Abstract
Background
Static hyperinflation is known to be increased during moderate acute exacerbations of chronic obstructive pulmonary disease (COPD) (AECOPD), but few data exist in patients with severe exacerbations of COPD. The role of dynamic hyperinflation during exacerbations is unclear.
Methods
In a prospective, observational cohort study, we recruited patients admitted to hospital for AECOPD. The following measurements were performed upon admission and again after resolution (stable state) at least 42 days later: inspiratory capacity (IC), body plethysmography, dynamic hyperinflation by metronome-paced IC measurement, health-related quality of life and dyspnea.
Results
Forty COPD patients were included of whom 28 attended follow-up. The IC was low at admission (2.05±0.11 L) and increased again during resolution by 15.6%±23.1% or 0.28±0.08 L (mean ± standard error of the mean, p<0.01). Testing of metronome-paced changes in IC was feasible, and it decreased by 0.74±0.06 L at admission, similarly to at stable state. Clinical COPD Questionnaire score was 3.7±0.2 at admission and improved by 1.7±0.2 points (p<0.01), and the Borg dyspnea score improved by 2.2±0.5 points from 4.4±0.4 at admission (p<0.01).
Conclusion
Static hyperinflation is increased during severe AECOPD requiring hospitalization compared with stable state. We could measure metronome-paced dynamic hyperinflation during severe AECOPD but found no increase.
Introduction
Chronic obstructive pulmonary disease (COPD) is currently the fourth leading cause of death and predicted to become the third by 2020.Citation1 Chronic airflow limitation is the defining characteristic of COPD. This limitation is caused by a mix of small airways disease and parenchymal destruction caused by chronic inflammation.Citation1
Important in the clinical course of COPD are episodes with worsening of respiratory symptoms from the stable state and beyond normal day-to-day variations, which require additional treatment.Citation1 These exacerbations are associated with viral or bacterial airway infections in the majority of cases. Most exacerbations are of mild or moderate severity; only about 4% is categorized as severe.Citation2 Severe exacerbations require hospital admission and are associated with increased mortality, morbidity, and health care costs.Citation3–Citation5
Apart from classification by severity (level of care) and infectious cause, little has been done to categorize exacerbations of COPD. Lopez-Campos and Agusti proposed a dual axes system for categorizing and thereby for treating exacerbations, classifying exacerbations on an axis of severity and of infectious or eosinophilic inflammation.Citation6 We believe that hyperinflation is another important component.Citation7 Hyperinflation is a better predictor of symptoms than most of our physiological parameters and also is a predictor of mortality in stable state.Citation8,Citation9 Furthermore different treatment strategies can be considered for hyperinflated patients.
Static hyperinflation is caused by entrapment of air during expiration, due to peripheral airway obstruction. This can be observed especially by destruction of alveolar attachments to small airways when the disease becomes more severe. Hyperinflation is characterized by increased functional residual capacity (FRC) and reduced inspiratory capacity (IC), resulting in increased dyspnea and limitation of exercise capacity.Citation1,Citation10–Citation12
During tachypnea and exercise, hyperinflation can increase further and this is called dynamic hyperinflation. Dynamic hyperinflation is at least partly caused by a shortening expiration time, thus preventing patients to exhale completely and thereby causing air trapping.Citation13–Citation15 Dynamic hyperinflation of the lungs is known to limit exercise capacity in stable COPD and to impact on the perception of dyspnea.Citation10,Citation16,Citation17
Only a few groups have attempted to study the course of hyperinflation during exacerbations. Parker et al included 7 hospitalized patients and 13 outpatients with moderate exacerbations.Citation18 They measured dyspnea and lung volumes with plethysmography. They found that after resolution of the exacerbation, some COPD patients showed an increase in IC (therefore decrease of hyperinflation) and improvements in dyspnea. Stevenson et al studied admitted patients.Citation19 They measured symptoms with a Borg dyspnea score and volumes with spirometry but lacked direct measurements of hyperinflation for instance with body plethysmography. Although both studies reported dyspnea changes in subgroup analyses, these studies did not analyze the temporal relation between changes in symptoms and changes in hyperinflation. These two studies provide a strong direction of thoughts regarding static hyperinflation. However, they did not completely answer the question whether and to what degree static hyperinflation is present during severe acute exacerbations of COPD and whether increased static hyperinflation is associated with more symptoms. Moreover, they did not assess dynamic hyperinflation.
