation ().
Exacerbations
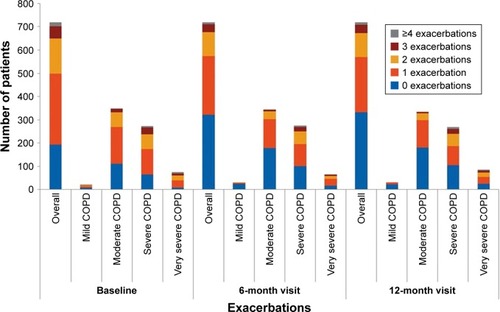
Most patients either had ≤1 or no exacerbation in the past year at baseline (69.4%) and at 6- (79.8%) and 12-month visits (79.3%; ). The proportion of patients who experienced ≥3 exacerbations was ~3 times higher in severe COPD groups than in moderate/mild groups (~15% vs 5%). The frequency of exacerbations strongly correlated with lung function and GOLD D (Pearson coefficient =1; P=0.01) at baseline and at 12 months. Patients with ≥2 exacerbations had a mean FEV1 reversibility value of ~5.5% and a mean COPD duration of ~10 months; up to 72% were men and exsmokers (47.7%). A strong correlation was noted between exacerbations and number of hospitalizations (Pearson coefficient =1; P=0.01) at baseline and at 12 months, indicating increase in hospitalizations with increase in exacerbation rates.
Comorbidities
Overall, the majority of patients had cardiovascular comorbidities at study inclusion (72.5%), followed by diabetes (9.6%), benign prostatic hyperplasia (3.0%), neurological or psychiatric disorders (2.2%), bronchiectasis (1.1%), gout (1.0%), status post-tuberculosis (0.9%), glaucoma (six patients 0.7%), and renal diseases (0.6%). Hypertension (25%) was the most common comorbidity in patients experiencing exacerbations.
COPD management
Patients previously diagnosed with COPD were on regular pharmacological treatment at baseline. Patients who were newly diagnosed had regular treatment initiated at baseline. The most frequently used medication classes, alone or in combination with other classes were as follows: SABA (61%–63%), ICS/LABA FDC (62%), or LAMA (56%–59%) at baseline and at 6- and 12-month visits (Figure S1), regardless of COPD severity. The level of adherence to GOLD recommendations was the highest in GOLD group D and the lowest in GOLD group A (Figure S2). Most of the patients (~75%) in GOLD group D received the recommended first-line treatment; however, deviation from GOLD guidelines was observed in ~17% of patients in this high-risk COPD group. Conversely, in most patients in GOLD groups A (~67%) and B (~63%), the medications prescribed by pulmonologists were not in line with the GOLD recommendations (only ~3% group A patients and ~24% group B patients received GOLD recommended first-choice therapy). ICS-containing regimens were used in 45.8%, 44.5%, and 43.5% of group A patients and in 59.8%, 62.8%, and 60.7% of group B patients at baseline, 6 months, and 12 months, respectively (Figure S2). A triple combination of ICS + LAMA + LABA was prescribed in few (up to six) patients; these patients had percentage predicted FEV1 values ranging between 45% and 68% and mMRC scores ≥2 (only one patient had an mMRC score of 1). Specifically, increased use of LABA + LAMA was observed in patients (19–25 patients) with more frequent dyspnea (mMRC score ≥2). Overall (for all GOLD groups), treatment approaches used in routine clinical practice produced no notable differences during the follow-up period in mean percentage predicted FEV1 levels (50.24% at base-line, 50.60% at 6 months, and 49.4% at 12 months), CAT (CAT scores: 14.5 at baseline, 13.9 at 6 months, and 13.8 at 12 months), and mMRC scores (the proportion of patients with mMRC ≥2 was 77.9%, 78.6%, and 78.4% at baseline, 6 months, and 12 months, respectively) over 12 months. On the other hand, both airflow limitation and symptoms were the influential factors in choosing COPD treatments at baseline (Pearson coefficient =1, P=0.002 and P=0.001, respectively) and at 12 months (Pearson coefficient =1, P=0.0001 [for both the factors]).
Health care resource utilization
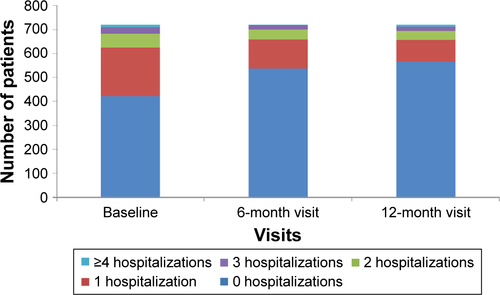
The majority of patients had not been hospitalized over the past 12 months (58.7% patients at baseline, 74.7% at 6 months, and 78.4% at 12 months; Figure S3). Approximately 75% of patients had visited a general practitioner most of whom (~55% patients) had one or two visits. Most patients had two visits to a pulmonologist’s office each year (≥90%). More than 90% of patients did not require COPD-related rehabilitations, physiotherapy, or balneotherapy at all visits.