This study was designed to confirm the presence of static hyperinflation in severe acute exacerbations and to analyze this in more depth with body plethysmography. Secondly, this study was aimed to assess dynamic hyperinflation. Furthermore, we hypothesized that improvement in dyspnea and quality of life during and after admission for an acute COPD exacerbation is closely related to changes in both static and dynamic hyperinflation.
Methods
Subjects
Patients admitted with an acute COPD exacerbation were eligible for the study. The inclusion criteria we employed were as follows: 40 years or older, doctor’s diagnosis of COPD based on an incompletely reversible airflow obstruction defined as 1) a post-bronchodilator forced expiratory flow in 1 second (FEV1)/forced vital capacity (FVC) <70% and 2) post-bronchodilator FEV1<80% predicted. An exacerbation was defined as a worsening of respiratory symptoms from the stable state and beyond normal day-to-day variations, which requires additional treatment. Excluded were patients with an X-ray-confirmed pneumonia, an indication for (non)invasive ventilation, admission to an intensive care unit, unstable angina pectoris or other clinically important cardiac comorbidity requiring admission to a cardiology ward. Additionally, we excluded patients who received any investigational new drug within the last 4 weeks prior to admission. In concordance with the Dutch law and approved by our ethics committee (Medisch Ethische Toetsingscommissie Universitair Medisch Centrum Groningen), informed consent was obtained verbally within the first 24 hours of admission, and written informed consent was obtained in the first 48 hours after the patient had had time and energy to read the paperwork. Data of patients who did not finally provide written informed consent were excluded from the study.
Design
This trial was registered in the World Health Organization approved International Clinical Trials Registry Platform, the Netherlands Trial Registry (NTR 4600). The study was conducted in the emergency room and pulmonary ward of a university teaching hospital in the Netherlands. Participants were tested after inclusion, prior to discharge, and after discharge in stable state at day 42 or later. Patients were allowed entry into the trial only once. Baseline characteristics were obtained, including X-ray, medication use, differential blood count, cultures and swabs for viral polymerase chain reaction. Treatment according to local guidelines included steroids (between 30 and 40 mg of prednisolone), antibiotics, antivirals, oxygen and bronchodilators, all as needed. Individual doses of each were titrated, and the decisions about admittance and discharge were made by the treating physician who was not involved with the study team.
The primary outcomes were the changes in static hyperinflation (as measured by IC via spirometry [IC]) during resolution of the COPD exacerbation and changes in health-related quality of life (primary: Clinical COPD Questionnaire [CCQ] and dyspnea [Borg score]).
The secondary outcomes were the changes in dynamic hyperinflation (as measured by IC during the metronome-paced dynamic hyperinflation test) during resolution of the COPD exacerbation, changes in health-related quality of life by COPD Assessment Test and Modified Medical Research Council dyspnea score (mMRC), changes in other static hyperinflation volumes such as FRC, residual volume, total lung capacity (TLC) as well as changes in FEV1 and FVC during the resolution of the exacerbation.
Procedures
Spirometry was performed on working days during admittance, post-medication; no pre-bronchodilator lung function was attempted. Once during admission and once in stable state, a body plethysmography (Jaeger MasterScreen, CareFusion) was performed as per European Respiratory Society (ERS)/American Thoracic Society (ATS) criteria.Citation20 When measuring static hyperinflation, the lung function technician aimed to measure the volumes at an elastic recoil pressure of the respiratory system of zero.
Metronome-paced hyperinflation was performed once during admission and stable state. Subjects were requested to breathe at a metronome-paced frequency of 40/minute during 30 seconds. Before increased pacing and immediately afterwards an IC maneuver was performed. Subjects were coached to maintain as much as possible a stable tidal volume. After at least 2 minutes, this measurement was repeated. Acceptability criteria of <150 mL and/or <5% were used (Oxycon Jaeger, CareFusion).
Pulmonary function testing during acute exacerbations is difficult for both patients and staff. If patients failed to produce a reliable value, the test was disregarded and was treated as missing value.
The diagnosis of an infection was established if either the culture or the nose swab tested positive. The swab used a polymerase chain reaction with primers for the 15 most common respiratory viruses in the Netherlands with a cutoff cycle threshold of 40.
Statistical analyses
Data were analyzed with IBM SPSS 24. Normally distributed data with 2 time points were assessed with paired t-tests. Variables with multiple time points were first assessed by ANOVA to obtain an f ratio. If the f ratio indicated a significant difference, a paired t-test was performed to assess the difference between admission and stable state. Unpaired t-tests were used to compare unpaired means. Bivariate correlations by Pearson were calculated to assess correlations between two variables.
No formal power calculation was deemed possible since no relevant data were identified adequately reflecting our target population. Based on previous studies we anticipated dropouts; our ambition to perform reliable measurements based on the ATS/ERS criteria would only increase that. Aiming to measure a difference of 100 mL of change in IC, we roughly estimated the necessity of 25 evaluable patients. This estimated sample size was approved by the ethics committee.
Results
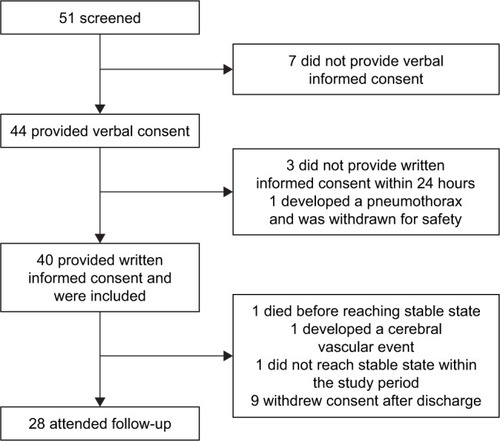
From September 2014 till April 2016 patients admitted to the respiratory ward from our tertiary university hospital with an acute exacerbation of COPD were recruited. Forty-four patients provided their verbal informed consent upon admittance, 41 of them provided written informed consent within the first 24 hours; one of them developed a pneumothorax and had to be excluded from the analysis, leaving 40 subjects to be included in the study. Of 12 patients, no follow-up was obtained largely due to not being in stable state or not able to attend the follow-up. A flowchart of the study is provided in . Baseline characteristics are provided in .
Table 1 Baseline characteristics
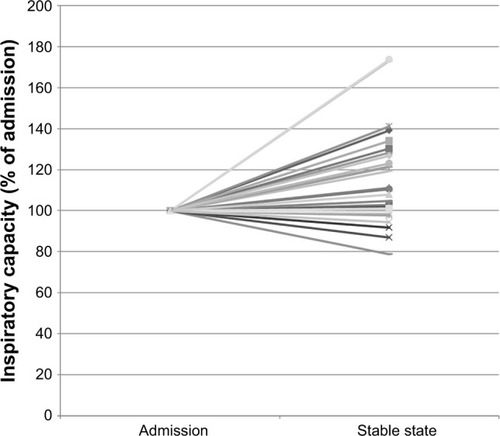
The primary endpoint, change in static hyperinflation measured by IC, showed an improvement of 0.28±0.08 L or 15.6%±23.1% (mean±standard error of the mean) from admission to stable state (p<0.01). This was accompanied by an improvement in CCQ of −1.7±0.2 points and in Borg score of −2.2±0.5 points. No correlation between the change in IC and change in CCQ (r=0.12, p=0.58) or change in Borg (r=−0.2, p=0.36) was found. The relative changes in the IC of each individual participant are plotted in .
Figure 2 The relative changes in the inspiratory capacity of each individual participant.
Of the secondary endpoints, static hyperinflation, measured as change in FRC by body plethysmography, improved significantly by 334±102 mL (p<0.01). Residual volume decreased significantly by 501±140 mL or as percentage predicted 22%±6% (p<0.01). As expected, TLC did not change during the resolution of the exacerbation. Dynamic hyperinflation (as measured by change in IC during a metronome paced test) did not change significantly during the recovery from the exacerbation. Symptoms improved during the resolution of the exacerbation in all questionnaires (). The change in dynamic hyperinflation did not correlate with the change in symptoms.