Factors influencing treatment choice
Overall, the patients’ demographic/clinical characteristics had no significant (P>0.05) influence on the treatment decisions or choices. However, selection of COPD treatment correlated with both airflow limitation and symptoms at baseline (Pearson coefficient =1, P=0.002 and P=0.001, respectively) and at 12 months (Pearson coefficient =1, P=0.0001 [for both the factors]). The COPD exacerbation history of the patients, in particular, seemed to be significantly correlated (P<0.05) with the treatment prescribed. Furthermore, use of ICS regimens varied significantly (P<0.05) based on gender, smoking status, COPD exacerbation history, and duration of disease.
Safety
Twenty-five of the 796 patients had reported at least one AE. The complete list of AEs by System Organ Class is provided in Table S2.
Discussion
This study prospectively assessed the COPD treatment paradigms in real-world clinical settings in Bulgaria. The study showed that the management approaches for COPD in routine clinical practice deviate considerably from GOLD 2013 recommendations, particularly more in low-risk patients (groups A and B) and less in high-risk patients (groups C and D). Most of the enrolled patients had an established COPD diagnosis, moderate airflow limitation (GOLD 2), and a greater number of symptoms and belonged to group D. A total of 27% patients were nonsmokers, suggesting that factors other than smoking, such as air pollution, occupational exposure, and passive smoking, had contributed to the development of COPD. This observation was consistent with an observational study in the Bulgarian population, reporting 26% of nonsmokers.Citation6 A similar trend was observed in DACCORD study in the German population (20% nonsmokers).Citation15
Short-acting β-agonists, ICS/LABA, and LAMAs were the more commonly used medications in routine clinical practice in Bulgaria. Notably, use of ICS-containing regimens was higher in patients belonging to GOLD groups A and B, which is outside the GOLD recommendations.Citation16 Similarly, an observational study in COPD patients in Bulgaria showed a substantial overuse of LABA/ICS in GOLD groups A (43% patients) and B (73% patients), which may imply a need for more education of health care professional with regard to GOLD recommendations for optimal use of ICS.Citation6 Furthermore, the findings were consistent with data on primary care prescribing patterns in the UK, wherein overtreatment with ICS was reported in ~50% of the study population,Citation17 and with real-life studies INCARE in Greece and DACCORD in Germany.Citation15,Citation17
Airflow limitation and symptoms were correlated with treatment choices, thus indicating that both lung function and symptoms were factors in selecting a COPD therapy. As symptoms are not evaluated using CAT and mMRC in local daily practice, pulmonologists rely only on verbal assessment of symptom severity, which could be inaccurate. The same could be true for exacerbation history. So, spirometry remains the single most influential determinant of treatment choice in routine clinical practice. On the other hand, the relatively long-term use of the ICS/LABA combination for the treatment of COPD, when they were one of the few choices available, is not to be neglected. The change in treatment practices usually lags behind scientific data.
It is worthwhile mentioning that the overall treatment strategies used by local physicians in Bulgaria showed no worsening in FEV1 levels, symptoms, quality-of-life scores, and exacerbation and hospitalization rates over 1 year. Maybe the short follow-up period influenced these results.
Almost half of the study population was classified as GOLD 2 (moderate COPD), and only a few (~4.5%) had mild COPD; this disproportionate distribution of patients reflects a certain level of underreporting. Presumably, many patients ignore early symptoms and visit the doctor only when symptoms deteriorate (specifically dyspnea). Another reason why COPD is diagnosed at a relatively later stage of disease could be that in Bulgaria, general practitioners do not perform spirometry and COPD patients are referred to a pulmonologist.Citation6 Nevertheless, the overall distribution was in line with that reported in other studies.Citation18–Citation20 As per GOLD, only patients experiencing ≥2 or ≥1 exacerbations leading to hospitalization are classified in GOLD groups D or C, respectively. Surprisingly, in this study, ~28% patients from GOLD group D and ~75% patients from GOLD group C had not experienced any exacerbation; this could have been due to missed reporting as is possible in routine clinical practice. The severity classification of COPD in this study population showed better correlation with mMRC scores and FEV1 levels; patients categorized into GOLD D had mMRC scores of 2/3/4 and a mean FEV1 value of 40% of the predicted value. Notably, half of the recruited patients had experienced exacerbations in 1 year before study entry. Hypertension was the most frequent comorbidity among COPD patients; this was consistent with the observational study in COPD patients in Bulgaria and other real-life studies that have reported high prevalence of cardiovascular-related comorbidities in COPD patients.Citation21,Citation22 Furthermore, a strong correlation was observed between exacerbation and hospitalization rates in this study. Risks of exacerbations and hospitalizations were high in patients with longer duration of COPD (>0.8 years).