Table 2 Recovery from severe acute exacerbations of chronic obstructive disease measured at admission, discharge and in stable state
We compared patients with additional hyperinflation during the exacerbation with patients without additional hyperinflation (). The additionally hyperinflated group was defined as those patients whose IC improved 100 mL or more after recovery. The hyperinflated group had larger ICs and lower mMRC dyspnea scores (both significant) in stable state and additionally tendencies for greater improvement in mMRC and Borg dyspnea scores (both nonsig-nificant) during resolution. Interestingly, the group without additional hyperinflation during exacerbation had a higher number of eosinophils in peripheral blood at admission and a lower body mass index.
Table 3 Differences between patients with additionally increased hyperinflation during their exacerbation versus patients without additional hyperinflation
No difference in dynamic hyperinflation between the groups was detected. Subdividing the groups based on IC/TLC greater or lesser than 0.25 (instead of on decreased IC) yielded similar results, independent of whether exacerbation or stable state data were used.
An exacerbation is often associated with a viral or bacterial airway infection. To assess the hyperinflation in relation to these infections, hyperinflation was analyzed in the subgroups based on the presence or absence of a viral or bacterial infection (). A significant change in static hyperinflation was observed in patients with a culture-positive bacterial infection, whereas no change was detected in the group without bacterial infection; these changes, however, did not statistically differ from one another (p=0.32). No difference was found in change in hyperinflation in patients with versus without a viral infection. No difference in dynamic hyperinflation was observed between any of the subgroups.
Table 4 Differences in hyperinflation between exacerbations with and without a viral or bacterial infection
Discussion
This study showed a more complete picture of the course of static and dynamic hyperinflation in patients hospitalized for acute severe exacerbations of COPD, and its resolution toward stable state. Static hyperinflation is increased during acute severe exacerbations compared with stable state. We were able to measure dynamic hyperinflation during the exacerbation but found no further increase (more than the increase in static hyperinflation). No correlation between change in hyperinflation and symptoms was found.
COPD exacerbations are the main cause for admissions of COPD patients, and hospital-related mortality and morbidity in COPD patients is high. Nevertheless, little is known about the physiology of such exacerbations,Citation3,Citation21–Citation24 and there is not really a universally accepted clinical definition of a COPD exacerbation nor of strict criteria when to admit.Citation3,Citation25 Although efforts have been made to better define and prevent exacerbations in several recent trials, the treatment of an exacerbation in the hospital has remained mostly unchanged for the last 2 decades.Citation1,Citation26–Citation29
Two previous studies assessed static hyperinflation in the setting of an acute exacerbation of COPD. Our results in inpatients are in line with the results of the study of Parker et al (n=20) who studied mostly outpatients with less severe exacerbations than in the current study.Citation18 The study of Stevenson et al (n=22) did study the same group of patients as the current study but provided only the change in IC as measured by spirometry, without the confirmation of body plethysmography. Our data confirm their results and extend them with additional measurements.Citation19
Assessment of dynamic hyperinflation has not been described earlier in the setting of an acute severe exacerbation of COPD to our knowledge. Patients were measured in this trial with a metronome-paced test aimed at changes in IC during tachypnea.Citation12,Citation30 No changes in dynamic hyperinflation between the exacerbation and stable state were found. Multiple explanations are possible for this result. It could indicate that patients with an exacerbation severe enough to require admission are limited by something else than hyperinflation, eg, airway resistance, mucus, hypercapnia or a change in ventilation/perfusion ratio. Another explanation could be that admitted patients already have a severely decreased inspiratory reserve capacity before admission and hardly have any room for further deterioration, as opposed to patients who do not need admission and in whom past data have shown decreasing ICs during exacerbations. Another explanation lies in the breathing frequency and tidal volume during the metronome-paced dynamic hyperinflation test. Due to ethical and practical concerns we imposed a frequency of 40, with a stable tidal volume during both tests. An alternative would have been to double their breathing frequency, which we deemed impossible for patients during severe exacerbations of COPD. A further increase in breathing frequency could have allowed to find an additional dynamic hyperinflation component. Next, measurement of static hyperinflation depends on being able to measure at zero elastic recoil level.Citation13 Although every attempt was made to achieve this, it is more difficult to achieve during severe exacerbations. If this elastic recoil cannot be achieved for the first FRC of the dynamic measurement, ie, before increasing the breathing frequency, dynamic hyperinflation (decrease in IC) during metronome pacing will be underestimated. Finally, one could discuss whether the test used in the current trial is the optimal standard to detect dynamic hyperinflation.Citation13,Citation31 Based upon ethical arguments, the study team chose not to perform the more commonly used and better validated exercise test during the acute distress of severe exacerbation requiring admission.