Results from the present study were viewed in line with GOLD 2013 recommendations, which were the latest guidelines at the time of protocol development. Recently published GOLD 2017 guidelines emphasize a few important revisions in the diagnostic and treatment approaches for COPD:Citation9 1) spirometry grades are now separated from ABCD combined assessment; 2) for GOLD group B patients who remain symptomatic despite monotherapy, step-up therapy with LABA + LAMA combination has been recommended as a preferred option; and 3) ICS treatment is considered as an alternate choice for groups C and D patients together with long-acting bronchodilators. Use of ICS triple therapy (ICS + LAMA + LABA) has been recommended only for group D patients who experience exacerbations despite treatment with long-acting dual bronchodilation or LABA/ICS combinations. It is important to take into account these changes in GOLD recommendations to optimize the treatment of COPD patients in clinical practice.
There are limitations to this study that must be acknowledged. As this was an observational noninterventional study, selection bias could be an important factor that might have influenced study outcomes. To overcome this bias, a large number of study centers across different parts in Bulgaria were included in the study. Moreover, consecutive enrollment of patients and the targeted number of patients above the calculated minimum sample size should allow the generalizability of data. The possibility of random error from both physician-reported and patient-reported data should be considered. Although not directly related to the outcomes of this study, it should be noted that medications administered to COPD patients in Bulgaria are typically paid for by the National Health Insurance Fund. It is also important to note that although national guidelines are often aligned with global guidelines for disease management, local clinical practice differences, usually based on historical reasons such as differences in the availability of medications, may contribute to nonadherence to global guidelines at a country level.Citation23,Citation24
Conclusion
COPD treatment prescribed in local clinical settings in Bulgaria was generally adequate. This study also shows that routine clinical practice for COPD in Bulgaria deviates from the GOLD 2013 strategy for low-risk patients (groups A and B), while the discrepancy was smaller in high-risk patients (groups C and D). Additional studies are warranted to better understand reasons for this deviation in Bulgaria.
Acknowledgments
The authors thank Ananya Chikramane, PhD, and Rahul Lad, PhD, of Novartis for providing professional medical writing support, which was funded by Novartis Pharma AG in accordance with Good Publication Practice (GPP3) guidelines. This study was funded by Novartis Pharma AG.
Supplementary materials
Figure S1 Treatment patterns over 12 months (efficacy analysis set).
Note: Data are presented as percentage of patients.
Abbreviations: FDC, fixed-dose combination; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; PDE-4, phosphodiesterase-4; SABA, short-acting β2-agonists; SAMA, short-acting muscarinic antagonists.
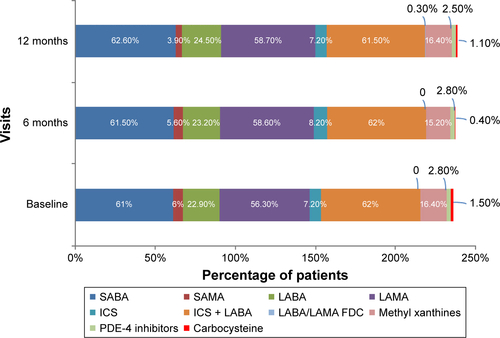
Figure S2 Long-acting inhaled therapy and agreement of treatment with GOLD guidelines (A and B) at baseline; (C and D) at 6 months; (E and F) at 12 months (efficacy analysis set).
Notes: Data are presented as number of patients (A, C, and E) and percentage of patients (B, D, and F). A: GOLD group A; B: GOLD group B; C: GOLD group C; D: GOLD group D.
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist.
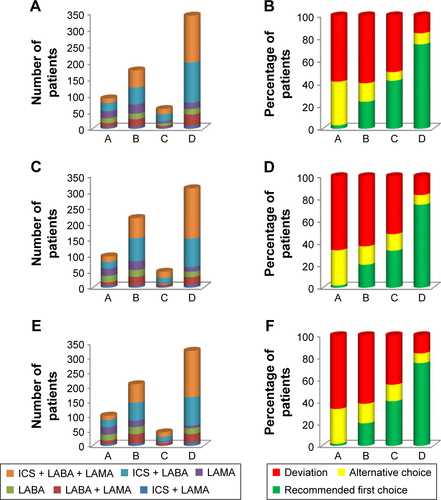
Table S1 List of study centers and contributors
Table S2 Adverse events and serious adverse events, according to system organ class
Disclosure
Yavor Ivanov has received honoraria for delivering lectures for Novartis, GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim Chiesi, and Roche, and reports no other conflicts of interest in this work. Ivan Nikolaev is a Novartis employee and reports no other conflicts of interest in this work. Imola Nemeth reports no conflicts of interest in this work.
References
- FooJLandisSHMaskellJContinuing to confront COPD international patient survey: economic impact of COPD in 12 countriesPLoS One2016114e015261827092775
- MorganADSharmaCRothnieKJQuintJKChronic obstructive pulmonary disease and the risk of stroke: a systematic review protocolBMJ Open2016611e011898
- SorianoJBRodriguez-RoisinRChronic obstructive pulmonary disease overview: epidemiology, risk factors, and clinical presentationProc Am Thorac Soc20118436336721816993
- World Health Organization (WHO)Chronic Obstructive Pulmonary Disease (COPD)2017 Available from: http://www.who.int/respiratory/copd/en/Accessed September 12, 2017
- European Lung White Book [webpage on the Internet]Chronic Obstructive Pulmonary Disease2013 Available from: http://www.erswhitebook.org/chapters/chronic-obstructive-pulmonary-disease/Accessed September 12, 2017
- KamushevaMDimitrovaMvan BovenJFClinical characteristics, treatment patterns, and socio-economic burden of COPD in BulgariaJ Med Econ201720550350928058859
- PavlovPIvanovYGlogovskaPPopovaTSBorisovaENozharovVNew epidemiological data on COPD in the region of PlevenThorac Med2012624550
- The Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD)2016 [homepage on the Internet]. Available from: http://www.goldcopd.orgAccessed September 12, 2017
- The Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD)2017 [homepage on the Internet]. Available from: http://www.goldcopd.orgAccessed September 12, 2017
- ManninoDMYuTCZhouHHiguchiKEffects of GOLD-adherent prescribing on COPD symptom burden, exacerbations, and health care utilization in a real-world settingJ COPD Found201523223235
- SenEGucluSZKibarIAdherence to GOLD guideline treatment recommendations among pulmonologists in TurkeyInt J Chron Obstruct Pulmon Dis2015102657266326715844
- SalinasGDWilliamsonJCKalhanRBarriers to adherence to chronic obstructive pulmonary disease guidelines by primary care physiciansInt J Chron Obstruct Pulmon Dis2011617117921468169
- KoblizekVPecenLZatloukalJReal-life GOLD 2011 implementation: the management of COPD lacks correct classification and adequate treatmentPLoS One2014911e11107825380287
- IvanovYGermanovaENikolaevIA one year non-interventional, observational study to explore the treatment paradigm of COPD in the routine clinical practice in BulgariaThorac Med2014628590
- WorthHBuhlRCrieeCPKardosPMailanderCVogelmeierCThe “real-life” COPD patient in Germany: the DACCORD studyRespir Med2016111647126775251
- VestboJVogelmeierCSmallMHigginsVUnderstanding the GOLD 2011 Strategy as applied to a real-world COPD populationRespir Med2014108572973624675239
- PriceDWestDBrusselleGManagement of COPD in the UK primary-care setting: an analysis of real-life prescribing patternsInt J Chron Obstruct Pulmon Dis2014988990425210450
- AgustiAEdwardsLDCelliBECLIPSE InvestigatorsCharacteristics, stability and outcomes of the 2011 GOLD COPD groups in the ECLIPSE cohortEur Respir J201342363664623766334
- HanMKMuellerovaHCurran-EverettDGOLD 2011 disease severity classification in COPDGene: a prospective cohort studyLancet Respir Med201311435024321803
- LangePMarottJLVestboJPrediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general populationAm J Respir Crit Care Med20121861097598122997207
- HaughneyJGruffydd-JonesKRobertsJLeeAJHardwellAMcGarveyLThe distribution of COPD in UK general practice using the new GOLD classificationEur Respir J2014434993100224176990
- RodriguezLAWallanderMAMartin-MerinoEJohanssonSHeart failure, myocardial infarction, lung cancer and death in COPD patients: a UK primary care studyRespir Med2010104111691169920483577
- MartinABadrickEMathurRHullSEffect of ethnicity on the prevalence, severity, and management of COP in general practiceBr J Gen Pract201262595e76e8122520773
- Asia Pacific COPD Roundtable GroupGlobal Initiative for Chronic Obstructive Lung Disease strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: an Asia-Pacific perspectiveRespirology200510191715691232