Hyperinflation might provide a target for therapeutic strategies in patients with severe exacerbations. Patients admitted with a severe exacerbation of COPD are most commonly treated with short-acting bronchodilators via nebulizers. Long-acting bronchodilators, both anticholinergics and beta-2-mimetics, have been shown in stable state to provide larger reductions in hyperinflation compared to short-acting bronchodilators, alongside greater increases in flows.Citation32–Citation37 Long-acting bronchodilators, however, are not commonly available via nebulizers. A recent Cochrane review showed that after several decades of treatment with nebulizers, there is still no evidence to favor nebulizers over regular pressurised metered dose inhalers with good instruction.Citation26 New studies should shed light on the potential of combined long-acting bronchodilators on reduction of hyperinflation during severe exacerbations requiring hospitalization. Such a trial could compare combined long-acting bronchodilators in currently available pressurised metered dose inhalers or dry powder inhalers versus short-acting bronchodilators by nebulizer, the latter being usual care in many hospitals. Based on the finding that hyperinflation is increased during exacerbations, we can speculate that the long-acting bronchodilators provide an early treatment for impending hyperinflation-predominant exacerbations of COPD, thus preventing some of them.Citation38,Citation39 Other strategies such as rehabilitation and noninvasive ventilation have been shown to reduce hyperinflation, while cognitive-behavioral strategies and perhaps even bronchoscopic lung volume reduction interventions could be further investigated as treatment of hyperinflation in selected patients during an acute event.Citation7,Citation40
Interestingly, patients who did hyperinflate during an exacerbation of COPD had higher ICs during stable state and fewer symptoms (CCQ, mMRC and Borg) both in stable state and during exacerbations. In other words, they had a better preserved inspiratory reserve capacity. This could perhaps also explain the lack of correlation between decrease in IC (worsening of hyperinflation) and increase in symptoms. One could argue that patients who do not hyperinflate during an exacerbation are those patients who in stable state already have a flow limitation and are less able to increase their IC. This could result in more symptoms both during and after the exacerbation. This explanation is supported by the nonsignificant observation that symptoms improve more during resolution in patients with additional hyperinflation during exacerbations.
Increased static hyperinflation was found in patients with a bacterial or viral infection. The patients without a cultured bacterial infection showed no increased static hyperinflation during their exacerbations. These changes, however, do not significantly differ. This might suggest that the presence or absence of increased hyperinflation is related with an infectious origin; however, more research and a larger sample size would be necessary before drawing conclusions from this subgroup analysis.
This study has several strengths but also has weaknesses that should be considered. A strong point of the study was that treatment decisions such as bronchodilator dose and discharge were made by the treating physician without influence from the trial team and without influence by the study measurement results. Another strong point of the trial is that we excluded patients with pneumonia. This will make the results from the trial more applicable toward exacerbations, since pneumonia might influence lung volumes. Patients who were in such distress that (non) invasive ventilation was required were also excluded in order to prevent bias due to inability to perform reliable pulmonary function tests. A weakness of the study is that it has been performed in only one center, potentially limiting the applicability of its results. Due to the severity of the exacerbation and disease, a relative high number of patients was not able or willing to provide reproducible pulmonary function tests or attend follow-up. Especially the intensive tests including the static and dynamic hyperinflation tests and spirometry repeatedly during the hospitalization were quite a burden on patients.
Static hyperinflation turned out to be an important feature of severe acute exacerbations of COPD in our population. We believe that this supports discussions whether the occurrence of hyperinflation should be incorporated in a new definition, since it is a common but not universal finding and opens a path toward a more precision medicine strategy in treatment. To our surprise, changes in hyperinflation were not directly correlated with symptoms although hyperinflated patients showed lower changes in symptoms. There must be other factors as well. Perhaps a model incorporating hyperinflation along with the current parameters of inflammation and respiratory infections will help to work on a future definition.
In summary, this study measured changes in static and dynamic hyperinflation during acute severe exacerbations of COPD requiring hospital admittance. The increases in static hyperinflation were anticipated based on two earlier studies, only partially performed in hospital with less severe exacerbations. They have now been confirmed with body plethysmography. We were bold enough to attempt at measuring dynamic hyperinflation during acute exacerbations in the hospital setting, but could not find a further increase over and above the change in static hyperinflation already induced by the exacerbation.
Acknowledgments
We thank Jantien Remmelink, Margot Klijnsma and Alice Niemeijer for the collection of data and for performing measurements. We thank Joost van den Aardweg for his support with some of the calculations. We would like to thank our complete pulmonary function team, especially Marga Star-Kroesen, Martijn Farenhorst, Margrietha Swieringavan der Veen, Yvonne Valkema-Tol, Wies Heins-Konigers and Jenny Stevens-van der Vinne for their time, flexibility and dedication to perform and schedule the pulmonary function tests during the study.
Disclosure
WHVG reports a grant for an investigator-initiated trial to University Medical Center Groningen from Novartis and an ERS short-term research fellowship, outside the submitted work. HAMK reports that his institution (University Medical Center Groningen) has received a fee per patient for recruitment in trials and a grant for investigator-initiated studies from GlaxoSmithKline, Novartis, and FLUIDDA. Additionally, his institution has received grants as well as consultancy fees from Novartis, AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline, all outside the submitted work. The authors report no other conflicts of interest in this work.
References
- VogelmeierCFCrinerGJMartinezFJGlobal Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD executive summaryAm J Respir Crit Care Med2017195555758228128970
- WedzichaJABanerjiDChapmanKRIndacaterol-glycopyrronium versus salmeterol-fluticasone for COPDN Engl J Med2016374232222223427181606
- WedzichaJACalverleyPMAAlbertRKPrevention of COPD exacerbations: a European Respiratory Society/American Thoracic Society guidelineEur Respir J2017503160226528889106
- MüllerovaHMaselliDJLocantoreNHospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohortChest20151474999100725356881
- DonaldsonGCWedzichaJAThe causes and consequences of seasonal variation in COPD exacerbationsInt J Chron Obstruct Pulmon Dis201491101111025336941
- Lopez-CamposJLAgustiAHeterogeneity of chronic obstructive pulmonary disease exacerbations: a two-axes classification proposalLancet Respir Med20153972973426165134
- van GeffenWHSlebosDJKerstjensHAHyperinflation in COPD exacerbationsLancet Respir Med2015312e43e4426679031
- MooreAJSolerRSCettiEJSniff nasal inspiratory pressure versus IC/TLC ratio as predictors of mortality in COPDRespir Med201010491319132520399631
- CasanovaCCoteCde TorresJPInspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2005171659159715591470
- O’DonnellDEElbehairyAFWebbKANederJACanadian Respiratory Research NetworkThe link between reduced inspiratory capacity and exercise intolerance in chronic obstructive pulmonary diseaseAnn Am Thorac Soc201714Suppl 1S30S3928398073
- MahlerDAO’DonnellDERecent advances in dyspneaChest2015147123224125560861
- CooperCBThe connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and functionAm J Med200611910 Suppl 1213116996896
- RossiAAisanovZAvdeevSMechanisms, assessment and therapeutic implications of lung hyperinflation in COPDRespir Med2015109778580225892293
- O’DonnellDELavenezianaPThe clinical importance of dynamic lung hyperinflation in COPDCOPD20063421923217361503
- O’DonnellDEHyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary diseaseProc Am Thorac Soc20063218018416565429
- LangerDCiavagliaCENederJAWebbKAO’DonnellDELung hyperinflation in chronic obstructive pulmonary disease: mechanisms, clinical implications and treatmentExpert Rev Respir Med20148673174925159007
- GuenetteJAWebbKAO’DonnellDEDoes dynamic hyperinflation contribute to dyspnoea during exercise in patients with COPD?Eur Respir J201240232232922183485
- ParkerCMVoducNAaronSDWebbKAO’DonnellDEPhysiological changes during symptom recovery from moderate exacerbations of COPDEur Respir J200526342042816135722
- StevensonNJWalkerPPCostelloRWCalverleyPMLung mechanics and dyspnea during exacerbations of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2005172121510151616166620
- MillerMRHankinsonJBrusascoVATS/ERS Task ForceStandardisation of spirometryEur Respir J200526231933816055882
- KoFWChanKPHuiDSAcute exacerbation of COPDRespirology20162171152116527028990
- HawkinsPEAlamJMcDonnellTJKellyEDefining exacerbations in chronic obstructive pulmonary diseaseExpert Rev Respir Med20159327728626013261
- WedzichaJASinghRMackayAJAcute COPD exacerbationsClin Chest Med201435115716324507843
- HurstJRVestboJAnzuetoAEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) InvestigatorsSusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- LeidyNKWilcoxTKJonesPWRobertsLPowersJHSethiSEXACT-PRO Study GroupStandardizing measurement of chronic obstructive pulmonary disease exacerbations. Reliability and validity of a patient-reported diaryAm J Respir Crit Care Med2011183332332920813886
- van GeffenWHDoumaWRSlebosDJKerstjensHABronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPDCochrane Database Syst Rev20168CD011826
- BathoornEGroenhofFHendrixRReal-life data on antibiotic prescription and sputum culture diagnostics in acute exacerbations of COPD in primary careInt J Chron Obstruct Pulmon Dis20171228529028144133
- BrillSEWedzichaJAOxygen therapy in acute exacerbations of chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis201491241125225404854
- van GeffenWHBruinsMKerstjensHADiagnosing viral and bacterial respiratory infections in acute COPD exacerbations by an electronic nose: a pilot studyJ Breath Res201610303600127310311
- GelbAFGutierrezCAWeismanIMNewsomRTaylorCFZamelNSimplified detection of dynamic hyperinflationChest200412661855186015596684
- KloosterKten HackenNHHartmanJESciurbaFCKerstjensHASlebosDJDetermining the role of dynamic hyperinflation in patients with severe chronic obstructive pulmonary diseaseRespiration201590430631326352833
- O’DonnellDECasaburiRFrithPEffects of combined tiotropium/olodaterol on inspiratory capacity and exercise endurance in COPDEur Respir J2017494160134828424359
- MahlerDAKerstjensHADonohueJFBuhlRLawrenceDAltmanPIndacaterol vs tiotropium in COPD patients classified as GOLD A and BRespir Med201510981031103926094050
- KerstjensHADesleeGDahlRThe impact of treatment with indacaterol in patients with COPD: a post-hoc analysis according to GOLD 2011 categories A to DPulm Pharmacol Ther20153210110825743376
- PowrieDJWilkinsonTMDonaldsonGCEffect of tiotropium on sputum and serum inflammatory markers and exacerbations in COPDEur Respir J200730347247817504798
- O’DonnellDEFlügeTGerkenFEffects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPDEur Respir J200423683284015218994
- CasaburiRMaltaisFPorszaszJ205.440 InvestigatorsEffects of tiotropium on hyperinflation and treadmill exercise tolerance in mild to moderate chronic obstructive pulmonary diseaseAnn Am Thorac Soc20141191351136125289942
- WedzichaJAAgustiADonaldsonGChuecosFLamarcaRGarcia GilEEffect of aclidinium bromide on exacerbations in patients with moderate-to-severe COPD: a pooled analysis of five Phase III, randomized, placebo-controlled studiesCOPD201613666967627159613
- WilkinsonTMDonaldsonGCHurstJRSeemungalTAWedzichaJAEarly therapy improves outcomes of exacerbations of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2004169121298130314990395
- van GeffenWHKerstjensHAMSlebosDJEmerging bronchoscopic treatments for chronic obstructive pulmonary diseasePharmacol Ther20171799610128527920